1 Introduction
Chrysanthemum is grown worldwide and is considered to be one of the most important ornamental plants. Although desirable traits have been introduced into Chrysanthemum by conventional breeding, there are limitations to such programs due to the limited gene pool and cross incompatibility [1]. Therefore, Chrysanthemum breeders have begun to exploit Agrobacterium-mediated transformation methods to develop novel cultivars to satisfy current market trends. Efficient genetic transformation of leaf explants of Chrysanthemum has been carried out by many researchers [2–4]. However, this approach requires more efficient in vitro regeneration procedures.
In vitro adventitious shoot regeneration is the most commonly used technique for creating new cultivars of Chrysanthemum, both in mutation breeding as well as in genetic transformation [5]. However, adventitious shoot regeneration of Chrysanthemum is affected by many factors, including interactions among plant growth regulators, dark incubation period, gelling agents, ethylene inhibitor, explant type, and plant genotype [5–7]. Moreover, an efficient protocol for one cultivar may not be easily adaptable to other cultivars. Therefore, standardization of efficient protocols for each newly released cultivar is necessary to promote micropropagation and genetic transformation. The present study was also conducted to determine the effects of plant growth regulators, dark incubation period, gelling agents, and silver nitrate on in vitro shoot regeneration from leaf segments of Chrysanthemum cv. Vivid Scarlet. The established protocol was applicable to shoot regeneration for four out of the six cultivars tested.
2 Material and methods
2.1 Plant materials
Unrooted cuttings of Chrysanthemum cv. Vivid Scarlet provided by the National Institute of Horticultural and Herbal Science (South Korea) were transplanted into a greenhouse at 25 °C under ambient long-day conditions. After 4 weeks, the shoot tips were taken and then thoroughly washed under running tap water with 5% of liquid detergent. They were subsequently surface-sterilized with a 70% (v/v) ethanol solution for 30 s and 1% NaOCl plus Tween 20 for 5 min, followed by five rinses with sterilized distilled water. The sterilized shoot tips were then cultured on plant growth regulator (PGR)-free Murashige and Skoog (MS) medium containing 1 g L−1 of activated charcoal to induce plant growth. After 6 weeks of culture, the youngest fully expanded leaves from the in vitro-grown healthy and uniform plants were used as a source of explants for further experiments.
2.2 Optimization of plant growth regulators (PGRs) and dark incubation period on shoot regeneration
To determine the best combination of plant growth regulators (PGRs) for optimal shoot regeneration, leaves were cut into 0.5–1-cm-long segments and cultured on MS medium supplemented with various concentrations of 6-benzyladenine (BA) and α-naphthaleneacetic acid (NAA), as shown in Table 1, along with 3 g L−1 of Gelrite. The explants were cultured under condition with 16 h photoperiod of 37 μmol m−2 s−1 for 45 days.
Effects of plant growth regulators on the percentage of shoot regeneration and the mean number of shoots per leaf explant of Chrysanthemum cv. Vivid Scarlet.
| PGRs (mg L−1) | No initial dark incubation | ||
| BA | NAA | Shoot formation % | Mean No. of shoots |
| 1 | 1 | 0b | 0c |
| 2 | 100a | 12.3a | |
| 3 | 100a | 8.0b | |
| 2 | 1 | 0b | 0c |
| 2 | 0b | 0c | |
| 3 | 100a | 8.3b | |
| 3 | 1 | 0b | 0c |
| 2 | 0b | 0c | |
| 3 | 0b | 0c |
To investigate the effects of the dark incubation period on shoot regeneration, explants were prepared as described above (0.5–1-cm-long segments), and the resulting explants were incubated for 10, 20, or 30 days in the dark, after which they were subjected to culture conditions with 16 h photoperiod of 37 μmol m−2 s−1 and kept for 45 days, including the initial dark incubation period.
