1 Introduction
The measurement of gross production, net production and respiration is essential for the definition of the trophic status of coastal aquatic ecosystems which are among the most geochemically and biologically active within the biosphere [1]. It is unfortunate that little data exist on the carbon and oxygen fluxes in coral reef sediments, while these areas play an important role in the decomposition and degradation of exogenous and autochnonous organic matter [2–4], and they cover very large surfaces of coral reefs [5]. For example, the soft-bottoms of the La Saline fringing reef at Reunion island cover an area of 770,000 m2 that represents 43% of the total reef surface (ARVAM – CAREX, 2003).
Community respiratory (CRQ) and photosynthetic (CPQ) quotients provide fundamental information on metabolic pathways. CRQ reflects the ratio between the community release of dissolved inorganic carbon (DIC) and the community uptake of oxygen through aerobic respiration, anaerobic respiration and chemical oxidation [6]. CPQ corresponds to the ratio of community gross oxygen production to community gross DIC fixation. These gross fluxes are calculated from net fluxes measured during the day, corrected from the effect of biological and biogeochemical processes. Knowledge of CRQ and CPQ allows the calculation of DIC fluxes from the O2 fluxes, which are less difficult to measure in situ [7–13]. Furthermore, CPQ and CRQ are required for the calculation of productivity and calcification with the pH-O2 technique, which is used in several metabolic studies [14–17].
Very often, coral reef studies have been made with the assumption that CPQ = CRQ = 1 [8,18,19] without attempting an experimental verification of this value. Generally, the main electron acceptor during respiratory process is oxygen (aerobic respiration). However, anaerobic respiration can contribute significantly to the overall metabolism in sediments [20,21]. DIC is released into interstitial water or overlying water, and a part of the reduced components (ferrous iron, sulphide, methane, reduced manganese and ammonia) produced during anaerobic respiration may be re-oxidized. Therefore, the oxygen fluxes at the water–sediment interface do not always reflect respiratory processes only, unlike the flux of DIC (corrected from calcification process), which is the final product of all respiration pathways [5]. In such a case, metabolic quotients differ from 1, which could affect data interpretation for trophic balance. For instance, Barnes and Lazar [16] estimated productivity and calcification for the shallow fringing reef at Eilat (pH-O2 technique) based upon two sets of CPQ / CRQ. Changing the value of CPQ = CRQ = 1 to CPQ = 1.1 and CRQ = 0.9 resulted in a decrease of community gross production of about 10%, and a decrease in community gross production to community respiration ratio of about 6%, leading to a misinterpretation of the autotrophic/heterotrophic status of the ecosystem. The decrease in community calcification was about 50%.
There are few data sets where parallel estimations of community oxygen and community DIC fluxes were made to establish metabolic rates. The aim of this study was to determine the community respiratory quotient and the community photosynthetic quotient for the sediments of a shallow fringing reef as conversion factors to obtain carbon fluxes from oxygen fluxes.
2 Materials and methods
2.1 Study site
Reunion Island (21°S, 55°E) is located in the south-western Indian Ocean. Coral reefs are limited to small and discontinuous systems scattered along the western coast. This study was conducted in a wide range of environment at 4 reef flat stations and 4 back reef stations (Fig. 1) of La Saline fringing reef (500 m wide, 1 to 1.5 m deep), which is the most extensive reef of the island (9 km long). Tides are semidiurnal and the range varies from 0.1 m (neap) to 0.9 m (spring). We performed our measurements during the two main seasons: in summer from January to April and winter from July to October [22]. The summer season is characterised by high seawater temperature and high rainfall.
