1 Introduction
The ability of a symbiotic microorganism to be transmitted from one host to the next is a life history trait which is fundamental for the maintenance of the symbiotic interaction over time. The two possible modes of transmission are vertical (i.e. given by a parent to its offspring via reproduction) and horizontal (i.e. by any other route, from direct intimate contact to indirect contamination via a vector or the environment). Mutualistic (i.e. beneficial) symbionts are typically transmitted vertically, while parasites or pathogens are mostly transmitted horizontally [1]. However, exceptions occur. Vertical transmission is not necessarily required to establish a stable mutualism between the symbionts and their host [2,3], while pathogenic symbionts can fluctuate between a vertical and a horizontal transmission [4,5]. Finally, cases of non-mutualistic symbionts with a day by day vertical transmission are known. These “hereditary foes” insure their transmission by manipulating the reproduction of their host, which earned them to be called reproductive manipulators or influential passengers [6–10]. The endosymbiotic bacteria of the genus Wolbachia have become the most famous example of this way of life [11]. However, despite of their efficiency, most of the different strategies used by Wolbachia do not insure the irrevocable association to the host, leading to some taxa losing their infection over time. Accordingly, some Wolbachia lineages have developed other mechanisms insuring a lasting association with the host, leading them to become permanent residents.
2 The Wolbachia
The discovery of Wolbachia dates back to 1924, when Hertig and Wolbach observed them in reproductive cells of the mosquito Culex pipiens [12]. The bacterium received its official name (Wolbachia pipientis) and description twelve years later [13]. To date Wolbachia pipientis is the only recognized species of the genus Wolbachia, family Anaplasmataceae, order Rickettsiales, class alpha-proteobacteria [11,14]. Their principal hosts are terrestrial arthropods, with an estimated 20–76% of all species infected, depending on the PCR technique used [15,16]. However, Wolbachia are also found in a much smaller taxonomic group: filarial nematodes [17]. Eight Wolbachia clades, or supergroups (named A to H) have been defined [14]. Clades A to D are considered the major supergroups with A and B Wolbachia found only in arthropods (mainly insects but also in terrestrial isopods) and the C and D Wolbachia found only in filarial nematodes [18,19]. Clade E was described more recently from Collembola [20]. Clade F broke established barriers by being found both in arthropods (bedbugs, crickets, lice, scorpions, spiders) [21–25] and in two nematode of the Genus Mansonella [26]. The two most recently described clades (G and H) have been found respectively in australian spiders [21] and termites [27], but the validity of clade G is disputed [28]. Finally, two new Wolbachia lineages have been described in fleas [29] and in another filarial nematode, Dipetalonema gracile [30], but their taxonomic status is not determined yet [17].
In Arthropods, Wolbachia are present in most tissues, including ovaries and testes [31] but in adult nematodes they are restricted to the hypodermal cells of the lateral cords and only in the reproductive cells of females [16]. Whatever the host, Wolbachia are only transmitted by females to their offspring, through the cytoplasm of the egg [16,32].
3 Wolbachia as reproductive manipulators in Arthropods
In terrestrial arthropods, Wolbachia manipulate reproduction to ensure their own vertical transmission to the next generation of host. Four mechanisms have been described, three of which are detrimental to the non-transmitting sex (i.e. the males): male killing, feminization and thelytokous parthenogenesis induction. The fourth mode of manipulation is called cytoplasmic incompatibility. It reduces the transmission of cytoplasmic lineages by females which are either not infected or infected by a different Wolbachia as the male which fertilizes their eggs.
(i) Male killing (MK): In Coleoptera, Lepidoptera and Diptera [33–35] Wolbachia kill the sons of infected females before they hatch. This mechanism increases the transmission of infected cytoplasm whenever infected sisters benefit from their brothers' deaths. Benefits might include a protein-rich first meal (which happens, for example, in ladybirds, where first instars eat dead siblings), reduced sibling competition for resources, or a reduced probability of inbreeding. The net result is that infected females produce fitter daughters than uninfected ones, allowing the infected cytoplasm to be more efficiently transmitted, thus spreading the infection.
(ii) Feminization (F): In terrestrial isopod crustaceans (i.e. woodlice) Wolbachia turn genetic males into functional females (reviewed in [36]). As a result, infected females produce twice as many daughters as uninfected ones, which increases dramatically the fitness of the infected cytoplasm relative to the wild type. This change of sex is obtained by inhibiting the development of the male endocrine gland in infected males. In absence of the masculinising hormone produced by this gland, the default development leads to a functional female [37]. Apart from crustacean isopods, Wolbachia-related feminization has been described in two insect species only, in the Lepidoptera Eurema hecabe [38] and the Hymenoptera Zyginidia pullula [39]. Since sex determination is not hormonal in these insects, the mechanism of action must however be different from that found in woodlice. Two earlier reports of feminization due to Wolbachia had been made, in the adzuki corn borer Ostrinia furnicalis [40] and the adzuki bean borer, Ostrinia scapulalis [34] However, further investigation revealed that these were case of Wolbachia-induced male killing [34].
(iii) Thelytokous Parthenogenesis Induction (TPI): In haplodiploid species such as Hymenoptera (wasps), Thysanoptera (Thrips) and Acari (mites), Wolbachia also turn males into females, making use of a particularity of haplodiploid sex determination (reviewed in [7]). Haplodiploid development results normally in unfertilized (haploid) eggs developing into haploid males (arrhenotokous parthenogenesis), while fertilized (diploid) eggs develop into diploid females. Depending on the host, the bacterium either wholly suppresses meiosis (apomixis), or aborts the final stage of the first mitosis, doubling the number of chromosomes (automixis). In both cases, unfertilised eggs develop into (heterozygous or homozygous) diploid females (thelytokous parthenogenesis) [7,41]. This leads infected females to produce twice as many daughters as uninfected ones, allowing their cytoplasm to be transmitted to four times as many granddaughters.