2.3 Evaluation of the role of the gelling agent on shoot regeneration
Leaf segments were cultured on a MS medium supplemented with 1 mg L−1 of BA and 2 mg L−1 of NAA, which resulted in the highest number of shoots per explant. To investigate the effects of the gelling agents on the capacity of shoot regeneration, the induction medium was solidified with 7 g L−1 of Agar (Duchefa Biochemie, The Netherlands), 4 g L−1 of Agarose (Cambrex, USA), 3 g L−1 of Gelrite (Duchefa Biochemie, The Netherlands), or 3 g L−1 of Phytagel (Duchefa Biochemie, the Netherlands). The explants were cultured under condition with 16 h photoperiod of 37 μmol m−2 s−1 for 45 days.
2.4 Effect of silver nitrate on shoot regeneration
A medium supplemented with 1 mg L−1 of BA, 2 mg L−1 of NAA, and 3 g L−1 of Gelrite showed the best results for shoot induction. Therefore, different concentrations (0–5 μM) of silver nitrate were added to this shoot induction medium to test its effects on the capacity of shoot induction from leaf segments. The explants were cultured under condition with 16 h photoperiod of 37 μmol m−2 s−1 for 45 days.
2.5 Evaluation of the role of gelling agents on rooting and plant growth of regenerated shoots in vitro
To investigate the effects of gelling agents on rooting and plant growth of regenerated shoots in vitro, regenerated shoots were transferred to a plant-growth regulator (PGR)-free MS medium solidified with the following gelling agents: 7 g L−1 of Agar (Duchefa Biochemie, the Netherlands), 4 g L−1 of Agarose (Cambrex, USA), 3 g L−1 of Gelrite (Duchefa Biochemie, the Netherlands), or 3 g L−1 of Phytagel (Duchefa Biochemie, the Netherlands). The explants were cultured under conditions with a 16-h photoperiod of 37 μmol m−2 s−1 for 45 days.
2.6 Experimental design and statistical analysis
All cultures contained 3% of sucrose, and each treatment consisted of 30 explants and three replicates. For shoot regeneration, the number of usable shoots (about 3 cm in height) formed per explant was recorded after 45 days of culture. Similarly, plant growth parameters were considered after 45 days of culture. All data were analyzed using ANOVA, and means were compared by DMRT (P > 0.05).
2.7 Analysis of the ploidy level
Plantlets obtained from hormone-free MS medium solidified with Gelrite were selected at random and subjected to flow cytometry, which was performed as described by Naing et al. [8]. Briefly, approximately 20 mg of tissue per sample were chopped using a sharp razor blade in a plastic Petri dish containing 500 mL of a nuclei extraction buffer (Partec, GmbH, Munster, Germany) to obtain a fine suspension. The samples were then filtered through a nylon mesh (50 μm), after which 2 mL of staining buffer (Partec, GmbH, Munster, Germany) were added and the suspension supplemented with propidium iodide. The ploidy level of each sample was then measured using a flow cytometer (CyFlow@ Ploidy Analyser, Partec, GmbH, Munster, Germany). A minimum of 2000 particles (total count) was analyzed in each sample, and the measurements were carried out using at least three samples. The leaf tissue from mother plants in the greenhouse was used as a hexaploid reference.
2.8 Effect of genotype on shoot regeneration
To determine the effect of the cultivar on shoot induction, 6-week-old in vitro grown expanding leaves of several cultivars (Borami, Peach Andy, Pink Pride, Star, Baeksun, Baekma, and Shinma) were cut into 0.5–1.0-cm-long segments, which were then cultured on a shoot induction medium supplemented with the combination of 1 mg L−1 BA and 2 mg L−1 NAA along with 3 g L−1 of Gelrite. The explants were maintained under culture conditions, as explained above.