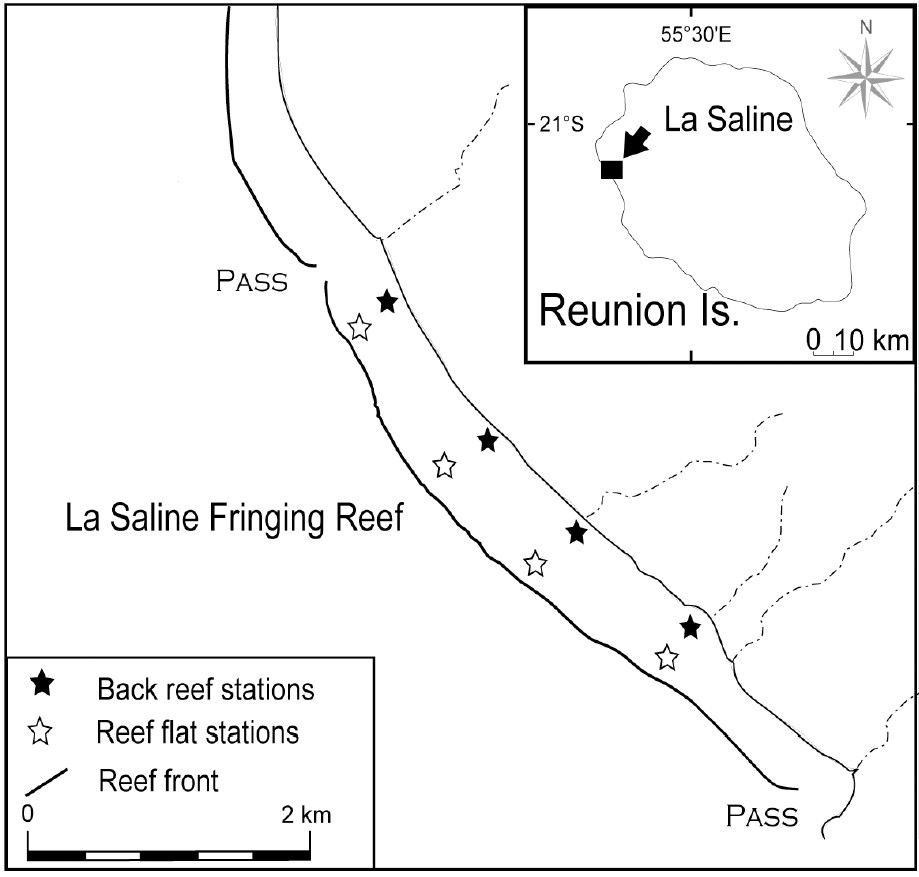
Location of the 8 sampling stations in La Saline fringing reef (Reunion Island, Indian Ocean).
2.2 Environmental parameters
Three environmental parameters were analysed in the water column: temperature, pH and chlorophyll a (ChloSW); and three parameters in sediments: granulometry, organic matter (OMSED) and chlorophyll a (ChloSED). Six water samples were collected simultaneously at each station over 3 days in summer 2004 (3 at 9 h and 3 at 15 h) and again in the same way in winter 2004. Water samples for chlorophyll a were filtered onto GF/F glass-fiber filters. After extraction (12 h at 4 °C) with acetone (90%), chlorophyll a was measured fluorimetrically (Turner 700), according to Welschmeyer [23]. Water samples for pH were collected and measured according to the method described in Section 2.3. Six sediment cores (2.9 cm diameter, 5 cm deep) were collected at each station concurrently to the flux measurements at the water–sediment interface (see Section 2.3). Three cores were used to determine total organic matter load, corresponding to the weight loss between sediment dried at 60 °C for 48 h and ashed at 550 °C for 3 h [24]. Three other samples were frozen at −80 °C and later used to measure chlorophyll a content of the sediments. Extraction was performed in 90% acetone for 12 h at 4 °C. After centrifugation, the optical density of the extracts was measured with a spectrophotometer (Kontron Uvikon 922) at 750 and 665 nm, and calculations made according to Lorenzen [25]. Granulometry was assessed on cores collected in triplicate at each station (4.3 cm diameter, 5 cm deep) in summer 2004. The sediment mean size was calculated in the φ value, according to Folk and Ward [26].
2.3 Fluxes at the sediment–water interface
Fluxes of O2 and DIC at the water–sediment interface were assessed using in situ benthic chambers [27]. During the experiments, the weather was calm; the swell was very low and no ripple formation was observed on the bottom. At each station and, in the course of the main two seasons (winter 2002 or 2003, summer 2001 or 2004), at least eight runs of triplicate incubations, lasting about 60 min, were performed over two or three diel cycles, in order to get the whole range of irradiance. Among these runs, two runs of triplicate incubations (generally one per day of sampling) were performed at night (around 20 h) to assess respiration. The benthic chambers were composed of a PVC ring pushed into the sediment to a depth of ca. 10 cm, covered with a transparent acrylic hemisphere. The volume of the enclosed water varied from 66 to 69 L. The water was continuously homogenised with adjustable submersible pumps at 2 L min−1. Dissolved oxygen, temperature and salinity were recorded with multiparameter probes (YSI 6920). A quantum sensor (LI-1400) was deployed inside one of the benthic enclosures to measure irradiance, defined as the photosynthetically active radiation (PAR, 400 to 700 nm) available for microphytobenthos. Between incubations, enclosures were opened for 60 min to restore ambient conditions. Further details about the experimental device are given in Clavier et al. [28].
