1. Introduction
In the animal and plant kingdoms, intraspecific communication is mainly mediated by chemical components that are released by a transmitter and that modify the behaviour of a receiver in an adapted fashion [1]. These chemicals—pheromones—can act at various distances (e.g., contact chemicals for gustation and long-range chemicals for olfaction) and mediate numerous behaviours such as sex recognition, copulation and gregarism [2].
In invertebrates, pheromones can be present in glands or directly on an individual’s cuticle and thus are easily releasable and accessible at long distances, at short distances or by contact with potential receivers [3]. Cuticular chemical compounds (CCCs) are detected by peripheral organs called sensilla, housing gustatory or olfactory receptor neurons and representing the first relay of chemical detection. Peripheral detection is followed by the central integration of chemical information, leading to the behavioural response of the receiver [4]. In terrestrial isopods, chemoreception is mainly ensured by the second antenna (the first pair being strongly reduced), on which is located the apical sensory organ, a tuft of sensilla gathering both the olfactory and gustatory functions [5, 6, 7].
Qualitative and/or quantitative modifications of CCC composition can be observed between sexes [3], but other factors (i.e., non-genetic factors), such as the reproductive status or health of an individual, can also alter CCC production [8, 9, 10, 11, 12, 13, 14, 15]. Many studies have shown the negative impact of pathogens or parasites on host odour [16], such as loss of its attractiveness or, to the extreme, becoming repulsive [13, 17, 18, 19], limiting the fitness of the infected host [20]. In those cases, the recognition and avoidance of infected partners by non-infected partners can be beneficial, directly by decreasing the probability of contracting the pathogens and indirectly by avoiding transmission to offspring (even increasing their resistance to it) [21]. The odour modification of an individual resulting from its infection by a pathogen can thereby inform a partner of its health status and thus represent an honest signal of its quality [22, 23].
Wolbachia pipientis (hereafter Wolbachia) is an intracellular alpha-Proteobacterium maternally transmitted in oocyte cytoplasm and spread in nearly 75% of terrestrial arthropods and nematodes [24]. In some species, Wolbachia infection leads to deleterious effects on host fitness: in Tribolium confusum, infected females produce fewer offspring than non-infected females [25]. In Drosophila simulans, Wolbachia strain wRi is responsible for a decrease in infected female fecundity [26]. A loss of performance (in terms of sexual individuals produced) is observed in Formica truncorum colonies exhibiting a high proportion of infected workers [27]. Wolbachia can also be responsible for breeding behaviour distortions, as in the spider mite host Tetranychus urticae, where uninfected individuals tend to mate together, while infected individuals promote sib matings in their progeny [28].
Wolbachia is also known to manipulate the reproduction of its host, securing its vertical transmission, through four major strategies: cytoplasmic incompatibility (decreasing the fitness of uninfected individuals), parthenogenesis, male killing and feminization (increasing the female proportion in the population) [29]. This last strategy describes the conversion of infected genetic males into intersexed functional females (male genotype, female phenotype) [29, 30].
In the terrestrial isopod Armadillidium vulgare (commonly called woodlouse), males discriminate between males and females at short distances [31]. Copulation is linked with assortative mating, and mating probability necessitates a stimulation duration beyond the threshold [32].
The sex ratio of natural A. vulgare populations is, however, biased towards females by the presence of two Wolbachia strains (wVulC and wVulM) responsible for the feminization of infected male embryos [33]. These infected individuals (Wb females from now on) suffer from decreased survival and lower fertility and immunity [34, 35, 36].
Wb females also show a decrease in their mating success [31, 37, 38]; however, the cause of this decrease is still unknown. Two main hypotheses can be formulated: (i) In the short range, the chemical differences in cuticular profiles between Wb females and Wb-free females may allow males to assess their respective health status [12], helping them properly orient their sexual behaviour towards uninfected females. In this scenario, we hypothesize that Wb-free and Wb female emit a “sex reversal”-specific odour discriminable by males. (ii) After male courtship starts, Wb females display aberrant behaviour leading to multiple mating interruptions [35, 38]. In this scenario, we hypothesize that Wolbachia presence alters Wb female olfactory detection of male compounds, potentially leading to aberrant behaviour and the interruption of mating at early stages.