3 Results
3.1 Optimization of plant growth regulators (PGRs) and dark incubation period on shoot regeneration
Leaf explants were cultured on MS medium containing different concentrations of BA and NAA under continuous light conditions without any dark initial period, after which morphogenetic changes in the explants were observed after 2 weeks of culture. Adventitious shoot primordia were visible on the surface of leaf explants (Fig. 1A). Development of shoots occurred over the next 3 weeks after initiation. Shoot regenerability of leaf explants was highly variable in response to PGR concentrations. Of the various PGR combinations tested, only a few combinations showed shoot regenerability, especially media containing higher concentrations of NAA than BA (Table 1).

(Color online). Plant regeneration through shoot organogenesis from leaf segments of Chrysanthemum cv. Vivid Scarlet. A. Formation of shoot primordia on leaf surfaces. B. Development of shoot primordia into shoots. C. Effects of various gelling agents on in vitro shoot growth and rooting. D. Acclimatization of in vitro regenerated plantlets to the greenhouse.
Shoot regeneration occurred for all combinations of PGRs when explants were subjected to an initial dark period of 10 days (Table 2). However, PGR combinations that showed shoot induction under continuous light condition showed negative effects on shoot regenerability when the explants were cultured with the initial dark period of 10 days. Reduction of shoot regeneration was also detected for all PGR combinations when explants were incubated for 20 days in the dark (Table 3). Total inhibition of shoot regeneration was observed for some PGR concentrations upon dark incubation for 30 days (Table 4). Therefore, PGR concentration and dark incubation period had interactive effects on shoot regeneration. Our results show that a dark incubation period of 10 days resulted in a more reasonable number of shoots per explant in most PGRs combinations compared to the other periods (0, 20, or 30 days). However, the highest frequency of shoot regeneration as well as the mean number of shoots per explant were obtained using a medium containing a combination of 1 mg L−1 of BA and 2 mg L−1 of NAA under continuous light conditions without any initial dark period. Thus, this medium composition and culture conditions were used in further experiments.
Effects of plant growth regulators and of the dark incubation period on the percentage of shoot regeneration and the mean number of shoots per leaf explant of Chrysanthemum cv. Vivid Scarlet.
| PGRs (mg L−1) | 10-day-dark incubation | ||
| BA | NAA | Shoot formation % | Mean No. of shoots |
| 1 | 1 | 80c | 2.7e |
| 2 | 80c | 6.0b, c, d | |
| 3 | 80c | 5.3d | |
| 2 | 1 | 90b | 6.7b, c |
| 2 | 60d | 5.3d | |
| 3 | 80c | 5.7c, d | |
| 3 | 1 | 90b | 7.0b |
| 2 | 100a | 6.7b, c | |
| 3 | 100a | 11.3a |
Effects of plant growth regulators and of the dark incubation period on the percentage of shoot regeneration and the mean number of shoots per leaf explant of Chrysanthemum cv. Vivid Scarlet.
| PGRs (mg L−1) | 20-day-dark incubation | ||
| BA | NAA | Shoot formation % | Mean No. of shoots |
| 1 | 1 | 0b | 0f |
| 2 | 0e | 2.0d, e | |
| 3 | 70b | 1.3e, f | |
| 2 | 1 | 60c | 5.7a, b |
| 2 | 70b | 3.7c, d | |
| 3 | 40d | 4.3b, c | |
| 3 | 1 | 80a | 2.7c, d, e |
| 2 | 60c | 3.3c, d | |
| 3 | 80a | 7.0a |
Effects of plant growth regulators and of the dark incubation period on the percentage of shoot regeneration and the mean number of shoots per leaf explant of Chrysanthemum cv. Vivid Scarlet.