Seawater samples were collected inside the chamber at the beginning and at the end of each incubation. They were immediately poisoned with HgCl2 (20 μL of saturated solution per 100 mL of sample) and stored in darkness at 5 °C pending subsequent potentiometric determination of pH and total alkalinity (TA). Samples intended for TA measurement were filtered through GF/C glass-fiber filters. DIC concentrations (mmol L−1) were determined from pH, TA, temperature and salinity [29]. pH was measured on to the total hydrogen ion concentration (mol kg SW−1) pH scale, using a Ross combination electrode (Orion 81-03) calibrated against Tris/HCl and 2-aminopyridine/HCl buffers in synthetic seawater [30]. To measure TA, inflection point titrations (Radiometer TIM 865) were performed on 20 mL subsamples (4 replicates) maintained at 25 °C (), and slowly neutralized with saline (0.7 M NaCl) HCl 0.01 M solution. TA was obtained from the second inflection point of the curve. The mean standard deviation of replicate measurements was less than 0.003 mmol L−1.
The DIC flux (FDIC; mmolC m−2 h−1) was calculated as the difference in DIC concentrations between the end and the start of the incubation, corrected from half the TA variation, to account for the effects of carbonate dissolution and precipitation [17]. O2 flux (FO2; mmolO2 m−2 h−1) was calculated as the difference in concentrations between the end and the start of the incubation.
2.4 Data analyses
The community respiratory quotient (CRQ) is defined as:
3 Results
3.1 Environmental parameters
Temperature, pH and chlorophyll a content of seawater showed significant seasonal variability, with the highest values in summer (Table 1). The pH differences reflect seasonal variations, as sampling was undertaken at the same hour of the day in summer and winter. In sediments, the highest chlorophyll a content was found in summer. The organic matter load remained constant over the year. The mean grain size in φ value was 0.61 (SD 0.38) which corresponds to coarse sand on the Wentworth scale [33]. The underwater maximum mean irradiance at the sediment level, calculated from data measured during benthic incubations, was 1532 μmol m−2 s−1 at about 11 h in summer and 995 μmol m−2 s−1 at about 12h30 in winter (Fig. 2). In summer, the daily curve was not symmetrical, and the maximum was before midday as clouds often appear at the beginning of the afternoon. The mean irradiance varied significantly according to the season (U-test; ). In winter, bottom communities received 33% less of light than in summer (respectively 21 and 31 mol m−2 d−1).
Mean temperature (T), pH and chlorophyll a concentration (Chlosw) in sea water, and mean benthic chorophyll a load (ChloSED), organic matter load (OMSED) and grain size (φ value) in La Saline reef sediments
| T (°C) | pH | Chlosw (μg L−1) | Grain size (φ value) | ChloSED (mg m−2) | OMSED (%) | |
| Summer | 28.75 | 8.184 | 0.22 | 0.61 | 42.90 | 3.64 |
| (2.84) | (0.018) | (0.09) | (0.38) | (28.58) | (0.99) | |
| Winter | 24.78 | 8.140 | 0.14 | – | 29.61 | 3.53 |
| (1.28) | (0.097) | (0.04) | (20.46) | (0.58) | ||
| p-value (U-test) | p<0.001 | p<0.05 | p<0.001 | – | p<0.01 | n.s. |

Daily evolution of the average irradiance at the bottom during summer and winter.
3.2 Respiratory and photosynthetic quotients
CRQ were 1.01 (SD 0.11) in winter and 1.15 (SD 0.07) in summer (Fig. 3). These two values were not significantly different (Z-test; ). The common CRQ for the two seasons was 1.08 (SD 0.05) and did not significantly differ from 1 (Z-test; ). The intercepts of the regression line in winter and summer were not significantly different (Z-test; ) but they significantly differed from 0 (Z-test; ). The mean DIC release was 1.21 mmolC m−2 h−1 when the oxygen uptake was zero.

Linear relationships between gross FO2 (release) and gross FDIC (uptake) during light incubation in winter (a) and in summer (b); and FDIC (release) and FO2 (uptake) during dark incubation in winter (c) and in summer (d).
