1 Introduction
Eutrophication of marine costal water and global warming lead to an important proliferation of marine microorganisms, producing a large variety of toxins that contaminate tissues of shellfishes and fishes. The displacement of human population to coastal areas and the expansion of commercial exchange all around the globe increase the occurrence of sea food poisoning [1–4].
Poisoning by tetrodontic fish consumption is a real problem, because it leads to a high mortality rate (>60% for puffer fish fugu [3], due to the presence of a neurotoxin: the tetrodotoxin (TTX) [5]), and numerous cases of intoxication were reported, particularly in Asia [2,4,6]. The puffer fish accumulates TTX and, to a lesser extent, saxitoxin (STX) in their body through the food chain [7]. TTX and STX are respectively produced by various species of marine bacteria and dinoflagellates. Usually, TTX accumulates in liver, gonads, intestines [8,9], but it was also found in the edible part (muscle and skin) of fishes [9,10]. In the course of the last decade, puffer fish Lagocephalus lagocephalus were often collected on the Tunisian coast. In this work, toxicity assessment of liver and edible part of this fish was investigated in Wistar rats. Rats were daily injected, for 10 days, with acidic extracts of Lagocephalus lagocephalus. To evaluate the toxicity of the treatment, clinical symptoms, mortality, weight of body, liver and kidney, activities of serum alanine amino transferase (ALT) (a marker of hepatic damage) and alkaline phosphatase were recorded. Because some marine toxins were found to induce an oxidative stress [11–13], lipid peroxidation and changes in the activity of antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase) were investigated in liver, kidney and blood cells.
2 Material and methods
2.1 Fish collection and acidic extract preparations
Specimens of the puffer fish L. lagocephalus (Linnaeus, 1966) were collected in different locations along the Tunisian coast and the southern golf of the Gabes region (Tunisia), between 2003 and 2006 (May to August). Immediately after collection, fishes were eviscerated and the liver was taken. Flesh (including muscles and skin) and liver were rapidly frozen at −20 °C until use.
The toxicity of organs was determined according to the standard bioassay method of Kawabata [14]. Flesh and liver of several fishes were pooled and 1 g from each group of organ was homogenized in 4 ml of 0.1% acetic acid, boiled on a water bath for 10 min, cooled, centrifuged (3000 rpm for 10 min); supernatants were stored at −30 °C.
2.2 Experimental design
Males Wistar rats weighing about 170–175 g were purchased from the Central Pharmacy of Tunisia (SIPHAT, Tunisia). They were housed at with light/dark periods of 12 h and a minimum relative humidity of 40%. Rats were fed with a commercial balanced diet (SICO, Sfax, Tunisia) and drinking water was offered ad libitum.
After acclimatizing to the laboratory conditions for one week, 45 rats were divided into three groups which were daily intraperitoneally (i.p.) injected with (1) 1 ml of a saline solution (0.9% NaCl)/100 g of body weight (BW) for the controls group (C) or (2) 1 ml of flesh extract/100 g for the (FT) treated group and (3) 1 ml of liver extract/100 g for the (LT) group. After 2, 5 and 10 days of treatment, 5 rats of each group were sacrificed under anaesthesia by i.p. injection of chloral hydrate.
The blood was collected without heparin by heart puncture, centrifuged (4000 rpm/15 min, 4 °C) and plasma and blood cells were kept at −30 °C.
The liver and kidneys were removed, weighed, rinsed with ice-cold saline and kept at −30 °C.
The frozen liver, kidney and blood cells samples were homogenized (Ultra Turrax T25, Germany) 1/2, w/v) in an ice cold buffer (TBS: 50 mM Tris, 150 mM NaCl, pH 7.4) and centrifuged (5000 g, 30 min, 4 °C); supernatants were frozen at −30 °C.
2.3 Biochemical assays
In blood plasma, activity of alanine aminotransferase (ALT) and alkaline phosphatase (ALP) was determined using commercial Biomaghreb kits (Tunisia).
In tissues, SOD activity was determined according to the colorimetric method of Beyer and Fridovich [15] using the oxidizing reaction of nitroblue tetrazolium (NBT): CAT activity was measured by the UV colorimetric method of Aebi [16] using H2O2 as substrate; glutathione peroxidase (GSH-Px) activity was measured by a modification of the colorimetric method of Flohe and Günzler [17] using H2O2 as substrate and the reduced GSH.
Lipid peroxidation was estimated by measuring the formation of thiobarbituric acid reactive substances (TBARS) according to the method of Buege and Aust [18].
The protein content of tissue extracts was determined using the method of Bradford [19].
2.4 Statistical analysis
The statistical analysis of the data was made using Student's t-test. All values are expressed as . Differences are considered significant at the 95% confidence level ().