(iv) Cytoplasmic Incompatibility (CI): Reproductive incompatibility between populations of the mosquito Culex pipiens was reported in the 1950s [42], but it took another twenty years before Wolbachia were identified as the causative agent [43]. In its simplest form (unidirectional incompatibility), CI occurs when infected males mate with uninfected females. Such a cross results in aborted fertilization while fertilization is normal if the female is also infected, or when an infected female mates with an uninfected male. Thus, in mixed populations, infected females have a reproductive advantage over uninfected ones, which leads to increased infection frequencies. A more complete reproductive isolation (bi-directional incompatibility) occurs when males and females are infected by different and mutually incompatible Wolbachia, leading to reduced hatch rates in both directions of cross (reviewed in [44]). The phenomenon is well characterized cytogenetically [45,46]. In incompatible crosses, paternal chromosomes fail to condense normally or fast enough, so that maternal chromosomes segregate on their own at the first mitosis. In diploid organisms, this aborted fertilization typically results in developmental arrest and death of the embryo (Fig. 1). In haplodiploids, where males naturally develop from unfertilised haploid eggs, CI-induced haploidy can lead to the death of the embryo but also to the development of a male offspring [47,48]. From the Wolbachia's point of view, both results are equivalent since they break the transmission line of an uninfected cytoplasm (males do not transmit their cytoplasm).
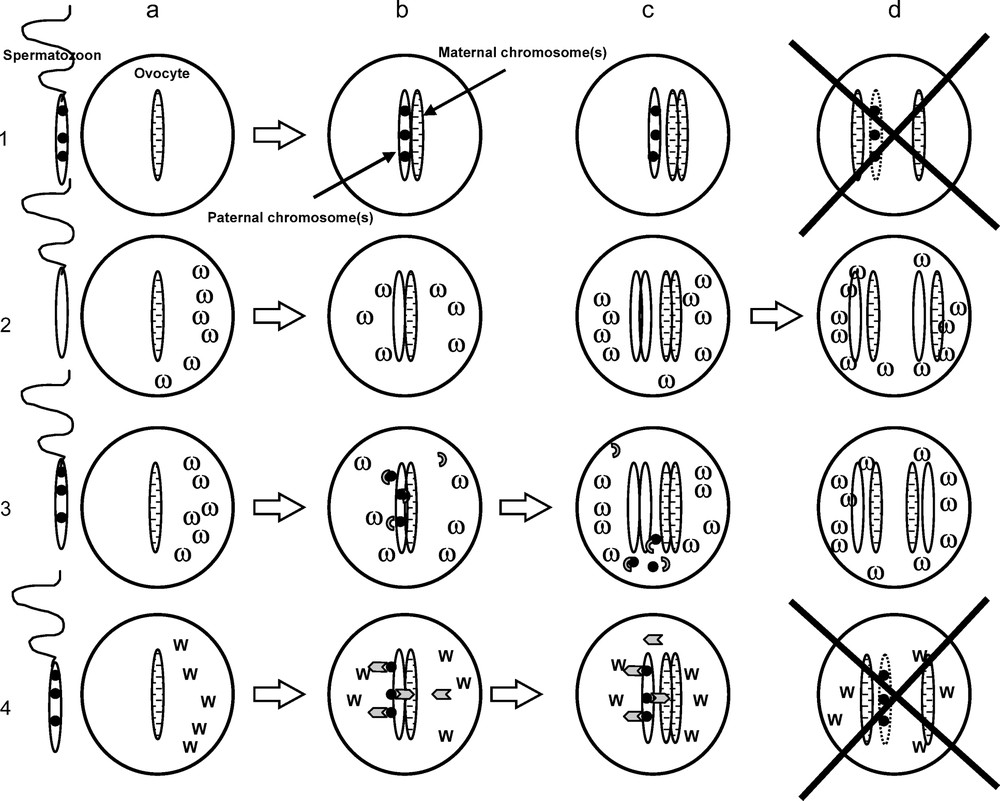
Expression of cytoplasmic incompatibility in a diploid organism according to the “poison-antidote” or “lock-key” model. 1: Cross between a male infected by Wolbachia and an uninfected female. The Wolbachia modify (●) the chromosomes borne by spermatozoids (a), after fertilization and fusion of male and female pronuclei (b), paternal chromosomes remain inactive so that only maternal chromosomes duplicate (c), the embryo aborts (d). 2: The reverse cross (uninfected male × infected female) is normal: incompatibility is only unidirectional. 3: Cross between a male and a female bearing the same Wolbachia strain. The Wolbachia modify (●) paternal chromosomes (a), after fertilization, the Wolbachia (ω) transmitted through the ovocyte produce antidote molecules (grey half-circle) which recognize and neutralise the chromosomal modifications (b), now restored in their function, paternal chromosomes duplicate along with maternal ones (c), the embryo is rescued (d). 4: Cross between two individuals bearing different Wolbachia strains (ω vs. W). After fertilization, the molecules of the antidote (grey arrows) produced by the Wolbachia (W) borne by the ovocyte do not correspond to the modifications (●) made by the Wolbachia (ω) on the paternal chromosomes, and fail to restore a normal function of these. Paternal chromosomes remain inactive and do not duplicate, unlike maternal ones (c) the embryo aborts (d). Since the reverse cross yields the same result, the incompatibility is called bidirectional.