In winter CPQ was 1.06 (SD 0.05) and did not differ significantly from 1 (Z-test; ); the intercept of the regression line differed significantly from 0 (Z-test; ). We observed an oxygen uptake of 2.8 mmolO2 m−2 h−1 when the DIC uptake was zero. In summer CPQ was 0.79 (SD 0.02) and differed significantly from winter and from 1 (both: Z-test; ). The intercept of the regression line was significantly lower than 0 and different from winter (both: Z-test; ). The oxygen uptake was 0.8 mmolO2 m−2 h−1 when DIC uptake is zero.
4 Discussion
While transport in fine sediments is restricted to diffusion, bioturbation, and bio-irrigation, advective pore-water flow, especially at high flow velocity, may represent an additional solute transport mechanism in coarse sand [34]. Pore-water transport may be due to percolation of the bottom by wave action [35] or influenced by topographical features of the sediment surface [36]. In our study, benthic experiments were performed in calm water when the flow velocity was minimal and for sediments without ripples or mound formation. Therefore, the fluxes measured resulted mainly from diffusive transport. The chamber-derived fluxes of coarse sand depends on the hydrodynamic properties within the chamber, since these determine the pressure gradients at the sediment surface, and hence the rate of advective solute exchange [37]. Water in each chamber was adjusted to the minimum value allowing stable measures from probes, and therefore our measurements correspond to the minimal fluxes recorded at the water–sediment interface.
The CRQ value of 1.08 (not significantly different from 1, whatever the season) is within the range of previous estimates of 0.8 to 3 reported in Table 2. It is lower than values found in coral reef sediments of New Caledonia [6,38]. The CPQ value of 1.06 in winter (not significantly different from 1) is consistent with literature (Table 2). Few authors reported CPQ lower than 1 for sediments [39], such as the CPQ value we found in summer (CPQ = 0.79). Such low values highlight a disequilibrium between FO2 and FDIC which can be induced by a number of processes.
Published values of community respiratory quotients (CRQ) and community photosynthetic quotients (CPQ)
| CRQ | CPQ | ||
| Plankton (shallow estuary, Basque Country, Spain) | 2.2 | [56] | |
| Atoll (French Frigate Shoals, Hawaii) | 1.04 | [40] | |
| Barrier reef (Moorea, French Polynesia) | 0.9 | 1.0 | [19] |
| Great Barrier reef (Yonge reef, Australia) | 0.8 | 1.1 | [19] |
| Various environments in coral reefs | 0.8 to 3.0 | 0.5 to 1.15 | [15] |
| Sediment (Bay of Brest, France) | 1.07* | [57] | |
| Sediment (Coral reef, New Caledonia) | 1.14 | 1.03* | [38] |
| Sediment (Coral reef, New Caledonia) | 1.17 | [6] | |
| Sediment (Coral reef, Reunion Island) | 1.08 | Winter: 1.06⁎ Summer: 0.79⁎ | Present study |
⁎ CPQ calculated from gross productions.
4.1 O2 bubbles, calcification and photorespiration
A low CPQ may be caused by the effect of hydrodynamism [15,40]. Atkinson and Grigg [40] attributed the curvilinearity of their relationship to breaking waves. They hypothesised that waves could have converted dissolved O2 into bubbles, resulting in an underestimation of the oxygen fluxes. Our measurements were carried out in benthic chambers where there exists a risk of bubble formation, due to the supersaturating of water in oxygen. However, we found no curvilinerarity in summer (or winter) relationship for La Saline sediments (Fig. 3 a and b) excluding the supersaturating seawater factor to explain our low CPQ.
Calcium carbonate precipitation or dissolution can affect DIC concentration and therefore CPQ [41]. The production of one mole of CaCO3 is a CO2-releasing process and requires the uptake of two moles of HCO3−; therefore total alkalinity decreases by 2 equivalents and DIC decreases by 1 mole for precipitation or the reverse for dissolution [17]. Boucher et al. [42] showed a light-induced CaCO3 precipitation in coral reef sediments, similar to that occurring in calcifying organisms. Anaerobic respiration is also a potential source of alkalinity for the water column [17]. However, the associated changes in total alkalinity during short-term incubation (60 min) are probably negligible [42] and nitrogen fluxes measured for La Saline sediments are low (see below). In our study, a relationship between irradiance and CaCO3 fluxes was found [43]. Therefore, CPQ was calculated from FDIC resulting from total DIC fluxes subtracted from calcification (1/2 of total alkalinity variation). The dissolution recorded by Oviatt et al. [41] contributed to 14% of DIC fluxes and had significant lowering effect on CPQ. The contribution of carbonate metabolism to DIC fluxes in our study reached 16% [43]. As calcification had been already integrated in our calculations, causes of low CPQ may be found in photosynthetic activity of primary producers.