To that end, we first evaluated the olfactory electrophysiological responses of A. vulgare male antennae to volatiles derived from Wb-free and Wb females using electroantennography recording [39, 40]. To detect potential biologically active unpleasant odours, cuticular compounds of Wb-free and Wb females were extracted in five successive solvents (water–methanol–hexane–dichloromethane–ethyl acetate) and presented to the male antenna.
We then tested whether Wolbachia infection impacted male detection by females by extracting male cuticular compounds and presenting them to Wb and Wb-free female antennae.
2. Material and methods
2.1. Rearing conditions
All experiments took place in the Ecology and Biology of Interactions Laboratory at the University of Poitiers, France. Individuals of Armadillidium vulgare (Crustacea, Isopoda, Oniscidea; Latreille, 1804) were kept on moistened compost in plastic boxes (26 × 13 cm) under laboratory conditions (20°, photoperiod of France, 46° 40′ N) and supplied ad libitum with fresh carrots and linden tree leaves. Specimens were derived from individuals initially collected in Helsingør (Denmark). In the current experiment, we used males (ZZ), females (ZW) later called asymbiotic females and feminized males (ZZ + Wo). To avoid inbreeding, gravid females were isolated, and their offspring were sexed. Males and females were then reared in different boxes before sexual maturity, allowing breeding control. All individuals used in this study were one-year-old and virgin.
2.2. Wolbachia assessment
Wolbachia presence was assessed by DNA extraction and PCR assays on dissected gonads or legs. Total genomic DNA was obtained by phenol/chloroform extraction and ethanol precipitation. PCR amplification was performed using a 650-bp segment of the WOSP gene, commonly used to amplify a Wolbachia surface protein present in various strains of Wolbachia (primers sequences: 81F; 5′-TGG-TCC-AAT-AAG-TGA-TGA-AGA-AAC-3′ and 691R; 5′-AAA-AAT-TAA-ACG-CTA-CTC-CA-3′) [41]. PCR cycling conditions were 95 °C for 2 min; 35 cycles at 95 °C for 1 min, 55 °C for 1 min, 72 °C for 1 min; 72 °C for 5 min; and then held at 4 °C. The gene fragment was finally revealed by ethidium bromide staining on agarose gel.
2.3. Scanning electron microscopy (SEM)
Scanning electron microscopy was carried out on the antennae of 1-year-old males, females and symbiotic individuals. We selected individuals just after anterior moulting and kept them individually in plastic boxes (∅ = 8 × 5 cm) lined with moistened paper. Two days after full moulting, both antennae of every individual were collected under a dissection microscope. Antennae were washed for 2 h in a fixing buffer at 4 °C (3% glutaraldehyde, 0.1 M sodium cacodylate, 1% NaCl, pH adjusted to 7.3) then 2 h in a washing buffer at 4 °C (0.26 M sucrose, 0.1 M sodium cacodylate, 1% NaCl, pH adjusted to 7.3) followed by 1 h in post-fixing buffer at room temperature (1.3% osmium tetroxide, 0.1 M sodium cacodylate, 1.8% NaCl). Antennae were then dehydrated in four successive acetone baths with increasing concentrations (50, 70, 90 and 100%), dried with a critical point bypass device (CDP 030 Balzers®) gradually replacing the acetone with CO2, and then fixed on a SEM adapted support with silver lacquer and platinum coated (metallizer SCD 005 BAL-TEC®). Observations were finally carried out on a JEOL 840A at a 10-kV tension.
To support the possible size dimorphism between the two antennae (right and left) of an individual, the sensilla density per μm2 for each antennal segment was determined according to [42]: the segment was compared to a cylinder of surface π⋅∅⋅h. Sensilla were counted on a portion corresponding to 1/3 of the visible surface (equivalent to 1/6 of the total surface of the segment). The density was finally obtained by dividing the total number of sensilla on the segment by its surface.