The bacterial molecules involved in CI are still unknown. The current paradigm is that of the modification-rescue model [32], according to which two phenomena must be distinguished. One (termed mod, for modification) occurs in the male germline, where it alters paternal chromosomes so that their behaviour will be aberrant when fertilizing an uninfected egg. The other (termed resc, for rescue) occurs in infected eggs, where it restores a normal development. Attempts have been made to translate mod and resc into more concrete factors. It has been argued that a lock-and-key model is the most likely to be valid, in which mod (the lock) and resc (the key) are controlled by different genetic determinants but interact directly with each other [49,50]. Depending on whether mod and resc functions are expressed, four phenotypes can be defined: (i) “invasive” [mod+; resc+] when Wolbachia induce CI and rescue from their own [mod+] effect; (ii) “helpless” [mod−; resc−] when Wolbachia do not induce CI nor are able to rescue from the [mod+] effect of other variants; (iii) “defensive” [mod−; resc+] when Wolbachia do not induce CI but are capable of rescuing the [mod+] effect of another variant; and finally (iv) “suicide” [mod+; resc−] when Wolbachia induce CI but are unable to rescue from their own [mod+] effect [49] (Fig. 2). The three first phenotypes have been found in nature and studied in the laboratory [51]. On the contrary, the ‘suicide’ phenotype is still theoretical, although the models do not preclude its existence [52,53].
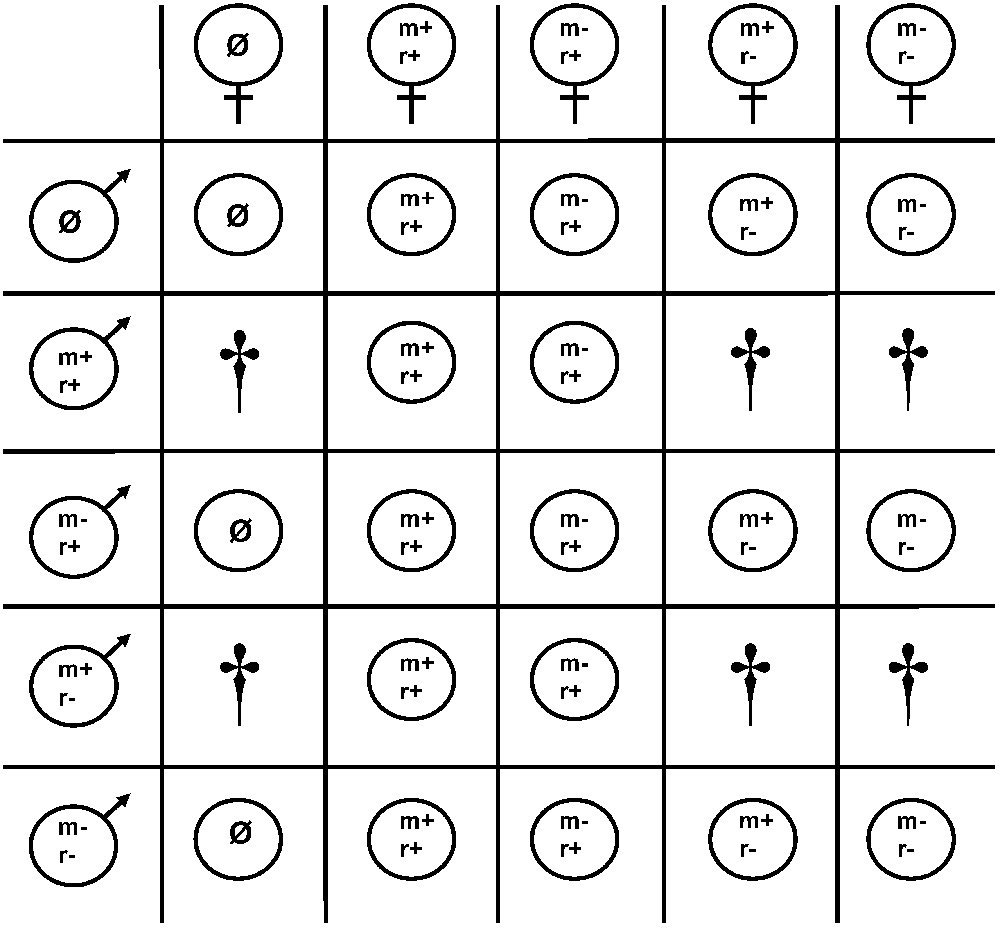
Cytoplamic incompatibility relationship between the four Wolbachia variants defined on the basis of their phenotype regarding the MODification and RESCue functions: Table showing the results of the different crosses. m+ r+: Invasive variant; m− r+: defensive variant; m+ r−: suicide variant; m− r−: helpless variant. Ø: Uninfected. Incompatible crosses are marked with a †, compatible crosses are shown by a bullet containing the phenotype of the Wolbachia variant maternally inherited by the viable embryo.