| PGRs (mg L−1) | 30-day-dark incubation | ||
| BA | NAA | Shoot formation % | Mean No. of shoots |
| 1 | 1 | 0e | 0c |
| 2 | 0e | 0c | |
| 3 | 0e | 0c | |
| 2 | 1 | 26.7c, d | 1.7a, b |
| 2 | 33.3b, c | 1.3b, c | |
| 3 | 36.7b | 2.0a, b | |
| 3 | 1 | 0e | 0c |
| 2 | 46.7a | 3.0a | |
| 3 | 23.3d | 1.3b, c |
3.2 Evaluation of the role of the gelling agents on shoot regeneration
The effects of the gelling agents on shoot formation from leaf explants of Chrysanthemum are presented in Fig. 2A and B. Shoot formation was observed in all media after 3 weeks, and the percentages of shoot formation were not significantly different among the gelling agents. However, the mean number of shoots formed per explant was dependent on the gelling agent according to the following order: Gelrite > Phytagel > Agar > Agarose. Shoots induced by Gelrite were likely to be more vigorous and healthy than those induced by other gelling agents. Explants cultured on Agarose medium curled upward and resulted in shoots with abnormal patterns, which was in contrast to explants cultured on other gelling agents. This result clearly indicates that Agarose had stronger negative effects on shoot regeneration compared to Phytagel or Agar, both of which induced lower shoot regenerability than Gelrite. Thus, Gelrite was the best gelling agent for increasing both the percentage of shoot formation as well as the number of shoots per explant.
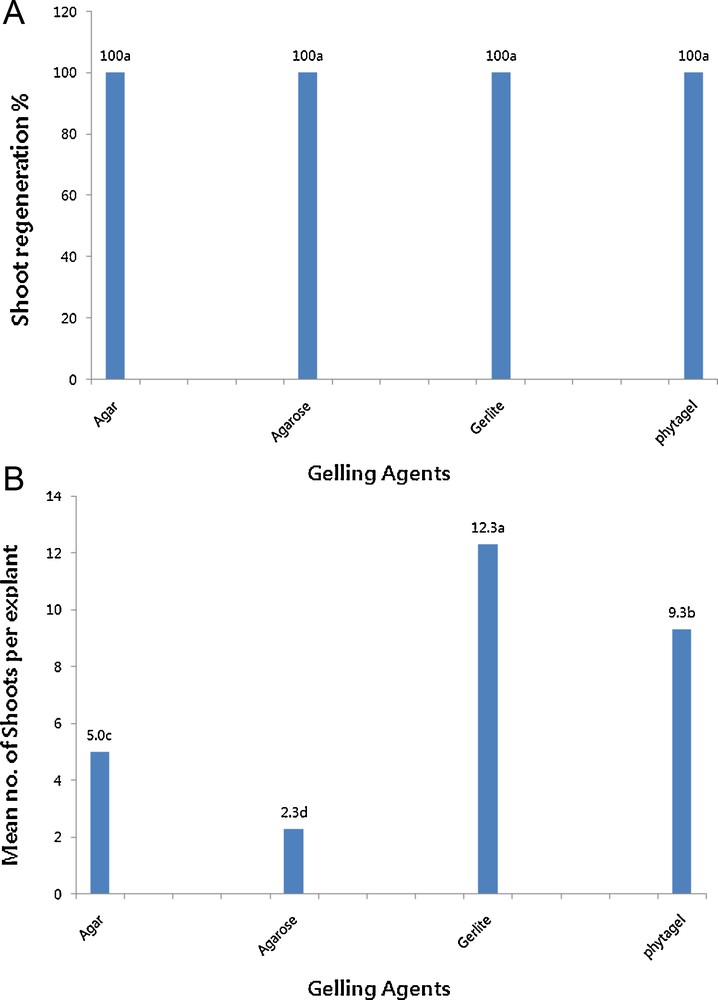
Effects of gelling agents on the percentages of shoot regeneration (A) and the mean number of shoots per (B) leaf explant of Chrysanthemum cv. Vivid Scarlet grown on a medium containing 1 mg L−1 of benzyladenine and 2 mg L−1 of α-naphthaleneacetic acid.