3 Results
3.1 Clinical manifestations
No mortality and no evident signs of neurotoxicity (trembling, respiratory difficulties) were recorded in rats treated with acidic extracts of L. lagocephalus. Only gastrointestinal disorders (diarrhoea) were observed.
After 10 days of treatment, the weight of the body, the kidneys and the liver of rats treated with liver extracts (LT) was found to be lower than that of controls (Table 1). Conversely, in the group of (FT) rats, no significant change of the weight of organs was observed. Only the body weight was lowered, as compared to controls, but to a lesser extent than in the (LT) group.
Effect of tissue extracts of flesh and liver of L. lagocephalus (1 ml/100 g, v/w) on body, liver and kidney weights of control and treated rats after 10 days of treatment
| Parameters | Controls | FT group | LT group |
| Body weights (g) | 202.5±10.33 | 187.25±7.89⁎ | 167.5±3.23⁎# |
| Liver weights (g) | 10.21±0.12 | 9.88±0.39 | 8.51±0.11⁎⁎# |
| Kidney weights (g) | 1.47±0.059 | 1.41±0.062 | 1.29±0.017⁎ |
⁎ ;
⁎⁎ significant from control.
# LT group vs. FT group: .
3.2 Alanine aminotransferase (ALT) and alkaline phosphatase (ALP) activities in blood plasma
ALT and ALP activities were both found to be inhibited in (FT) and (LT) rats, as compared to control, the decrease of activity being more important in (LT) rats (Table 2).
Effect of tissue extracts of flesh and liver of L. lagocephalus (1 ml/100 g, v/w) on alanine aminotransferase (ALT) and alkaline phosphatase (ALP) activities in blood plasma of control and treated rats
| Parameters and treatments | ALT (U/L) | ALP (U/L) | |
| After 2 days | C | 55.25±4.93 | 354.5±22.95 |
| FT | 70±20 | 297.5±29.83 | |
| LT | 22.75±2.75⁎⁎ | 221.75±29.58⁎ | |
| After 5 days | C | 40.5±0.5 | 319.5±6.5 |
| FT | 30.5±1.5⁎ | 280±2⁎ | |
| LT | 20.34±0.34⁎⁎## | 250±5.77⁎⁎# | |
| After 10 days | C | 59.5±17.5 | 327.5 ±3.5 |
| FT | 37±3⁎ | 289.5±3.5⁎ | |
| LT | 35±3.05⁎ | 233±9.29⁎⁎# |
⁎ ;
⁎⁎ significant from control.
# LT group vs. FT group: ;
## LT group vs. FT group: ; ALT and ALP as U/L.
3.3 Estimation of lipid peroxidation levels (TBARS) in blood cells and liver and kidney tissues
As compared to controls, a significant increase of the TBARS levels was found in blood cells as well as in liver and kidney tissues of FT and LT rats (Fig. 1). The increase of the lipid peroxidation appeared generally higher in LT rats than in FT rats, and more precisely in blood cells (5th and 10th days of treatment) and kidney cells (10th day).
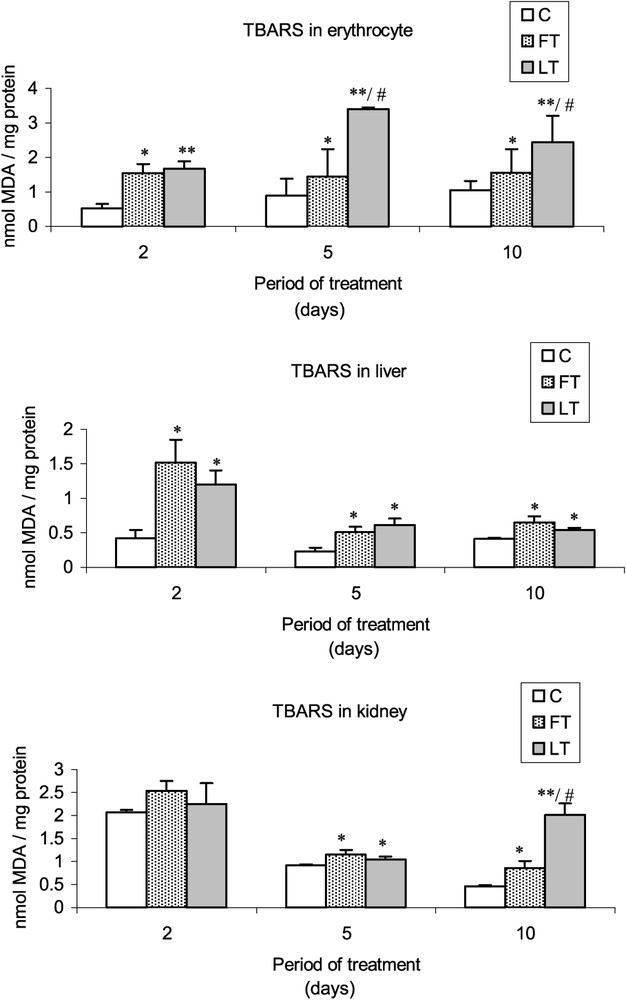
Effects of tissue extracts of flesh and liver of L. lagocephalus (1 ml/100 g, v/w) on the erythrocyte, hepatic and renal TBARS levels of treated rats vs. control rats.