4 Why are some Wolbachia-host associations transient?
In arthropods, despite of the efficient mechanisms used by Wolbachia to insure their transmission, despite of all their evolutionary successes, the fact that the infection of a host population or species by a reproductive manipulator Wolbachia will become permanent can never be taken for granted. The main reason is that the association is typically facultative from the perspective of the host, being in a few cases beneficial but much more often neutral or even deleterious [54,55]. Accordingly, the prevalence of the infection is rarely total in host populations and polymorphic populations are the rule in Drosophila melanogaster or Drosophila simulans for CI-Wolbachia [51,56], in isopods for F-Wolbachia [57] or in the butterfly Hypolimnas bolina for MK-Wolbachia [58]. Moreover, apart from the facultative nature of this symbiosis, different processes, due to the bacterium or the host, can result in the infection being lost over time.
(i) Infection cost: In contrast to most other maternally transmitted parasites, the manipulation of host reproduction allows the spread of Wolbachia even if they induce a fitness cost on their female host. The effect of this cost on the ability of Wolbachia to invade a host population has been modelled in the case of CI [59]. The models determine an unstable infection frequency threshold, which level depends on the infection cost and the strength of the CI phenotype, below which the infection will decrease and be lost. It is only if the infection level gets over this threshold (because of migration or drift) that Wolbachia will invade the population until meeting a stable (and higher) equilibrium frequency. This second equilibrium can be close to fixation but can never be complete since vertical transmission of infected cytoplasm is not perfect, leading to at least a few uninfected individuals produced at each generation [59]. In some CI-Wolbachia, infection costs have been found, usually related to Wolbachia density in the host [60,61]. In the case of F-Wolbachia, the cost is due to the fact that males do not find neo-females (i.e. infected genetic males) as attractive as genetic females leading to lower levels of fertilisation in neo-females [62].
(ii) Loss of the sex which cannot transmit the Wolbachia: In the case of MK or F phenotypes, Wolbachia can fall victim to their own success if they invade the host population completely. Indeed, both phenotypes result in the eradication of males, which (in non-thelytokous species) will results in the extinction of the host population and the Wolbachia it harbours [63].
(iii) Infection loss in the case of CI-Wolbachia: The origin of [mod−] variants has been postulated by theoretical models [64] according to the process described on Fig. 3. One prediction of this model is the existence of [mod−; resc+] variants. Indeed, such a variant has been found in a D. simulans population sampled in Tanzania, at the foot of Mt Kilimanjaro [65]. This variant (wKi, posteriorly referred to as wMa [66]) is compatible with wNo, a [mod+; resc+] variant found in D. simulans [51]. The same phenotype has been described in variant wMau, infecting part of Drosophila mauritiana populations [67] and more recently in variants infecting drosophila species in the Yakuba complex [68]. Due to deterministic effects or because of drift, this evolution process will result in gradual infection loss, following the extinction of the [mod+] phenotype.
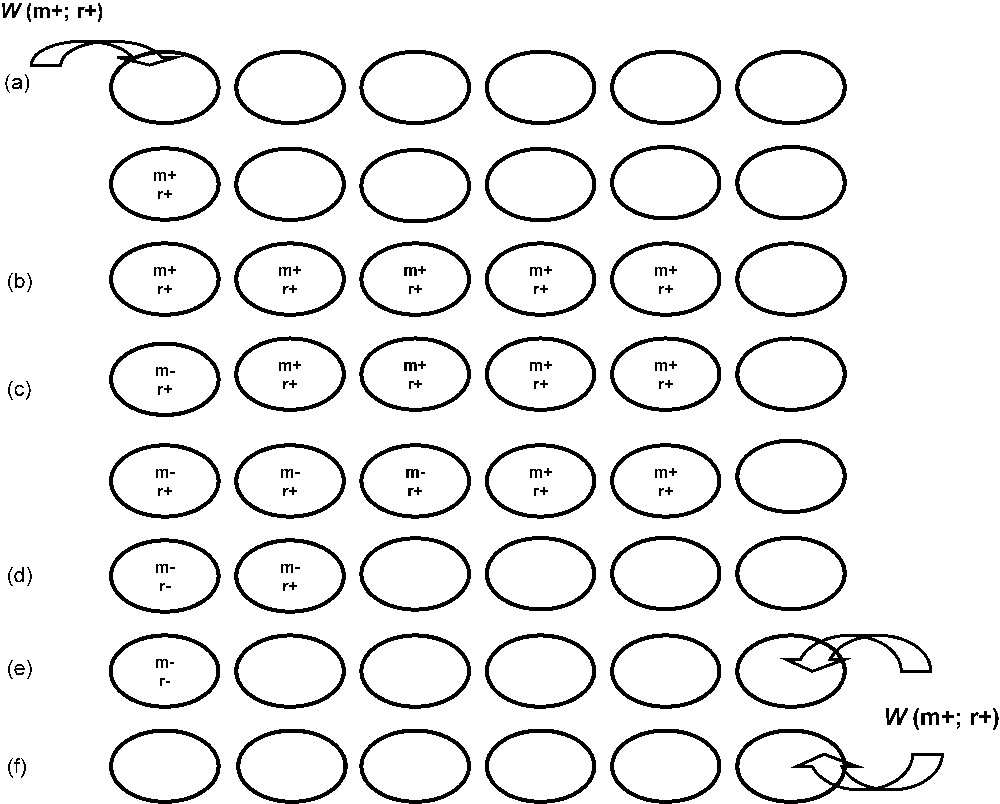
Evolutionary pathway leading to the loss of a CI-Wolbachia infection. (a) Through a horizontal transfer, initial infection by a invasive [mod+; resc+] CI-Wolbachia in a new host. (b) Through selection pressure of CI against uninfected cytoplasm, the invasive Wolbachia spreads to the entire population. (c) By mutations, appearance of defensive [mod−; resc+] variants. Due to deterministic effects or because of drift, the frequency of the defensive variants can increase leading to a possible loss of the invasive variant. (d) In this case, because there is no selective pressure against uninfected cytoplasm any more and because Wolbachia transmission to the offspring is not perfect, the frequency of uninfected cytoplasm increases. In the meantime, since the resc+ capability is not useful any more in absence of the ancestral invasive variant, helpless [mod−; resc−] variants, appeared by mutations, are not counterselected. (e), (f) In absence of any selective pressure in favour of the infection, Wolbachia infection is lost. A new cycle can start if a new horizontal transfer of an invasive CI-Wolbachia occurs.