2.4. Silver staining
The presence of porous sensilla on A. vulgare antennae was investigated by silver staining [43]. Two one-year-old males, females and feminized males (naturally infected females) in anterior moulting were placed in plastic boxes (∅ = 8 × 5 cm) on moistened paper. Twenty-four hours after moulting, both antennae of all individuals were excised and cleaned with a soft brush soaked in 70% ethanol. After air-drying, antennae were immersed in a mixture of 70% ethanol and 1 M silver nitrate for 24 h at 4 °C and protected from light. Antennae were then cleaned with two successive ethanol baths of 1 h each (90 and 100%). They were finally placed overnight in xylene (Merck®, 95% purity) and then mounted on microscope slides with xylene-free mounting medium (Diamount, DiaPath®) for optical microscope observations (Eclipse Ci-S model, Nikon®). Observations focused on the tip of the antenna, displaying the apical sensory organ [44], and the sensilla/aesthetascs distributed over the antenna [45].
2.5. Extraction of cuticular compounds
2.5.1. Experiment 1. Assessing male antennal response towards female cuticular compounds
We extracted the cuticular profiles of 15 A. vulgare asymbiotic and 15 naturally infected females. Females were freeze-killed for 20 min at −20 °C prior to extraction. Chemical extractions were carried out using five solvents of various polarities: water (polarity = 1), methanol (polarity = 0.762), hexane (polarity = 0.009, considered apolar), dichloromethane (polarity = 0.309) and ethyl acetate (polarity = 0.228) (Acros Organics®) [46]. Each individual was successively immersed in 1 ml of solvent under constant agitation for 30 min with a 30-min drying period under a hood between each solvent. The water extracts were lyophilized at −80 °C and resuspended in 50 μl. Methanol, hexane, dichloromethane and ethyl acetate extracts were dried under high-purity nitrogen flow (N2) up to 50 μl.
2.5.2. Experiment 2. Assessing female antennal response (asymbiotic and naturally infected) towards male cuticular compounds
Based on the results of Experiment 1, we modified the protocol for the second experiment. We extracted the cuticular chemical profiles of 72 A. vulgare males. Males were transferred to filter paper for cleaning 3 h prior to extraction and then freeze-killed 2 h prior to it. The cuticular chemical profiles of 3 batches of 24 males were extracted with solvents of different polarity: dichloromethane, ethyl acetate, and hexane. Batches were first immersed in 5 ml of Milli-Q water for 30 min, then dried for 30 min under a hood and finally immersed in 5 ml of solvent. Regular agitation was performed during both immersions. Extracts were dried up to 600 μl under nitrogen flow and stored at −20 °C.
2.6. Electroantennography (EAG)
EAG allows the recording of voltage variation across an antenna (differential between the base and the tip) after the detection of a chemical or a mixture of chemicals [47]. Here, we measure EAG recording with an isolated antenna connected on both extremities of the electrodes on a closed circuit [39, 48]. For each replicate, the left or right antenna of 1-year-old individuals was randomly sampled by excision at its base and mounted between the electrodes of an EAG recording probe (Syntech®). Electrical contact was ensured by an electrode conductive gel (Signa gel®, Parker), and special attention was given to the position of the antenna (placed in an arc at the end of the two electrodes), allowing it to sag over time without getting bogged down. The recording device was composed of an electronic filter (high-pass filter to 0.05 Hz) with a stimulus controller (CS-55, Syntech®) and an acquisition interface device (IDAC-2, Syntech®). The electroantennogram profiles were visualized using EAD/2014 software (Syntech®).
During the whole experiment, the antenna is exposed to a stable moistened air flow carried from the stimulus controller, passing through a humidifier and delivered via a glass tube surrounding the antenna. Antennae were exposed to a series of chemical extracts presented in random order deposited on rectangle filter papers (2 × 0.2 cm, Whatman™, GE Healthcare). After solvent evaporation, the filter papers were introduced into a glass Pasteur pipette (150 mm, WWR®) equipped with a rubber pipette bulb. Stimuli presentations were performed by sending the odours (by pressing) into the glass tube carrying the air flow, ensuring reception of the volatile compounds by the antenna. Every stimulus, of 1 s each, was spaced at least 30 s apart to avoid any adaptation or sensitization of the antenna [49].
Four replicates were performed per antenna, with each consisting of 3 controls of volatiles from filter papers exposed to pure solvent followed by 3 filter papers exposed to solvent plus cuticular extracts. After the antenna mounting, a control composed of pure methanol was presented before and after the presentation of 15 stimuli to ensure the full reactivity of the antenna. In total, we used 15 male antennae to assess their response towards female-borne volatiles and 14 female antennae (7 per condition: asymbiotic and naturally infected) to assess their response towards male-borne volatiles.
