1 Introduction
Information on the diversity and abundance of Neotropical polistine wasps is relatively sparse despite their wide distribution and ecological importance as insect predators. Nest site selection is intimately related to fitness because of its obvious impact on colony survival and offspring production. Inclement weather and predation are the two primary causes of nest mortality in most social wasps, so that the choice of habitat characteristics permitting protection from adverse weather, and concealment and avoiding predators, has presumably been paramount. Consequently, selected nest sites are supposed to be related to highest success. To find protection from inclement weather, numerous species build their nests under large leaves or overhanging rocks. Predation by vertebrates, particularly birds, can be intense, so that many wasp species select nest sites that permit them to camouflage their nests, even varying the shapes of the nests according to where they are built [1–3].
Ants, particularly army ants, exert such strong predation pressure that they are considered to be the main driving force in the evolution of Neotropical social wasps, to the point of influencing their nest architecture. For example, primitive Polistinae have nests with a pedicel onto which the workers deposit substances repellent to ants, and the nests of more evolved species have an envelope to protect the combs [2,4–6]. Wasps may choose sites where attacks by ants are unlikely: for instance, the probability of a wasp nest being discovered by ants is reduced if placed on a plant growing in an open area (army ants mostly hunt in the forest), on a branch rather than on the trunk, or under a leaf rather than on a branch [4]. Wasps place their nests on buildings for the same reason. Other wasps form nesting associations with arboreal ants as a means of being protected from army ants [7–10].
This field study was conducted in French Guiana with the aim of obtaining an overall view of wasp diversity along forest edges and to see to what extent the different wasp species construct their nests: (1) under natural shelters (plants with large leaves or overhanging rocks or large branches); (2) in areas not likely to be in the path of army ants; (3) in association with arboreal ants; or, (4) on the contrary, in association with plants not likely to be selected by arboreal ants. We therefore recorded the distribution of plant species along the forest edges studied and noted if arboreal ants were present or not. We verified whether some wasp species select plants sheltering arboreal ants, and inversely whether other wasp species select plants not attractive to arboreal ants.
2 Material and methods
This study was conducted between December 1997 and November 2005 in the surroundings of the HYDRECO Laboratory field station at Petit Saut, French Guiana (5°03′39″N; 53°02′36″W) during parts of 13 one month-long surveys, resulting in a total of 845 wasp nests monitored.
2.1 Baseline survey (1997–1998)
In Amazonian forests with tall, dense vegetation the “line census” technique, consisting in looking for wasp nests in forest edges (i.e., along rivers, streams and trails), is considered to be the most appropriate technique for surveying social wasp diversity [11,12]. We therefore used this technique in French Guiana where we had already noted the constant presence of wasp nests along forest edges [13].
To assess the diversity of polistine nest sites along forest edges, we firstly conducted a baseline survey between December 1997 and June 1998 along ca. 5 km of dirt road and small streams that penetrate the pristine forest. We thoroughly inspected each plant taller than 0.75 m, as well as hollow logs and shelters formed by erosion (i.e., overhanging rocks or dirt). Plants sheltering wasp nests were prepared for identification at the Herbarium of Cayenne. Wasps were identified and voucher specimens were deposited in the American Museum of Natural History, New York. Each time wasps shared a plant with arboreal ants (i.e., the plant also sheltered an ant nest), the latter were identified and voucher specimens deposited in the Laboratório de Mirmecologia, CEPLAC, Itabuna, Bahia, Brazil.
Wasp species richness was estimated using EstimateS 7.5 software [14]. The Mao Tau function was used to create a species accumulation curve (500 randomizations of the sampling order without replacement). We calculated the number of uniques and duplicates and four incidence-based, non-parametric estimators of total species richness (Jacknife 1 and 2, ICE and Chao). Non-parametric estimators are known to perform well with most data sets [15]. We based our calculations on the estimated number (15,235) of plants. It should be stressed that we were interested in seeing the general pattern (e.g., increase, leveling off, decrease) of the parameter curves and that this pattern is independent of the number of empty samples. The same is true for the final value of the parameters.