(iv) Host resistance against the infection: The previous phenomena described which can lead to infection loss are due to the bacterium itself. However, host resistance mechanisms can either directly cause or create favourable circumstances for this loss [33]. Male-killing, feminization and thelytokous parthenogenesis induction all result in female-biased sex-ratios, and males get rarer as infection frequency increases. Because the rarest sex always has the highest reproductive success, producing females rather than males in female-biased populations is costly for host nuclear genes. Any nuclear gene that would eliminate the infection or repress its effect would thus increase in frequency. The intensity of the conflict depends on the frequency of infection: the more biased the sex-ratio, the stronger the cost of sex-ratio distortion. Evidence of host resistance has been found against feminization in the woodlice Armadillium vulgare [69] and against Male-Killing in the butterfly Hypolimnas bolina [70]. However by avoiding the elimination of the male sex, the selection of these Wolbachia suppressor genes can, under certain conditions (intragenomic conflict and/or coevolution, strong population structure) favour in fine the selfish element, i.e. Wolbachia [71]. Concerning CI, predictions have been formulated as follows [59]: for females, bearing Wolbachia is advantageous because it protects their eggs from CI-induced mortality. By contrast, bearing CI-Wolbachia is always deleterious for males, because it reduces their fertility in crosses with uninfected females. The direction of selection thus depends on infection prevalence. When Wolbachia prevalence is low, the cost suffered by infected males is very high (since they will mostly mate with uninfected females) while the benefit to infected females is low (because they will rarely mate with an infected male). On the contrary, when prevalence of the infection is high, costs suffered by infected males are low (because they will rarely mate with uninfected females) while the benefit to infected females is very high (because they will mostly mate with infected males). Overall, costs and benefits of bearing Wolbachia will balance when infected and uninfected individuals are equally frequent, which is only a transient stage because of drift. Indeed, as soon as infection frequency passes 50%, selection will favour nuclear genes increasing female transmission rates. By contrast, nuclear genes reducing levels of embryonic mortality in crosses between infected males and uninfected females are always selected for because a few uninfected individuals are always produced at each generation. Accordingly, host factors that would allow infected males to exclude Wolbachia from testes, or to resist the mechanism of CI in embryos are always advantageous for the host. Wolbachia exclusion from testes is well documented in D. melanogaster. In this species CI does not seem to be much expressed in the field, although it can be detected in the laboratory with important variations (reviewed in [51]), especially if very young males are used [72]. Clearly, D. melanogaster can lower CI levels, as showed by two independent injection experiments between D. melanogaster and D. simulans. In the first the wRi variant was transferred from D. simulans into D. melanogaster. While the bacterium induced near 100% CI in its natural host, embryonic mortality was only 30% in the D. melanogaster trans-infected line [73]. In the second experiment the natural D. melanogaster variant (wMel) was injected into D. simulans. While in the donor D. melanogaster line CI level was about 30%, it jumped close to 100% in D. simulans injected lines in a few generations. Moreover, CI levels were not correlated to bacterial densities in eggs or in males if the whole body was considered, but they were significantly correlated with the proportion of infected sperm cysts in the testes. D. simulans males from trans-infected lines had more than 80% of their sperm cysts infected, versus 8% only in the donor D. melanogaster line [74]. It thus appears that D. melanogaster strongly represses the expression of CI. Another example of a control by the host is found in the Yakuba complex of Drosophila species. The three species of the complex (Drosophila yakuba, Drosophila santomea and Drosophila teissieri) are each infected by a Wolbachia variant (respectively wYak, wSan and wTei). In its natural host, each variant does not express a CI phenotype [68], and this is also the case when D. simulans lines are artificially transinfected with wYak or wSan [75]. In stark contrast, young D. simulans males from lines transinfected with wTei induce 100% embryonic mortality in crosses with uninfected females [75]. Like the occurrence of [mod−; resc+] variants, the repression of the CI phenotype by the host can result in the infection being lost. The rescue of the CI phenotype by the host itself would be another possibility. Although such a case has not been found yet, it is likely to exist, either from mutation in nuclear genes of the host or, more drastically, by integration of bacterial resc genes in the genome of the host. The latter option is not as far fetched as it looks. Indeed, a near complete Wolbachia genome was very recently found embedded in nuclear genes of its drosophilid host (Drosophila ananassae) [76], which constitutes the most important lateral gene transfer between a prokaryote and an eucaryote known to date. It is not possible yet to tell whether the genome in question is that of the Wolbachia variant found naturally in the cytoplasm of this species [77] or if it is a trace of another ancestral infection. But if this bacterium is of the [mod+] type, it would be very interesting to know what happens when a line (trans-)infected with this Wolbachia is crossed with uninfected females harbouring the bacterial DNA insert. The expression of a [resc+] phenotype by these females would be a remarkable example of a gain of function by the host resulting from the borrowing of a symbiont's gene (resc) allowing to resist a selective pressure due to the symbiont (via the mod function).