2.7. Statistical analysis
2.7.1. Scanning electron microscopy
For SEM, we calculated the mean of the sensilla density on both antennae for the same sex and compared it using Wilcoxon signed rank test.
2.7.2. Comparisons of antennal perception
Mean responses to a solvent (responses to the water control and water extracts of asymbiotic and naturally infected females, for example) were compared using Kruskal–Wallis tests followed by post hoc tests.
The difference in perception between asymbiotic and naturally infected female antennae towards compounds from the male cuticle was evaluated with non-parametric independent tests (Wilcoxon rank sum test).
3. Results
3.1. Scanning electron microscopy of A. vulgare antennae
The A. vulgare antenna (4.59 ± 0.06 mm) is composed of six segments (Figure 1) covered with trichodea sensilla and aesthetacs (Figures 2 and 3). The apical extremity of the antenna (segment I) is capped with the sensorial apical organ, formed by a tuft of sensilla whose taste function has been investigated through silver staining (Figure 3).
Overview of A. vulgare antennae.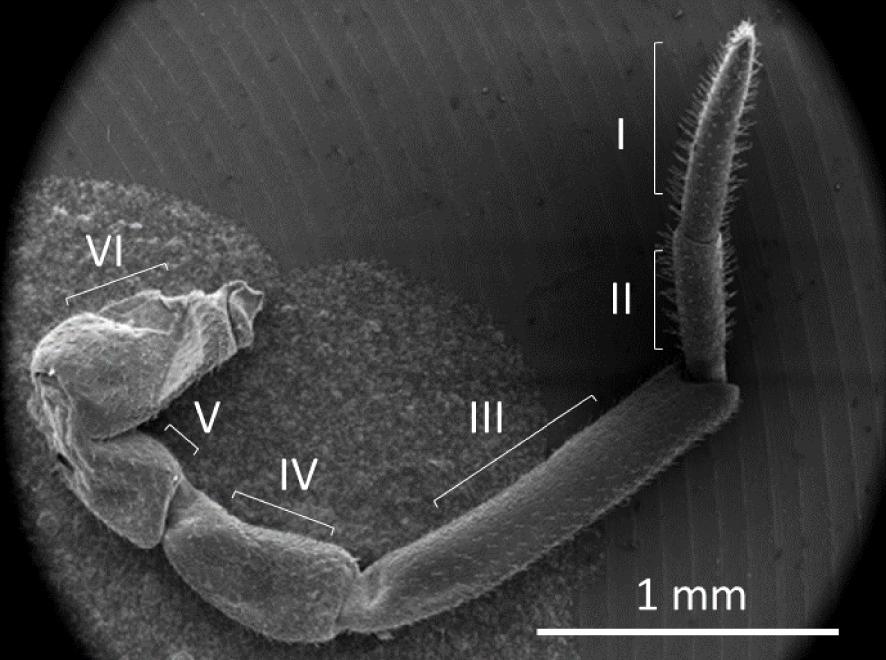
Visualization of unimodal olfactory sensilla present along the A. vulgare antenna. (A–C) Scanning electron microscopy. (D–F) Silver staining; the black colour at the base (E and F) seems to indicate the entrance of volatile molecules.
A. vulgare sensorial apical organ located at the end of segment I (see Figure 1) observed by scanning electron microscopy (A) composed of apical sensilla (B). Their argyrophilic property (C) allows us to classify them as taste sensilla.
Counting sensilla allowed us to estimate sensilla density (sensilla/μm2) on antennae for males, females free of Wolbachia and naturally infected females (Table 1). Antenna fixation and silver lacquering did not allow accurate counting of sensilla density on the two basal segments, and observations were mainly carried out on segments I–IV. The mean lengths of the segments were I (0.59 ± 0.039 mm), II (0.41 ± 0.029 mm), III (1.34 ± 0.089 mm) and IV (0.66 ± 0.035 mm) (Table 1).