3.3 Effects of silver nitrate on shoot regeneration
To examine the stimulatory effects of silver nitrate on shoot regeneration from leaf explants of Chrysanthemum, explants were cultured on a shoot induction medium solidified with Gelrite. As shown in Fig. 3A and B, the presence of silver nitrate markedly reduced shoot regeneration compared to control. In addition, leaf burning was observed on shoots treated with 1 or 5 μM of silver nitrate. Elevation of silver nitrate concentrations up to 10 or 25 μM completely inhibited shoot regeneration. Based on these findings, silver nitrate had highly negative effects on shoot regeneration from leaf explants of Chrysanthemum.
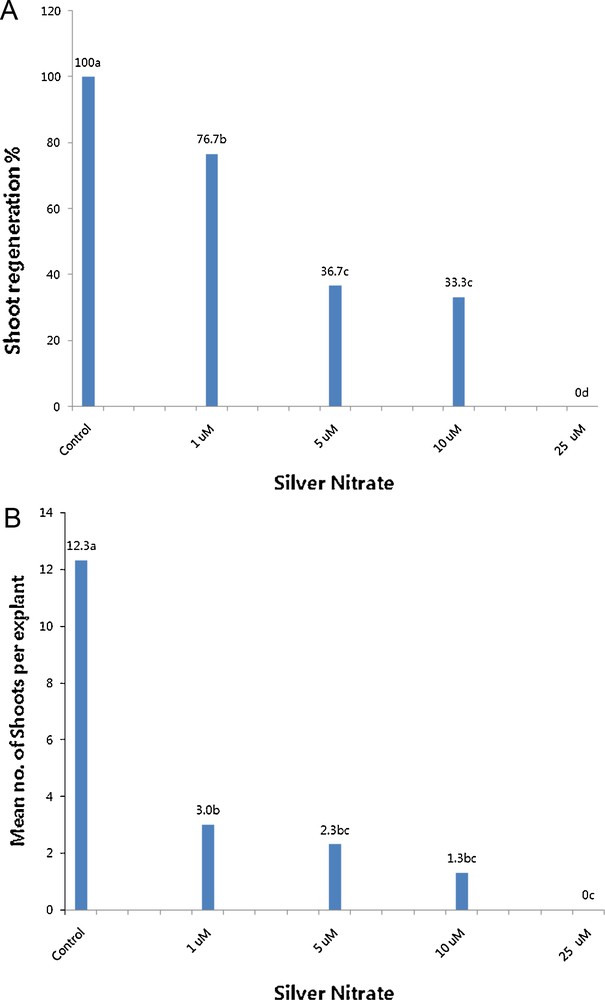
Effects of silver nitrate on the percentages of shoot regeneration (A) and the mean number of shoots per (B) leaf explant of Chrysanthemum cv. Vivid Scarlet grown on a medium containing 1 mg L−1 of benzyladenine and 2.0 mg L−1 of α-naphthaleneacetic acid, and 3 g L−1 of Gelrite.
3.4 Evaluation of the role of gelling agents on rooting and plant growth in vitro
The data presented in Table 5 show that plant growth parameters were influenced by the type of gelling agent. Shoots grown on Gelrite showed better parameters, especially for number of roots, plant height, and fresh weight, compared to the other gelling agents (Fig. 1C). However, Agar produced the highest average number of leaves per plantlet as well as it showed superior effects on most parameters compared to Phytagel and Agarose. Similar to its effect on shoot regeneration, Agarose had negative effects on rooting and plant growth. Further, Gelrite resulted in the greatest number of shoots per explant along with improved plant growth compared to the other gelling agents. Plantlets that reached approximately 4–5 cm in height were transferred to an acclimatization pot (Fig. 1D), and more than 90% of plants survived in the greenhouse.