5 What are the permanent host-Wolbachia associations?
The power of the sex-manipulation effects that Wolbachia cause in arthropods allows them to compensate for the disadvantage of a possible infection cost for the host. But there are cases where the phenomenon observed can only be explained by a mutualistic effect of the bacteria, even if its mechanism is not always elucidated. In one case, in the parasitoid wasp Asobara tabida, the bacterium has indeed become an obligate symbiont for its host. We shall also see that among the four sex-manipulation mechanisms, thelytokous parthenogenesis can lead to a stable and permanent infection status. Finally, the “obligate symbiont strategy” is nowhere as clear as in the other host group of Wolbachia: filarial nematodes.
(i) Irreversibility of the TPI-Wolbachia infection: Contrary to male-killing or feminization, the induction of thelytokous parthenogenesis does not lead to a population crash if the male sex is lost from the population. Despite of the possible inbreeding depression associated with asexual reproduction, the action of TPI-Wolbachia frequently occurs in parasitoid wasps and this reproduction mode appears as potentially irreversible. Indeed, although it is usually possible to obtain male offspring again by curing thelytokous parthenogenetic females from their Wolbachia, the males thus produced are in many cases unable to produce offspring sexually. The symptoms can be either the inability to produce viable sperm, to court females, or the inability of females to be receptive to male courtship or to store sperm (reviewed in [7]). In those cases at least, the populations appears to be indefinitely locked in parthenogenesis mode. It has been remarked that the highly female-biased sex ratio resulting from a spreading TPI-bacterium infection might select for mutations causing uninfected females to produce more male offspring [78]. However, this would not stop the spread of infected cytoplasm at all. Quite the contrary, these mutant females would produce less daughters able to transmit uninfected cytoplasm, thus further increasing the relative fitness advantage of infected cytoplasm. Moreover, once the infection reaches fixation, the production of uninfected lineages would become impossible. Indeed, rare uninfected females would either die before finding a male or find a male having lost the ability to fertilise them. In both cases, only haploid males would be produced, unable to transmit the uninfected cytoplasm to the next generation. A particular scenario is that of a functional virginity mutation (uninfected females refuse mating, thus producing all-male broods, which can spread efficiently their nuclear genes). Such functional virginity genes have been detected in the parasitoid wasp Telenomus nawai [79]. However, even the fixation of such a “behavioural virginity” gene in the population would not stop the infection at all, since (i) uninfected functional virgins abandon the transmission of their uninfected cytoplasm by producing only sons, and (ii) virginity in an infected female leads to an all-female brood anyway.
(ii) The [mod-] CI-Wolbachia: The progress of Wolbachia studies has revealed more and more populations and species which members of both sexes harbour Wolbachia which do not express CI, hence of phenotype [mod−]. Such cases are well illustrated in drosophilids. In D. simulans, wAu is a cosmopolitan variant found in Australia, Africa and America, with relatively low frequencies of 5–33% in wild populations [51,56,80]. It has been thought that the wAu infection was very ancient which would have explained its cosmopolitan nature and its low frequency in populations [66]. However, this hypothesis has been completely turned on its head recently, the wAu infection being now considered as the most recent in D. simulans [56], having occurred possibly following an horizontal transfer from a neo-tropical drosophila into D. simulans less than three centuries ago [81]. If such is the case, the whole process described in Fig. 3 would have taken place several times for this variant, within 5000 host generations. However, the persistence of [mod+] clones cannot be ruled out, which would maintain a background level of the infection in populations. As reported above, wAu seems to induce weak levels of CI in some D. simulans populations from Florida [82] but it is the only such case described. Alternatively, the driving force for the maintenance (and/or the spread) of wAu could be a positive fitness effect on its hosts. Twice already, such effects have been searched for extensively, but were not found [66,80]. The neo-tropical species Drosophila willistoni harbours a similar variant (wWil). Curiously, this variant is found in 17 of the 21 populations sampled between 1974 and 2002 (with 100% of the flies infected) while it is totally absent from the eight populations sampled earlier (1941 to 1971). The hypothesis of a very recent invasion of D. willistoni (i.e. in the 1970s) is privileged by the authors compared to the possibility that older laboratory stocks may have lost the infection [81]. The main argument in favour of this interpretation is that in D. willistoni, vertical transmission of the bacterium is perfect thanks to an extreme tissue tropism of wWil toward the germline of its host [81]. However, if a perfect vertical transmission can explain permanence, it does not explain the initial spread of the infection following an horizontal transfer. The hypothesis of a very recent invasion by a [mod−] variant thus seems paradoxical. Another example of [mod−] Wolbachia is found in the three drosophila species forming the Yakuba complex [68]. Investigations about putative positive effects of the bacterium in D. yakuba show that the variant wYak behaves as a neutral or nearly neutral variant, maintained thanks to a perfect vertical transmission [83]. Here again, no beneficial effect has been found yet. Finally, in D. melanogaster, the variant wMel revealed five distinct variants, all of a [mod−] phenotype (at least in the natural population). An analysis of old laboratory stocks together with wild-caught flies indicates that one of these variants has replaced the other globally within the last century [84], but the cause for this sweep is unknown. The search for beneficial effects of wMel on the host have brought conflicting results, with either no effect at all [85,86] or a positive effect on longevity or on fecundity [87–89]. However, the discrepancy between these results may lie in the different variant studied, but since this information is not available yet is it not possible to conclude. In any cases, the omnipresence of the [mod−] variants (whether an intrinsic quality or because of host repression), their distribution pattern, and the means by which they have spread remain elusive.