Sensilla density on the right and left antennae of different A. vulgare sexes
| Segments | Counted sensilla | Total number of sensilla | Surface of segments (μm2) | Density (sensilla/μm2) | Chi2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | Right | Left | |||
| Wolbachia-females | I | 48 | 34 | 288 | 204 | 185,849 | 227,959 | 0.0013 | 0.00075 | |
| II | 40 | 31 | 240 | 186 | 196,705 | 124,149 | 0.0012 | 0.0015 | ||
| III | 58 | 52 | 348 | 312 | 562,486 | 865,417 | 0.00062 | 0.00036 | ||
| IV | 27 | 40 | 162 | 240 | 442,510 | 509,451 | 0.00037 | 0.00047 | ||
| Total | 1038 | 942 | 1,387,552 | 1,726,977 | 0.00075 | 0.00054 | 3.17 × 10−5; P > 0.05 | |||
| Wolbachia-free females | I | 34 | 30 | 204 | 180 | 291,482 | 257,368 | 0.0006 | 0.00062 | |
| II | 22 | 29 | 132 | 174 | 235,969 | 207,751 | 0.00056 | 0.00084 | ||
| III | 64 | 60 | 384 | 360 | 1,300,726 | 1,159,775 | 0.0003 | 0.00031 | ||
| IV | 36 | 49 | 216 | 294 | 661,625 | 687,490 | 0.00033 | 0.00043 | ||
| Total | 936 | 1008 | 2,489,803 | 2,312,386 | 0.00038 | 0.00044 | 4.43 × 10−6; P > 0.05 | |||
| Males | I | 32 | 34 | 192 | 204 | 244,453 | 273,825 | 0.00067 | 0.00062 | |
| II | 26 | 20 | 156 | 120 | 220,134 | 256,111 | 0.00071 | 0.00047 | ||
| III | 45 | 39 | 270 | 234 | 1,159,326 | 1,056,725 | 0.00023 | 0.00022 | ||
| IV | 36 | 22 | 216 | 132 | 617,350 | 658,544 | 0.00035 | 0.0002 | ||
| Total | 834 | 690 | 2,241,264 | 2,245,206 | 0.00037 | 0.00031 | 6.18 × 10−6; P > 0.05 | |||
For all individuals, comparisons between left and right antennae were not significant (all Chi2 p > 0.05) based on total density, thus allowing the random selection of left or right antennae for further EAG experiments.