To sort the samples (i.e. plant species plus shelters other than plants; see Appendix 1, in Supplementary material) according to the wasp species they host, we used the Self-Organizing Map algorithm (SOM Toolbox version 2 for Matlab®). The SOM algorithm is a learning procedure which transforms the multi-dimensional input data constituted here by 61 nodes (one per wasp species) connected to the 13 “samples” (i.e., the 11 most common plant species sheltering wasps, all the other plants pooled together, and shelters other than plants) into a two dimensional map (shown as 20 hexagonal cells). SOM plots the data so that samples that are similar according to wasp species distribution are found together on the grid, while samples that are very different are far from each other (see [16]). We used the ordination process detailed in [17], and to assess whether a map was properly trained, “topographical error” was used as a measure of map quality ([16]; see an example in [18]).
We also conducted a comparison for which the null hypothesis is that the frequency of each of the 13 “samples” among those sheltering wasp nests is not significantly different than its frequency in the forest edge (the reference sample). Plants corresponding to this situation are likely to furnish wasps with good nesting conditions. When the null hypothesis is rejected and the representation of a plant among those sheltering wasp nests is significantly higher than its representation in the control lot, the plant is thought to furnish wasps (or certain wasp species) with particularly good nesting conditions. On the contrary, if the difference is significantly lower it is supposed that the plant furnishes wasps with poor nesting conditions with, as the most extreme case, plants that never shelter wasp nests. Wasp nest distributions were analyzed using a generalized linear model [19] with a binomial error (proportion data, Chi-square statistics) using the host plant frequencies as denominator vector. Models with all host plant species were fit to the data (full models). Afterwards simplified models grouping certain host plant species were adjusted to the data and only those that were statistically similar to the full model (Chi-square test) were retained (simplified models).
In order to have a control lot for this and other comparisons we identified and monitored all plants taller than 0.75 m growing along both sides of 250 m of dirt road and 250 m of streams in four different zones of the area studied (total of 2 km; 6094 plants monitored corresponding to an estimated 15,235 plants for the 5 km of forest edges studied).
2.2 Long term survey (1998–2005)
Because the nests recorded during a snapshot survey result from site selection combined with differential mortality, we conducted a long-term survey (1998–2005). Each time we found a wasp nest of eight frequent wasp species we recorded (1) the host plant species (or what supported the nest) and (2) the species of its ant occupants, if any. The same types of statistical comparisons described above were conducted.
2.3 Isolated trees (1999)
We hypothesized that certain wasp species are particularly attracted to certain small trees if they are isolated. As opposed to those growing in the comparatively very dense forest edge, these trees are subjected to more climatic variation, but dry quickly after the rain. They seldom shelter arboreal ants while columns of army ants rarely venture into these open areas. We therefore conducted an additional survey where we recorded the distribution of such isolated trees (<2 m tall), growing more than 5 m from the forest edges, along 4 km of dirt road (880 trees identified and monitored), noting each time those trees that sheltered a wasp nest and identifying the wasps. For the most frequent wasp species recorded, using Chi-square tests (GraphPad Prism 4 Software), we compared their frequency with those noted during the baseline survey. As the latter was conducted along 5 km of forest edge while the plant distribution was only monitored over 2 km, we calculated N, the theoretical number of plant individuals for each species using the formula where n is the number recorded over 2 km of forest edge.
3 Results
3.1 Diversity of wasp species
During the baseline survey we recorded 424 nests belonging to 61 wasp species (see Appendix 1). Despite the fact that this study was conducted on ca. 15,235 plants, the species accumulation curve did not plateau and the number of uniques (e.g., rare species found only once during sampling; 22 out of 61 species) did not decrease (Fig. 1). The estimated total species richness ranged from 83 (Chao2 and Jackknife1) to 94 species (Jackknife2) according to incidence-based, non-parametric estimators. Only Chao2 produced a stable value, regardless of the sample-size, at the end of the survey (Fig. 1). The other estimates should be considered as minimal values. Therefore, one can conclude that the survey recorded 61 out of a minimum of 83 species present in the local wasp assemblage, corresponding to a sample representing approximately 73.5% of the species.