(iii) From parasite to mutualist?: If, to explain the permanence of a [mod−] infection, the hypothesis of a mutualistic effect leaps to the mind, this hypothesis is not required in the case of [mod+] Wolbachia, because the CI phenotype is sufficient to explain both their initial spread and their permanence in host populations, regardless of fitness costs for the host. However, in a recent paper whose heading reads “From parasite to mutualist:…”, a mutualistic effect of a [mod+] Wolbachia has been reported [90]. This variant (wRi, found in D. simulans) induces at least 90% of embryonic mortality in incompatible crosses [51]. In a study published 20 years ago, a reduced fecundity had been found in laboratory stocks established from Californian populations where the variant was very rapidly spreading [91] Twenty years later, in the same populations, infected females show an increased fecundity compared to aposymbiotic ones [90]. The authors underline that this result is in agreement with theoretical models since the fixation of the infection is supposed to lead to a selection of those clones advantaging the host [59]. This theory is supported by several other Wolbachia-induced fitness benefits, such as fecundity advantages in the parasitoid wasp Trichogramma bourarachae [92], a potential increase in longevity in D. melanogaster [87,89] and increased fecundity and longevity in the mosquito Aedes albopictus [93]. However, the majority of investigations for Wolbachia-related fitness effects in insects have either failed to find any effect or found negative effects of the infection (reviewed in [85]). In any case, the putative beneficial effects described here remain within the framework of a secondary (i.e. not obligate) symbiosis – from the host point of view – which is not true of the symbiotic associations described further below.
(iv) Mutualism or trickery?: Asobara tabida is a parasitic wasp infected by three Wolbachia strains. Two of these strains induce CI. Eliminating these two variants using a moderate antibiotic treatment does not cause any deleterious consequence for the host. In sharp contrast, eliminating the third variant (wAtab3) from females during the early larval development renders them sterile: they cannot produce mature oocytes [94]. Another case of symbiont-dependant oogenesis has also been described in the stone beetle Coccotrypes dactyliperda [95]. This beetle is infected with both Wolbachia and Rickettsia but the specific role each of the bacteria plays in the oogenesis remains to be determined. A vital dependence between an insect host and its Wolbachia has been observed in the moth species Ostrinia scapularis and O. furnacalis [96]. Here, Wolbachia induce a classical male killing phenotype, but an antibiotic treatment of mothers leads their aposymbiotic daughters to die. How these hosts have become dependent on symbionts in the course of evolution remains an open question, but different scenario have been proposed in the case of A. tabida. One hypothesis is that the wAtab3 strain produces during oogenesis a toxic molecule that is first accumulated in the cytoplasm of the egg and then neutralized by the production of an antidote by the same Wolbachia during the development of the embryo. Accordingly, in females having lost the wAtab3 variant following an antibiotic treatment as adults, the toxin has been produced by the mother but is not neutralized by the antidote in the offspring. The result is that oogenesis is specifically inhibited in female offspring, leading to sterility [97] Another hypothesis that could explain how A. tabida became dependent on wAtab3 for oogenesis is that the host species A. tabida accumulated irreversible modifications in one or more nuclear gene essential for oogenesis. This model assumes that the wAtab3 strain had the special capability to act on host oogenesis, and that this action interfered with the oogenetic control of the host [98]. Now, is it pertinent to consider these obligatory associations as mutualistic? It has been proposed that two interdependent symbiotic partners do not necessarily form a mutualistic association in the sense that each partner does not necessarily benefit from the other [99]. Such “mutualistic” interactions would more correctly been considered as a “trickery” [100]. However, even if the bacterium has initially succeeded in trapping its host for its own benefit (like in the case of A. tabida), the obligate relationship created should, in the long term, select for bacterial clones bringing true benefits to the host, leading to a primary symbiosis such as the one observed in filarial nematodes.
(v) Wolbachia in filarial nematodes: The first observations of “innenkorpe” (central body) in tissues or unusual light mitochondria in oocytes of the dog heart-worm Dirofilaria immitis were published in the late 1960s and early 1970s. Their bacterial nature was suggested a few years later and confirmed by the description of intracytoplasmic bacteria in Onchocerca volvolus and the transovarial transmission of intracellular microorganism (possibly Ricketsiales) in Brugia malayi (reviewed in [101]). It took another twenty years before these bacteria were formally identified as Wolbachia [102], and few years later their key functional role in nematodes was identified following antibiotic treatments directed against their filarial hosts. The association is a primary symbiosis: it is obligatory for both partners. The removal of Wolbachia from the nematode leads to the sterility or developmental arrest in the host depending on the host species (reviewed in [16]). Accordingly, infection prevalence is total. Although Wolbachia are not harboured in a specialised organ per se, they are only found in the female reproductive system, as well as in the hypodermal cells within the lateral chords (in both sexes). A strict congruence is observed between the phylogeny of host nematodes and that of their Wolbachia [19], which suggests a long history of co-speciation without horizontal transfers between host species, in sharp contrast with the situation found in arthropods [18]. This lack of transfers is further corroborated by the absence of multi-infections and a recombination rate which is close to zero in these Wolbachia [103]. The smaller size of wBm, infecting the nematode B. malayi, compared to wDm infecting D. melanogaster (1.08 vs. 1.26 Mb), and its smaller number of coding genes (806 vs. 1271) might further reflect the difference between a true mutualist compared to a parasite [104]. Another observation also reinforces this view. Positive selection was detected in the gene coding for WSP of arthropod-borne Wolbachia, unlike what occurs in filarial-borne Wolbachia, reflecting the parasitic character of the former and the mutualistic nature of the latter [105]. Finally, from the metabolic point of view, wBm may provide its host with essential metabolites like riboflavin and flavin adenine dinucleotide, heme, purine and pyrimidine nucleotides while reciprocally it is likely that wBm receives from its host some amino-acids necessary to its bacterial metabolism [104]. Thus, contrary to the “trickery” observed in A. tabida, all the evidence supports the notion that a truly mutualistic relationship has developed between Wolbachia and their filarial hosts: there are benefits on both sides [105]. Also, considering the difference of hosts (arthropods vs. nematodes) and of symbiotic relationship (parasitism vs. mutualism) it was recently proposed to recognize the C and D Wolbachia as distinct species, respectively as Wolbachia volvulus and Wolbachia malayi [20].