Algal respiration includes both ‘dark respiration’ similar to heterotrophic respiration and photorespiration. The latter that decreases CPQ [44] occurs when RUBISCO (ribulose 1,5-biphosphate carboxylase/oxygenase) principal function as a carboxylase is substituted by oxygenase function [45]. The oxygenase function of RUBISCO is favoured under increasing oxygen levels, increasing temperatures, and high light [46]. No relationship was found between CPQ (either divided by ChloSED or OMSED, or not) and seawater O2 concentrations, DIC/O2, light or temperature (results not shown) either in summer or in winter suggesting the absence of photorespiration for La Saline sediments. Some studies demonstrated the existence of photorespiration in marine algae [47,48] but the rate is generally low or insignificant due to the existence of a CO2-concentrating mechanism [45,49] which preserves RUBISCO from high environmental O2 concentrations. However, we cannot rule out photorespiration in this study because higher temperature and irradiance in summer compared to winter, in which CPQ = 1, could induce photorespiration.
4.2 Anaerobic metabolism
Oxic respiration is generally considered to be the main pathway of organic matter degradation in coastal sediments [5]. However, other metabolic pathways, including sulphate reduction, denitrification, methanogenesis, and iron or manganese reduction, may have a more or less important contribution in some environments [5], especially in coral reef sediments where decomposition of detritus is essentially a microbially mediated process [50]. Aerobic metabolism appears to prevail at the beginning of the night in La Saline sediment (CRQ = 1). Nevertheless, the intercept of regression line in darkness indicates that DIC are produced (29 mmol m−2 d−1) without O2 uptake (Fig. 3 c and d). This anaerobic respiration appears to be steady whatever the rate of aerobic respiration. This rate is in the range of literature values reported in Table 3 and close to the sulphate reduction rate which is the major anaerobic respiration process in coral reef sediments, although the rates are low compared to most over marine environments [50]. As DIC is produced without oxygen consumption, anaerobic sulphur metabolism in sediments can contribute to higher CPQ and therefore does not explain the low summer CPQ in La Saline.
Anaerobic fluxes in carbonate sediments of coral reef ecosystems
| Sulphate reduction mmolS m−2 d−1 | ||
| GBR North Section (coarse sand) | 0.6 to 2.1 | [58] |
| GBR Arlington and Sudbury Reefs (mud) | 0.7 to 4.4 | [20] |
| GBR Heron Island | 29 to 42 | [59] |
| GBR Heron Island | 12.0 to 19.3 | [2] |
| GBR Davies Reef | 3.5 | [21] |
| GBR Lizard Island | 2 | [60] |
| Denitrification | ||
| GBR North Section (coarse sand) | 110 to 1280 μmolN m−2 d−1 | [58] |
| GBR Arlington and Sudbury Reefs (mud) | 84 to 991 μmolN2 m−2 d−1 | [20] |
| GBR North Section (close to mangrove) | 2.6 to 3.5 mmolN m−2 d−1 | [61] |
| Shiraho fringing reef (bare sand) | 41 to 156 μmolN m−2 d−1 | [62] |
| GBR Hopkinson Reef | 2.88 to 10.8 μmol m−2 d−1 | [63] |
| GBR Bowl Reef sediments | 4.8 to 319.2 μmol m−2 d−1 | [63] |
| Puerto Rico | 1.2 to 2.4 mmolN m−2 d−1 | [64] |
| Methanogenesis | ||
| GBR Arlington and Sudbury Reefs (mud) | 16.4 to 89.5 μmolCH4 m−2 d−1 | [20] |
| Grand Bahamas Island Hydro-Lab coral reef | ||
| (coarse sand) | 0.4 to 2.4 μmol m−2 d−1 | [65] |
| Manganese or Iron reduction | ||
| GBR Arlington and Sudbury Reefs (mud) | No detectable flux | [20] |
Other metabolic processes producing a release of DIC without simultaneous oxygen uptake are iron or manganese reduction, methanogenesis and denitrification. Iron or manganese reduction and methanogenesis are minor contributors (Table 3), with less than 1% following Alongi et al. [20]. Several metabolic processes involving nitrogen can also affect metabolic quotients, including the nitrification/denitrification process [41]. CPQ is affected by nitrogen species taken up by primary producers [51]. CPQ is not modified by NH4 uptake but is increased by NO3 uptake [52] as shown by the equation:
| (1) |
| (2) |
| (3) |