Effects of gelling agents on in vitro plant growth and rooting of Chrysanthemum cv. Vivid Scarlet.
| Gelling agents | Mean no. of leaves | Mean no. of roots | Plant height (cm) |
Fresh weight (mg) |
| Gelrite | 6.7b | 12.3a | 5.7a | 466.7a |
| Phytagel | 6.3b | 7.7c | 4.3b | 273.3c |
| Agar | 9.3a | 9.7b | 5.1a, b | 453.7b |
| Agarose | 6.0b | 9.7b | 3.5c | 235d |
3.5 Analysis of the ploidy level
Histograms based on the mother plant (control) and plantlets derived from hormone-free medium are shown in Fig. 4A and B. The flow cytometry results show that peaks of the analyzed samples occurred at nearly the same positions as those of standard hexaploid leaf cells (control), which indicates a high level of genetic stability. In addition, the means of the peaks were almost the same as that of the control. These findings demonstrate that the regeneration method had no effect on the induction of polyploids.
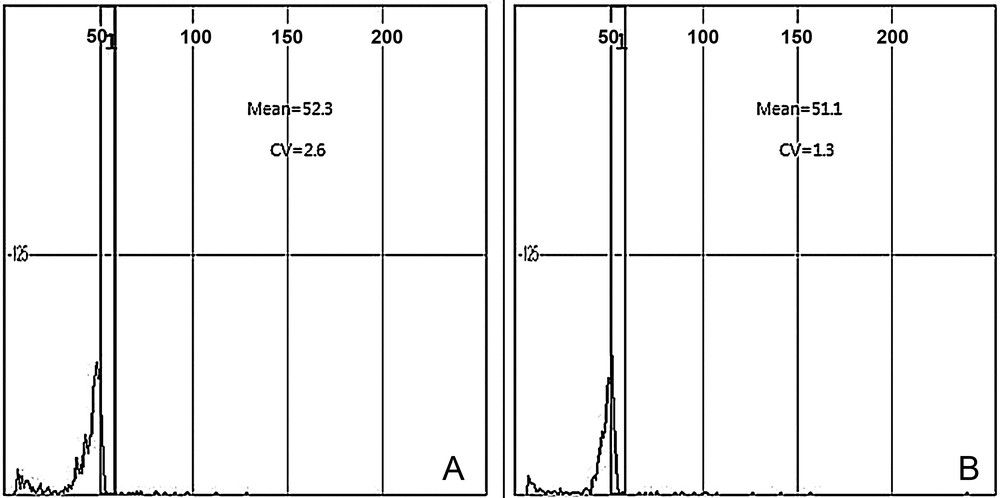
Analysis of the ploidy level using flow cytometry analysis of Chrysanthemum cv. Vivid Scarlet, mother plants (control) or regenerated plants via shoot organogenesis. A. Histogram of mother plants (control, hexaploid) planted in the greenhouse. B. Histogram of shoot organogenesis-obtained plant derived from medium containing 1 mg L−1 of benzyladenine and 2 mg L−1 of α-naphthaleneacetic acid solidified with Gelrite.
3.6 Effects of genotype on shoot regeneration
To examine the applicability of the established protocol to the other Chrysanthemum cultivars, shoot organogenesis was investigated in six commercially important cultivars. Shoot formation was detected in four of the cultivars, and the mean number of shoots per explant for each cultivar is presented in Table 6. The highest mean number of shoots per explant was observed in cv. Borami, followed by cv. Star, Pink Pride, and Peach Andy. No shoot formation was detected in cv. Baeksun or Baekma. Based on the results, the selected combination of PGRs could stimulate shoot formation in four out of six cultivars.