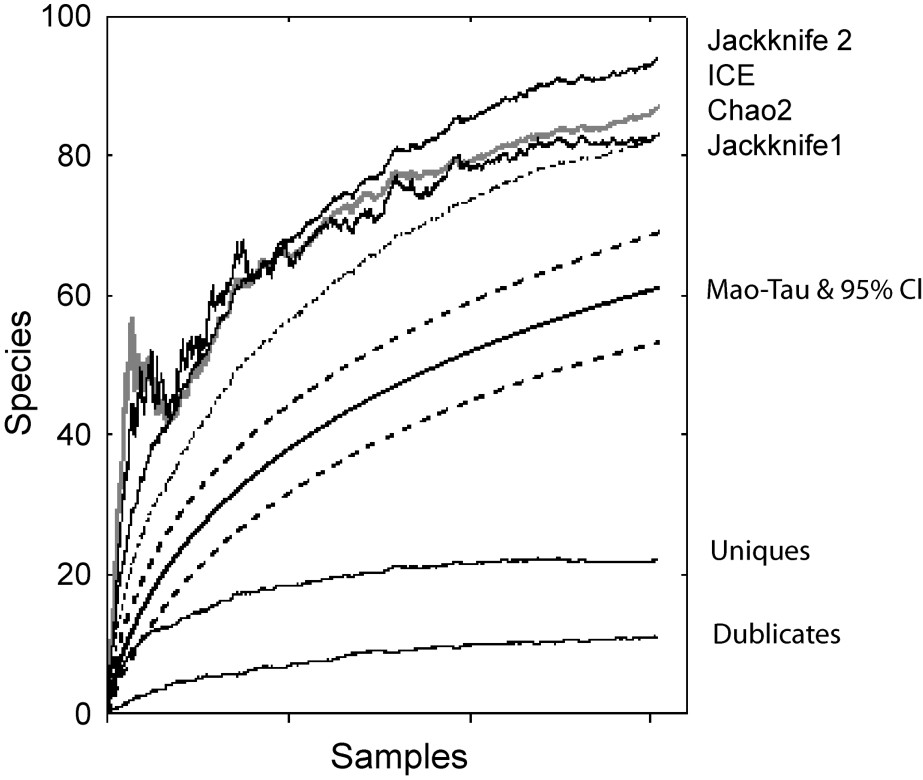
Species accumulation curve or “SAC” (Mao Tau and its confidence limits), number of species present in only one (uniques) or two samples (duplicates), estimates of total species richness based on four non-parametric estimators (Jackknife 1, Jackknife 2, Chao2, Incidence-based Coverage Estimator “ICE”).
3.2 Plant species sheltering wasp nests
After training the SOM with wasp species occurrences, the topographical error was null. The map thus reflects the typology of the input data very well, and so is relevant for subsequent interpretation [16]. On the map found in the upper left corner of Fig. 2, we can distinguish five clusters within which the 13 principal cases of wasp nest recorded are included (see also Appendix 1). The small maps show the ecological characteristics of the 17 most frequent wasp species (five nests recorded or more). One can distinguish wasp species strongly associated with (1) Clusia grandiflora (Polistes pacificus, Mischocyttarus injucundus, Charterginus xanthurus, Polybia scrobalis and mostly P. bistriata), (2) Vismia guianensis (Brachygastra myersi and B. smithii), (3) Vismia sessilifolia (Protopolybia emortualis), and (4) wasps selecting a shelter other than a plant for nesting (Polistes canadensis and P. versicolor). The nests of other wasp species (M. lecointei, Apoica pallens, Angiopolybia pallens, B. scutellaris, Polybia micans, P. occidentalis and P. rejecta) were found on several plant species, including those grouped in the cluster “other plants”.