Since sediments are heterotrophic [43] an other source of nitrogen is exogenous dissolved and particulate organic matter. Organic matter input needed to balance production and respiration is at most 53 mmolC m−2 d−1 in summer. The ratios of particulate and dissolved organic matter in seawater are respectively () and () [43]; therefore, 5 mmolN m−2 d−1 (high estimation calculated from the average ratio) are brought by exogenous material and sustain gross production in the sediment. Assuming that this pool of nitrogen is ultimately denitrified through the nitrification/denitrification pathways:
| (2) + (3) |
4.3 Daily evolution of respiration
Community respiration has generally been measured at the beginning of the night and the 24 h values are estimated assuming that respiration is constant [40,53]. However, the fact that community light respiration exceeds dark respiration seems unquestionable [5,54]. Several processes can produce daily variations in community respiration such as variations in microphytobenthic biomass [55] or in oxygen penetration into the sediments (for more details, see [28]). At La Saline reef, Clavier et al. [28] demonstrated a daily cycle of community respiration in sediments, with values at the end of the day 2.8 and 3.8 higher than the fluxes at the end of the night for oxygen and DIC, respectively. In order to test the influence of a daily respiration variation on CPQ, this pattern was included in our calculations, instead of assuming that respiration is constant. This was done for the back reef of Planch'Alizés station, the same studied by Clavier et al. [28] in summer 2003. Benthic parameters were relatively similar between the two studies: 4.32 (SD 0.51) and 4.32 (SD 0.32)% for OM content; 0.44 (SD 0.27) and 0.41 (diameter scale) for the main grain size; 82.3 (SD 30.5) and 47.4 (SD 18.1) mg m−2 for chlorophyll a content for the present study and Clavier et al. [28], respectively. Community gross productions were calculated with community respiration values obtained from the third-order polynomial regression proposed by Clavier et al. [28] (Fig. 4) and reported in Table 4.
For the present study, the CPQ reached 0.82 (significantly inferior to unit: Z-test, ) in back reef of Planch'Alizés in summer. When taking the daily evolution of the respiration into account, the new CPQ equals to 0.91 and is not significantly different to 1 (Z-test, ), which is a value more commonly found for sediments (Table 3). The variation range of community respiration in terms of carbon is higher than in terms of oxygen, resulting in a daily evolution of the CRQ with a minimum at 6h00 and a maximum value at 18h00 (Fig. 5 in [28]). In other words, the amount of DIC release compared to oxygen uptake increases at daytime; at the end of the day CRQ is close to unity. Finally, more than the daily evolution of the respiration, it is the discrepancy in the daily evolution of respirations in terms of carbon and in oxygen that caused a mistake in CPQ calculations.
5 Conclusion
This study confirms that total DIC fluxes, corrected from calcification, are closely coupled to the oxygen fluxes through community respiration and community production at the water–sediment interface in coral reef ecosystems. The CRQ measured in the sediments of La Saline reef from a large data set () suggests that DIC release and oxygen uptake are balanced, but anaerobic processes are highlighted by the intercept of relationship. The low CPQ in summer is not affected by anaerobic respiration, but results from the discrepancy in the daily evolution of community respiration in terms of carbon and oxygen. These results provide evidence that simultaneous benthic community production and respiration measurements are needed for long term integrated data sets, instead of daily or seasonal budget calculations made from limited measures of community respiration.
Acknowledgements
We gratefully acknowledge the team (Christine Payet, Sybille Cavaciutti, Perrine Mangion, Francky Saint Ange, Flora Mazzeo, Jonathan Flye Sainte Marie, Gwennaëlle Bucas, Charlotte Roby, Marc Touchard, Carine Marques, Emmanuelle Grondin) who helped us in field and laboratory works. This research was financially supported by the Regional Council of Reunion Island.