Efficiency of shoot organogenesis from leaf explants of different Chrysanthemum cultivars grown on MS medium containing 1 mg L−1 of BA and 2 mg L−1 of NAA after 30 days of culture.
| Cultivars | Shoot formation % | Mean No. of shoots per explant |
| Borami | 100a | 10.3a |
| Baekma | 0b | 0c |
| Baeksun | 0b | 0c |
| Peach Andy | 100a | 6.1b, c |
| Pink Pride | 100a | 7.3b |
| Star | 100a | 9.7a, b |
4 Discussion
Previous researchers have reported the effects of various combinations of PGRs on shoot regeneration of Chrysanthemum. However, the effects of dark incubation on shoot regeneration of Chrysanthemum have not been explored much, except by Park et al. [7]. In this study, higher NAA concentrations than BA induced shoot regeneration from explants cultured under light conditions without any initial dark period. Lu et al. [9] earlier reported that a medium containing 5.37 μM NAA and 2.22 μM BA is the most effective for shoot regeneration of Chrysanthemum. In addition, high-frequency shoot regeneration from leaf explants of Chrysanthemum has been observed in medium containing a higher concentration of auxin than of cytokinin [10], and a similar combination was used to induce adventitious shoot regeneration from leaf segments of Chrysanthemum [11]. Efficient genetic transformation of Chrysanthemum using leaf explants was also achieved using a higher concentration of auxin than of cytokinin [2–4], and Kaul et al. [12] also reported that leaf explants require a higher concentration of auxin for shoot regeneration. These results are in line with our own study, in which 1 mg L−1 of BA and 2 mg L−1 of NAA resulted in the highest frequency of shoot regeneration as well as mean number of shoots per explant. It seemed that the endogenous level of auxin contained in leaves of Chrysanthemum is too low to induce shoot regeneration.
However, explants that initially did not induce shoot regeneration under light conditions produced shoots when cultured in the dark for 10 days; it might be due to an accumulation of auxin. On the contrary, combinations resulting in shoot induction under light conditions showed inhibitory effects. Similarly, combinations that induced shoot regeneration upon initial darkness for 10 days also showed inhibitory effects when explants were subjected to darkness for more than 10 days, and greater inhibitory effects were observed with a longer dark incubation period (30 > 20 > 10 days). Higher auxin accumulation is more likely than optimum level to inhibit shoot regeneration. In support of this, Hitmi et al. [13] reported that a higher than optimal level of auxin distinctly inhibits shoot regeneration of Chrysanthemum. According to our study, a 10-day dark incubation period achieved higher shoot regeneration in most combinations compared to the other time periods (0, 20, 30 days). Park et al. [7] found that 12 to 18 days of dark incubation resulted in greater shoot induction from petal explants of Chrysanthemum. These observations could be attributed to differences in PGRs, explant type, and genotype.
The effects of various gelling agents on shoot organogenesis parameters are presented in Table 5. The highest frequency of shoot induction as well as the highest number of shoots per explant was observed using the Gelrite medium. Phytagel was the second effective gelling agent, followed by Agar and then by Agarose. Our results are similar to the findings of Lim et al. [5], who reported that Gelrite is the most suitable gelling agent for promoting shoot regeneration from leaf explants of Chrysanthemum cv. Borami. However, Agarose, which resulted in the lowest shoot regeneration in this study, has been reported to induce greater shoot regenerability than Agar. Lee et al. [6] found that the same combination of PGRs reported by Lim et al. [6] possesses higher shoot regeneration capacity on Agarose than on Agar, whereas a different PGR combination shows superior growth on Agar than on Agarose. Therefore, PGRs and gelling agents might have interactive effects on shoot regeneration. In this study, leaf explants grown on Agarose showed the weakest shoot regeneration. It may be due to the concentrations of PGRs used. Further, explants on Agarose curled upward during shoot regeneration, resulting in abnormal shoots since there was no sufficient surface for the growth of shoot primordia. However, the effects of Phytagel on shoot regeneration from leaf explants of Chrysanthemum have not been reported. As a possible explanation of these findings, other solidified media may have poor diffusion properties. This can limit the movement of nutrients, resulting in a reduction of the number of shoots per explant compared to Gelrite. Bornam and Vogelman [14] also reported that the physical state of the medium can affect the diffusion of PGRs and nutrients.