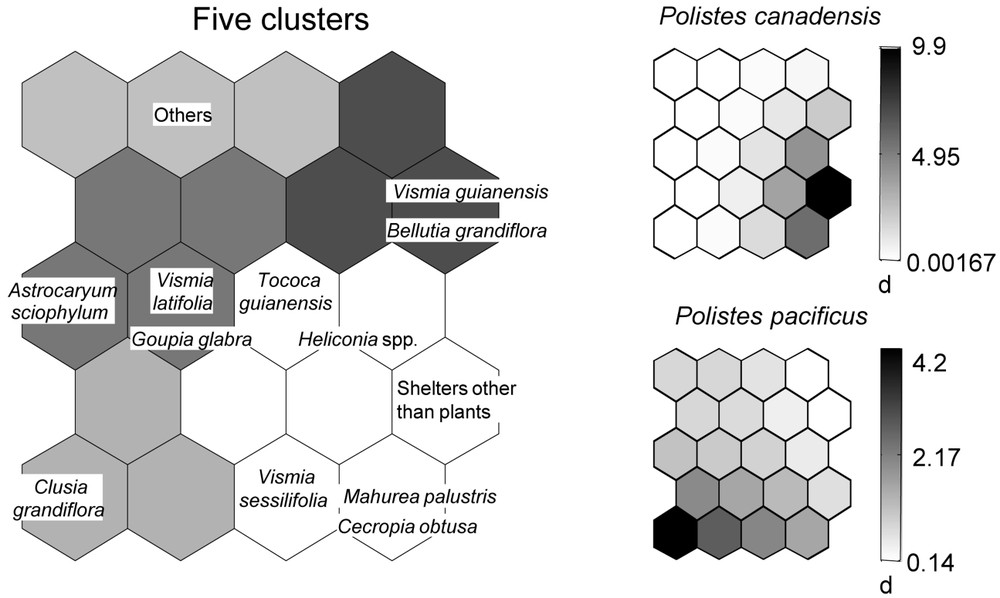
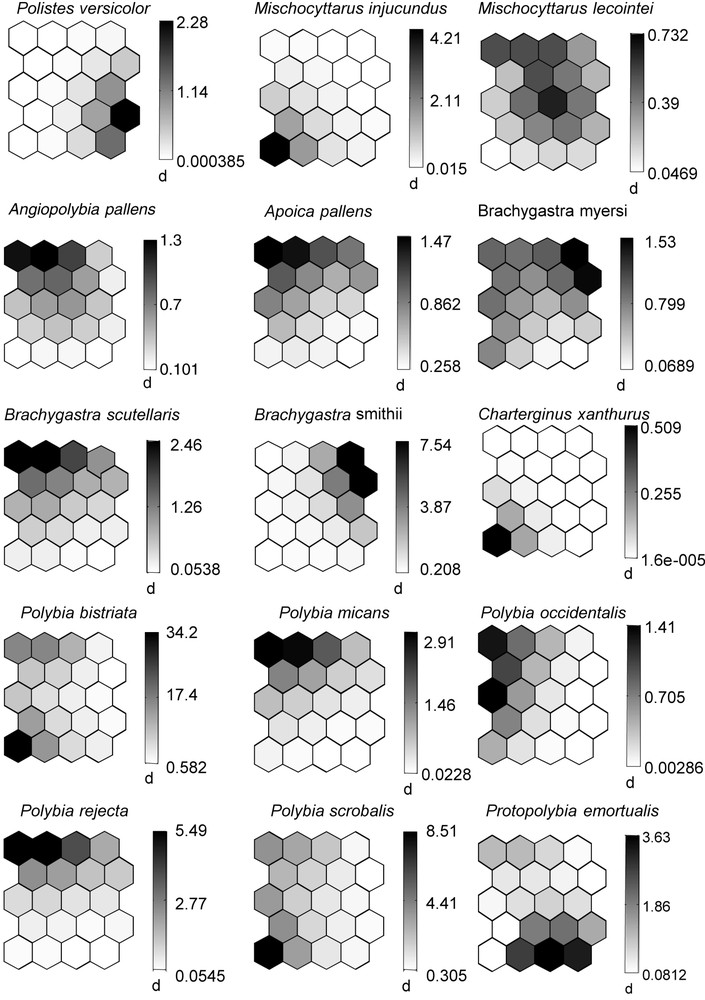
Self-organizing maps (SOM) permitting visualization of the selection of nest site by the selected wasp species (at least five nests recorded). Left, distribution of the plant species (plus shelters other than plants) according to the presence or absence of wasp species. Plants belonging to the same hexagons or represented by hexagons that are close together on the map are expected to have similar wasp species assemblages, the contrary being true for plants represented by hexagons separated by a large distance. Each small SOM represents one wasp species. The shaded scale on the right illustrates the probability of occurrence according to the number of nests recorded (dark = high probability of occurrence, light = low probability of occurrence). The rest of the figure is shown on the next page.