Antenna electrical responses were recorded for male and female A. vulgare towards conspecific volatile cues extracted with different solvents. An example of the amplitude of the antenna response is provided in Figure 4.
Electroantennogram of asymbiotic female antennae towards control solvent and male volatile extracts. (A) Timestamps of stimulus delivery. (B) Amplitude of antennal response. C: Control solvent; T: male extract; Di: dichloromethane; Hex: hexane; EA: ethyl acetate. Square sides represent an amplitude of 2 mV.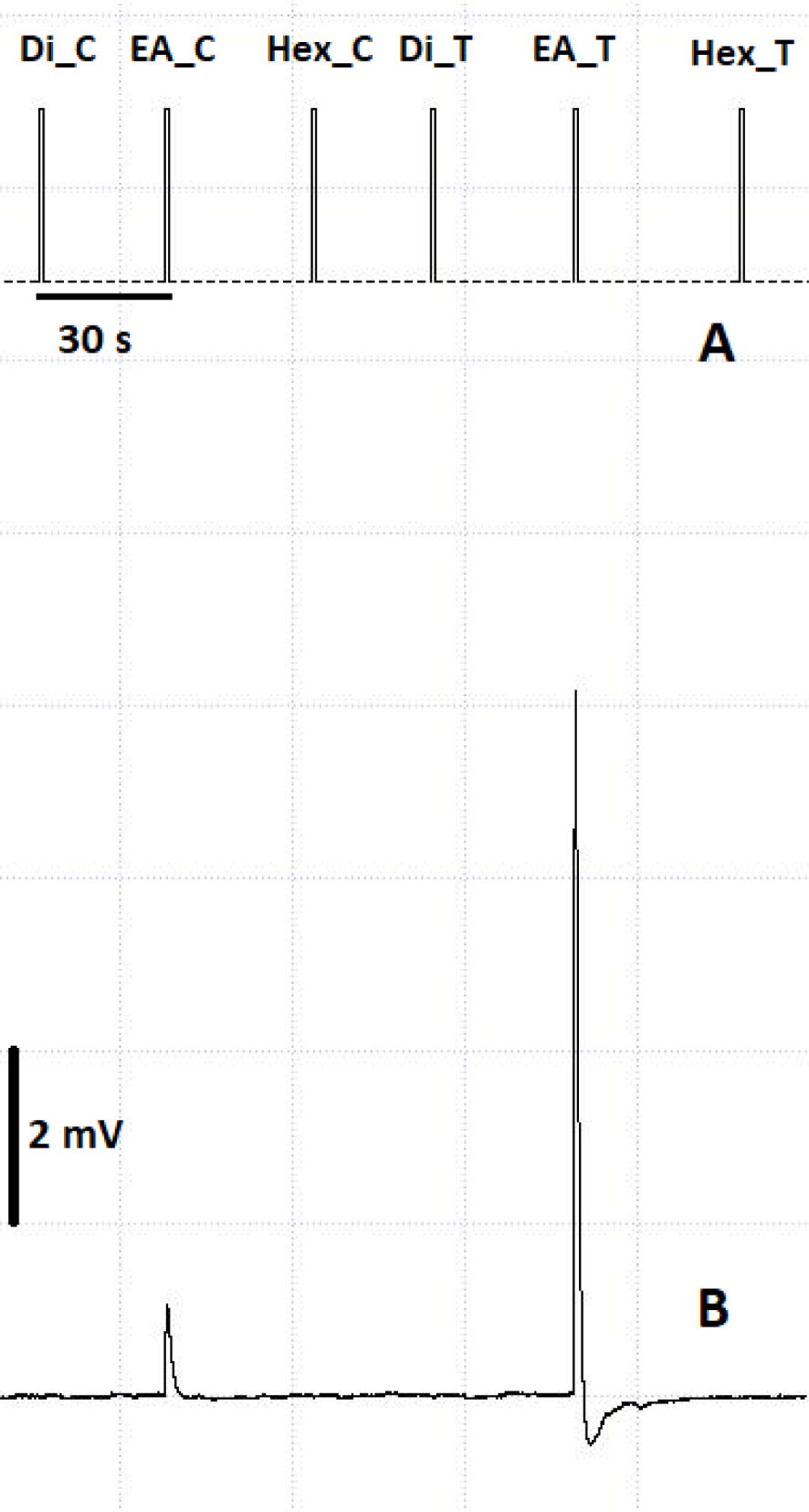
3.2. Assessing male antennal response towards female cuticular compounds
We recorded male antennal responses towards cuticular compounds extracted from asymbiotic and naturally infected females with methanol, hexane, dichloromethane or ethyl acetate (Figure 5). Male antennae showed the same responses to female extracts and pure solvent for methanol, hexane and dichloromethane (KW(2df) = 1.53, p = 0.46; KW(2df) = 1.89, p = 0.39; KW(2df) = 0.39, p = 0.83, respectively). However, the antennal response was significantly different between extracts made with ethyl acetate (KW(2df) = 63.28, p < 0.0001), with extracts of naturally infected females eliciting stronger responses than pure solvent and asymbiotic female extracts (Bonferroni test, z = 7.95, p < 0.0001; z = −3.79, p < 0.0001, respectively), and extracts from asymbiotic females eliciting stronger responses than pure solvent (Bonferroni test, z = 4.15, p < 0.0001).