The effects of silver nitrate, an ethylene inhibitor, on shoot regeneration have been studied in Chrysanthemum [6,15]. Xiaohan et al. [15] observed that the presence of silver nitrate (10–20 μM) markedly promoted shoot regeneration of Chrysanthemum when explants were cultured on media containing a higher amount of cytokinin than of auxin. Moreover, it was observed that a higher than optimal concentration of silver nitrate had no effect on the average number of shoots per explant. Similarly, Lee et al. [6] found that addition of 1 μM of silver nitrate increased the number of shoots per explant of Chrysanthemum when cultured on a medium containing equal amounts of cytokinin and auxin. Even at a concentration of 10 μM, silver nitrate did not inhibit shoot regeneration. Moreover, concentrations of silver nitrate up to 100 μM did not completely inhibit shoot regeneration. However, in the present study, silver nitrate exerted highly negative effects on shoot regeneration from explants cultured on medium containing 1 μM silver nitrate, whereas 25 μM of silver nitrate completely inhibited shoot regeneration. A possible explanation for this discrepancy could be that a higher concentration of cytokinin was used in the study by Xiaohan et al. [15], as cytokinin is known to stimulate ethylene production in vitro. Thus, addition of silver nitrate (10–20 μM) would result in the absorption of ethylene and promote shoot regeneration. In a study by Lee et al. [6], 1 μM of silver nitrate stimulated shoot regeneration in the presence of equal amounts of cytokinin and auxin. In this study, the concentration of cytokinin was lower than that of auxin, and the addition of silver nitrate had negative effects on shoot regeneration. A significant interaction between cytokinin and silver nitrate in Chrysanthemum has been reported [15]. Alternately, these observations could be attributed to differences in endogenous ethylene levels among the various genotypes. Cho and Kasha [16] earlier reported that silver nitrate as well as other ethylene inhibitors may stimulate embryogenesis in species containing high endogenous levels of ethylene, but inhibit embryogenesis in species with lower endogenous ethylene levels.
Until now, no report has investigated the effects of gelling agents on in vitro plant growth parameters of Chrysanthemum. In our study, Gelrite was best suited for shoot regeneration and was able to promote superior plant growth parameters of Chrysanthemum. This observation could be explained by the fact that Gelrite seemed to diffuse nutrients that favor in vitro rooting and plant growth. Recently, Gelrite has been increasingly used as a gelling agent due to its advantages.
The genetic variation observed in Chrysanthemum has been reported [17,18]. In the present study, flow cytometry demonstrated genetic stability between the mother plant (control) as well as the in vitro regenerated plants of Chrysanthemum cv. Vivid Scarlet. Miñano et al. [19] also observed that most Chrysanthemum cultivars show high genetic stability when tissue culture is employed for shoot multiplication. Martín et al. [20] mentioned that genotype is one of the most important factors affecting genetic variation in Chrysanthemum.
The effects of genotype specificity on shoot organogenesis of Chrysanthemum have been reported [5,21,22]. Recently, Lim et al. [5] observed that shoot formation from leaf explants of Chrysanthemum is highly dependent on genotypes, whereas only one out of eleven cultivars exhibited shoot induction. However, the PGR combination used in this experiment was applicable to four out of six cultivars. Thus, we suggest that our combination would be useful for genetic transformation of cultivars.
5 Conclusion
Shoot regeneration from leaf explants of Chrysanthemum cv. Vivid Scarlet was optimized by examining the effects of PGRs, dark incubation period, gelling agents, and silver nitrate. Influences of specific concentrations of PGRs, dark incubation period, and gelling agent on efficient shoot regeneration in vitro were observed. Further, a negative effect of silver nitrate was also revealed. In addition, the most suitable gelling agent for rooting and plant growth was determined to be Gelrite. In this study, the established protocol was applicable to shoot regeneration of other cultivars.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
This work was supported by Bio-industry Technology Development Program, Ministry for Food, Agriculture, Forestry and Fisheries, and Kyungpook National University Research Fund in 2012, Republic of Korea.