3.4 Analysis of SOD, CAT and GSH-Px activities in blood cells and liver and kidney tissues
As shown in Fig. 2, acidic extracts of flesh and liver of L. lagocephalus induced a significant decrease of SOD activity in FT and LT rats, which was more significant in blood cells than in liver or kidneys. Moreover, the treatment led to a decrease of catalase (Fig. 3) and GSH-Px (Fig. 4) activities, which was stronger at the 5 and 10th day. Conversely, at the 2nd day, we found that catalase and the overall GSH-Px activities were increased in blood cells.
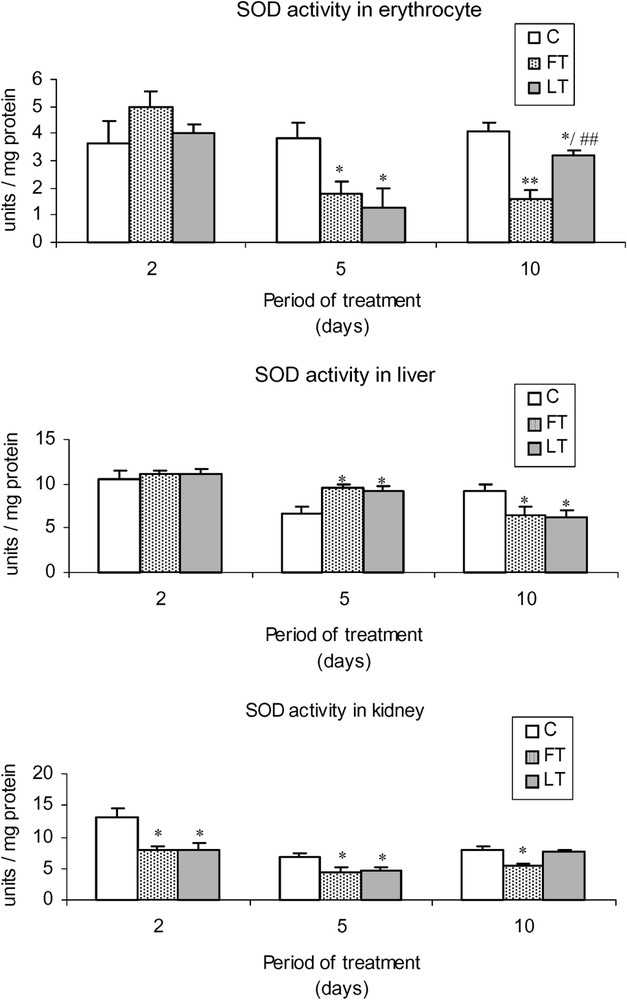
Effects of tissue extracts of flesh and liver of L. lagocephalus (1 ml/100 g, v/w) on the erythrocyte, hepatic and renal SOD activities of treated rats vs. control rats.

Effects of tissue extracts of flesh and liver of L. lagocephalus (1 ml/100 g, v/w) on the erythrocyte, hepatic and renal CAT activities of treated rats vs. control rats.