6 What past? What future?
The Wolbachia, maternally transmitted endosymbiotic bacteria, have developed two “opposite” strategies to conquer their hosts: (i) secondary (i.e. not obligate) symbiosis in arthropods, where they have become efficient sex-manipulators; and (ii) primary (i.e. obligate) symbiosis in filarial nematodes, where they have become efficient mutualists. This begs the question: which strategy came first? In a recent study, the authors used partial genome sequences available from wOvo, the variant from clade C found in Onchocerca volvulus, to identify 42 orthologous genes in wOvo, wMel (clade A), wBm (clade D) and related anaplasmataceae. The phylogenetic analysis suggests that the common ancestor of Wolbachia was probably an intracellular sex-parasite, because the root of the tree robustly falls between clade A (arthropod-associated Wolbachia) and clades C and D (nematode-associated Wolbachia) [106]. We can therefore propose an evolutionary scenario: vertical transmission of the sex-parasite ancestor (which probably expressed a CI phenotype) allowed it as a first step to invade its host species and to be transmitted as a mutualist symbiont without being one. Then, Wolbachia may have “tricked” an ancestral filarial nematode into becoming indispensable for oogenesis or development (the stage now observed in A. tabida). With time, a truly mutualistic relationship evolved, explaining the co-evolution found in nematodes. However, did Wolbachia develop this privileged relationship with filarial nematodes (but not with arthropods) by chance alone? If not, what is so special about nematodes? It has been proposed that because horizontal Wolbachia transfers do not occur in nematodes, the only available strategy to be maintained in the host was mutualism [100]. Indeed, if CI-Wolbachia are systematically lost at some stage or another during evolution, only the capacity to jump horizontally to naïve hosts can allow them to start a new cycle, which might be the pattern found in arthropods. That being said, is the mutualistic phenotype of nematode-associated Wolbachia irreversible? The answer seems to be in the negative, since some filarial nematodes do not harbour any bacteria [107]. Two hypotheses may explain this pattern: (i) Wolbachia infected the ancestor of all extant filarial nematodes but was secondarily lost in some lineages during co-speciation; (ii) Wolbachia infected independently some nematode lineages only (which does not mean that they cannot be lost secondarily in some cases). Although current data do not allow one to exclude formally one of these two hypotheses, the very close phylogenetic relationship between infected and uninfected nematode species fits better with the notion that Wolbachia would have been lost in some host lineages during the course of evolution [107]. Filarial nematodes are not strictly associated with Wolbachia clades C and D since two species of the genus Mansonella are infected by a clade F variant, a Wolbachia clade also found in Arthropods. This pattern would be due to a recent horizontal transfer, although the direction of transfer is not known yet [26]. It remains to be seen if this Wolbachia variant behaves as a mutualist. Finally, although Wolbachia was believed to be absent from non-filarial nematodes [108,109], this is not the case anymore: a variant belonging putatively to clade G was recently detected in Angiostrongylus cantonensis, a non-filarial species [110].
Regarding Arthropod-associated Wolbachia, cytoplasmic incompatibility is considered as the ancestral phenotype [18], not only because it is the most widespread but also because all other phenotypes lead to the rarefaction (or eradication) of males: if males had been rare or absent, how could CI have appeared in the first place? Accordingly, the three phenotypes leading to a strong sex-ratio distortion might have appeared from an ancestral variant causing CI, but not the other way round. An interesting consequence is that the Wolbachia functions allowing feminization, make killing or thelytokous parthenogenesis have not necessarily eliminated the ancestral CI capability in these Wolbachia. This capability might still be present but hidden since males are not available to express it, until the phenotype of sex-ratio distortion is suppressed as is the case in some populations of H. bolina [111]. Are arthropod-associated Wolbachia bound to remain sex-manipulators forever? On the one hand, their capability to jump between species and their sex-manipulation phenotypes allow them to compensate for the risk of being lost considering the facultative nature of their infection (from the host point of view), but on the other hand, what about the [mod−] phenotypes found in arthropods? Are they condemned to disappear, replaced by the next [mod+] arrived by horizontal transfer? Is the A. tabida “mutualist” variant an exception? Our vision of arthropod-associated Wolbachia might be biased by the fact that sex-manipulation phenotypes are so spectacular that they might hide more subtle positive effects. A better knowledge of Wolbachia-arthropod physiological interactions would be necessary in that respect.
In conclusion, Wolbachia strategies are exceptionally diverse, spanning the whole range from drastic sex manipulation to gentle mutualism, but in evolution the end justifies all means, and this bacterium is the most spectacular success story among symbionts, representing probably the largest biomass of this functional group.