Male antennal response (mV) towards pure solvent and asymbiotic and naturally infected female cuticular extracts.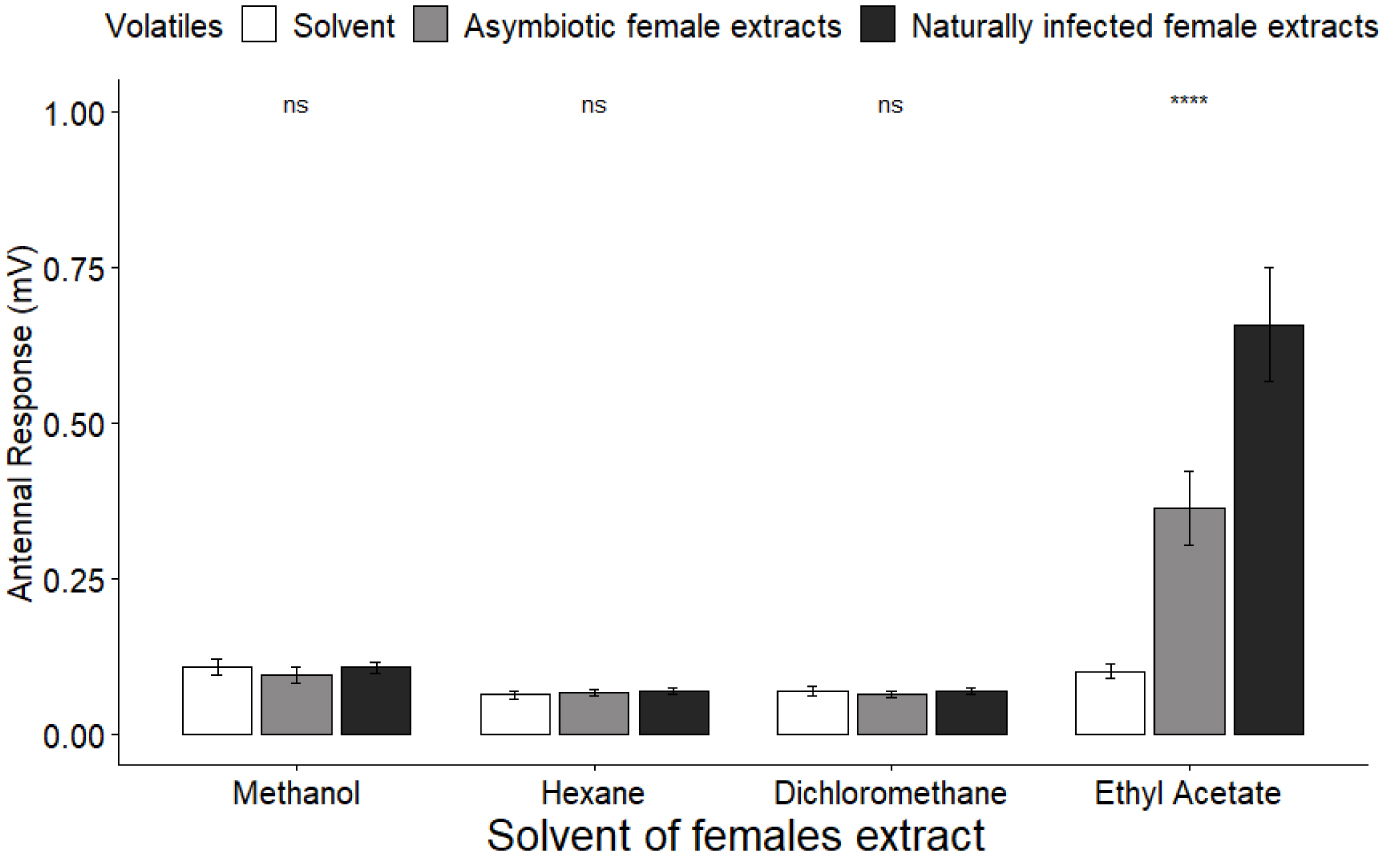
3.3. Comparison of antennal response of asymbiotic and naturally infected females towards male extracts
We then compared the antennal response of asymbiotic and naturally infected females towards male cuticular extracts. No significant differences were found in the antennal response of asymbiotic and naturally infected females (Figure 6) towards the ethyl acetate extract, dichloromethane extract and hexane extract (Wilcoxon rank sum test, W = 346.5, p = 0.6496; W = 483, p = 0.7212; W = 447.5, p = 0.8193, respectively).
Asymbiotic and naturally infected female antennal response (mV) towards male cuticular extracts.