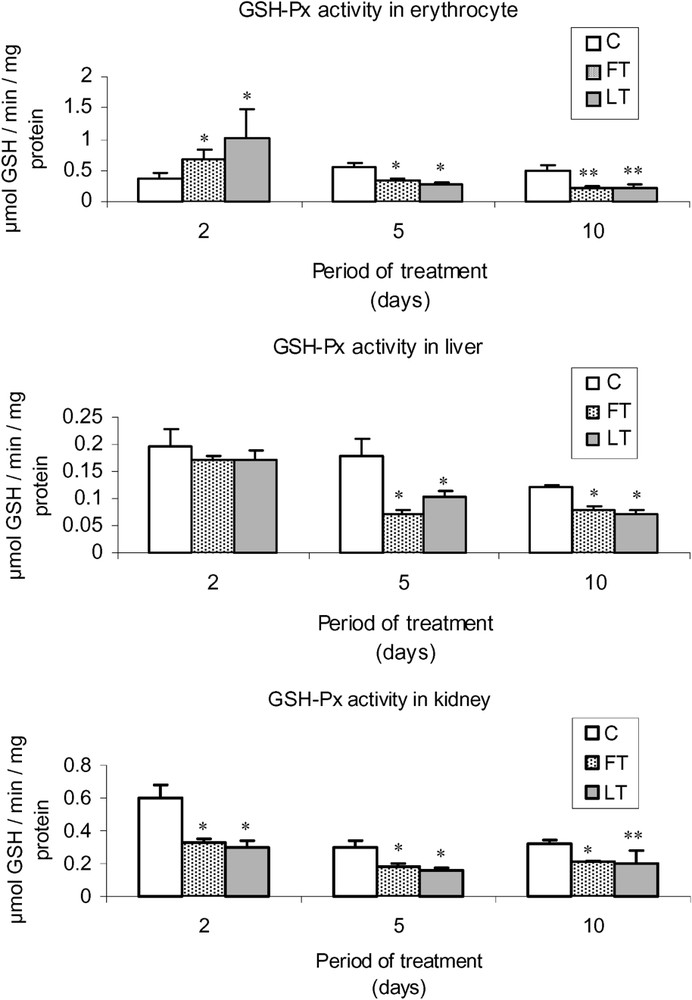
Effects of tissue extracts of flesh and liver of L. lagocephalus (1 ml/100 g, v/w) on the erythrocyte, hepatic and renal GSH-Px activities of treated vs. control rats.
4 Discussion
This study showed that flesh and liver acidic extracts of L. lagocephalus, intraperitoneally injected to Wistar rats for 10 days, led to a decrease of the body, liver and kidney of treated rats. This unquestionably shows the toxicity of such acidic extracts of L. lagocephalus. Of course, a part of toxicity can result from the injection of acetic acid, since controls were given injections of NaCl alone. Nevertheless, as toxicity of liver extracts was higher than that of flesh extracts, this can be related to the presence of toxins, at least in the liver, in these experimental conditions.
In other experimental conditions, the toxicity of L. lagocephalus flesh was demonstrated in rats fed for 2 months with a diet containing 10% of raw or cooked flesh [20].
Moreover, this study showed that acidic extract toxicity resulted in an oxidative stress. Indeed, treatment induced, in liver and kidney, as well as in almost all blood cells of the rats, a decrease in antioxidant enzyme activities (SOD, CAT, GSH-Px), which probably explains the concomitant increase in TBARS levels. These effects are not specific, since they are reported in numerous toxicity studies [11,13,21], but they confirm the presence of toxin(s) in the puffer fish extracts.
The fact that no signs of neurotoxicity were recorded suggests that neurotoxins are not implicated, or that concentrations are too low. Accordingly, Poletti et al. [22] assert that tetrodotoxin (TTX), brevetoxin (neurotoxic shellfish poisoning, NSP), and ciguatoxin (ciguatera fish poisoning, CFP) were not been found in the Mediterranean, at least until 2003. Near the coasts of Africa, TTX was detected in some specimens of puffer fish collected from the Red Sea, in Egypt [9,23]. So, we cannot exclude that climatic changes and maritime transports through the Suez Channel favoured the propagation and multiplication of TTX-producing bacteria, or of other toxic micro organisms, in the Mediterranean Sea.
Other toxins than TTX could account for the observed effects, such as, for example, saxitoxin, which was reported to contaminate puffer fish [7].
Insofar as we observed diarrhoetic episodes in rats treated with acidic extracts of pufferfish, we hypothesised that these effects could be related to diarrhoetic toxins such as okadaic acid and its derivatives. These toxins are produced by dinoflagellates that are present in the Mediterranean and contaminate more generally shellfish. They are powerful inhibitors of serine; threonine phosphatases and our work show that acidic extracts of L. lagocephalus induced a decrease in alkaline phosphatase activity in rat blood plasma. In addition, we observed that plasma ALT activity was decreased. Interestingly, Solter et al. [24,25] showed that ALT activity decreased with subchronic exposure to the hepatoxin microcystin-LR, which is also a potent inhibitor of serine/threonine phosphatases. In our study, the decrease in the serum ALT activity in treated rats suggests that this effect could be the result of a down-regulation of enzyme synthesis. This involves that there was no hepatocellular damage or necrosis. Accordingly, no insignificant change in hepatic histology was recorded (not shown).
In summary, this study shows that the flesh of the puffer fish L. lagocephalus collected along the Tunisian coast can be contaminated with toxic compounds able to induce gastrointestinal disorders and an oxidative stress. Further studies are needed to identify the implicated toxin(s), and regular monitoring of microbial contamination in the Mediterranean should be planed.