3.4. Assessing female antennal response towards male cuticular compounds
We recorded female antennal responses towards male volatiles extracted with three different solvents, with the responses to each pure solvent used as controls. Male volatiles extracted with ethyl acetate elicited significantly higher responses than the control (Wilcoxon signed rank test, V = 15.5, p < 0.0001). However, female antennae responded equally towards male dichloromethane and hexane extracts in comparison with their respective control solvents (Wilcoxon signed rank test, V = 631.5, p = 0.3219; V = 511, p = 0.4322, respectively). Furthermore, the antennal response was significantly different between extracts of male cuticular compounds obtained with different solvents (KW(2df) = 99.517, p < 0.0001). The ethyl acetate extract elicited significantly higher female antennal responses than the dichloromethane and hexane extracts (Bonferroni test, z = −8.938, p < 0.0001; z = 8.304, p < 0.0001, respectively).
4. Discussion
This study is the first attempt to characterize the olfaction of terrestrial isopods through electroantennography. Few electroantennographic studies have been carried out on crustaceans with convincing results on the costal and vent-dwelling shrimps Palaemon elegans and Mirocaris fortunate [50, 51], on daphniids [52], on the coconut crab Birgus latro [53] and on the hermit crab Coenobita clypeatus [54]. We recorded a positive EAG response, which is uncommon in insects but not surprising in crustaceans. Such a pattern has been reported in shrimp (Palaemon elegans [51]), in the terrestrial hermit crab (Coenobita clypeatus [54]) and in a coconut crab [53], even if for the latter, its overall responses are quite similar to those of insects. In addition, polarity has also been reported to vary according to the chemical properties of the odourant [53, 54]. In C. clypeatus, these differences seemed to be associated with related behaviours and could be the result of different pathways involved in signal transduction [54]. Here, we report electroantennography to be a reliable tool to study olfaction in terrestrial isopods.
We recorded the antennal response of male, asymbiotic and naturally infected female A. vulgare towards cuticular compounds from the opposite sex extracted with different solvents. We found that ethyl acetate-extracted compounds increased the antennal response in the tested antennae of both sexes. These compounds may be volatile cues of interest for intraspecific recognition and communication. In fact, male antennae responded more strongly towards naturally infected female cuticular compounds than towards compounds from asymbiotic females. A. vulgare males were previously found to prefer asymbiotic females over Wolbachia-infected females [35, 38], and it was further shown that both females’ cuticles had distinct chemical profiles [35]. Thus, the female compounds that we extracted with ethyl acetate could contribute to males assessing female infection status or changes in attractiveness.
The fact that male antennae react to the ethyl acetate extract of asymbiotic females may imply that the chemical(s) involved in the recognition of infected female cuticular compounds is (are) naturally produced by females but in a quantity dependent on their infectious state. It is possible that Wolbachia alters its host’s signal synthesis pathway [55] or issuance organ by modifying, for example, the infected females’ cuticle properties and composition [56, 57, 58], thus releasing more molecules.
It is also possible that Wolbachia produces its own signal directly in the excretory/secretory apparatus of the host [59, 60], modifying the chemical profile of infected females in a qualitative fashion.
We also compared antennal responses between asymbiotic and naturally infected females towards males’ cuticle extracts obtained with different solvents. Similar responses were found between both kinds of females. Therefore, the presence of Wolbachia does not seem to influence antennal perception of volatiles of the opposite sex, indicating that the aberrant behaviour displayed by infected females during mating is not caused by an alteration in the detection of male odour [35, 38].
Such a lack of impact of endosymbionts on antennal perception has already been reported in the pea aphid Acyrthosiphon pisum, for which the bacterial symbiont Hamiltonella defensa did not alter the perception of its alarm pheromone [61]. However, in Drosophila simulans, Wolbachia has been shown to enhance the olfaction of its hosts towards food-related cues [62]. It has further been suggested that Wolbachia might do so by mediating the expression of genes encoding odourant receptors [63], thus directly impacting antennal perception.
According to sexual selection theory, variation in female quality (infected vs. uninfected) in the natural population of A. vulgare favours male mate choice [64], and their preference for uninfected females is thought to slow the dispersal of Wolbachia [38]. Our results constitute a new indication that male selection is mainly mediated through the detection of chemical variation among sexes. Identification of these chemicals and behavioural tests to confirm their bioactive role are still needed.
Conflicts of interest
Authors have no conflict of interest to declare.
Acknowledgements
We wish to thank Alexandra Lafitte for isopod management and Maryline Raimond for microscopy advice.





 CC-BY 4.0
CC-BY 4.0