Overall, only four wasp species were very frequently associated with arboreal ants: Mischocyttarus lecointei was noted on myrmecophytes (i.e., Tococa guianensis and Hirtella physophora sheltering Azteca bequaerti and Allomerus decemarticulatus colonies, respectively); 18 out of the 19 Polybia rejecta recorded were associated with Azteca chartifex; 9 out of 10 Angiopolybia pallens were recorded with various ant species; and all 18 Protopolybia emortualis were associated with Dolichoderus bidens.
A comparison between plant species sheltering wasp nests () and the control lot () resulted in non-significant differences for six plant species (Fig. 3). Among them, Vismia guianensis and Astrocaryum sciophylum did not shelter arboreal ants. Mahurea palustris and Goupia glabra sheltered ants only occasionally, while Cecropia obtusa is a myrmecophyte known to shelter Azteca alfari or A. ovaticeps colonies in French Guiana. Finally, ca. 1% of the Vismia sessilifolia examined sheltered Dolichoderus bidens, a dominant arboreal species that builds polydomous carton nests under the leaves of supporting plants. All Protopolybia emortualis and most other wasp nests associated with this plant were noted in the presence of D. bidens.
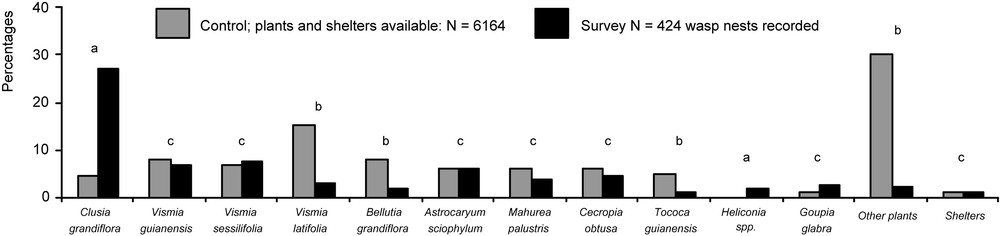
Plant species sheltering wasp nests along 5 km of dirt road and streams compared to the reference sample of plant species from the same areas. Statistical comparisons. Full model ; P < 10−5; simplified model ; P < 10−5; model adjustment difference (; NS) permitted us to present comparisons where letters indicate significant differences between cases of nest site selection (P < 0.05). The proportion of the targeted plant species among trees sheltering wasp nests vis-à-vis those from the reference sample was “a”: significantly higher (plant species selected by wasps); “b”: significantly lower (plant species not selected by wasps); “c”: not significantly different (neutral situation).
Otherwise, Clusia grandiflora was the only species for which the frequency among plants sheltering wasp nests was significantly higher than its local frequency along the edges (Fig. 3). Furthermore, none of the C. grandiflora individuals monitored in this study or in additional surveys sheltered arboreal ant nests ( individuals in total). Consequently, this plant species, which is not attractive to arboreal ants for nesting (although workers can occasionally forage on the foliage), seems very attractive to wasps, particularly Polybia bistriata (Fig. 2).
Lastly, among the plants that rarely sheltered wasp nests (their frequency among plants sheltering wasp nests was significantly lower than in the control lot) we noted V. latifolia, Bellutia grandiflora, T. guianensis and other species grouped as “other plants” (Figs. 2 and 3).
3.3 Long-term survey
The long-term survey permitted us to fine-tune previous data for eight wasp species (Fig. 4). We confirm that Polistes canadensis never nests on a plant (58 additional nests recorded; not included in Fig. 4). This species is also frequently found under the eaves of houses. All of the seven other species build their nests on plants ( wasp nests). (1) Polistes pacificus, Mischocyttarus injucundus and Polybia scrobalis surinama build their nests under the shelter of the large leaves of plants devoid of arboreal ant nests (mostly Clusia, but also Heliconia and Astrocaryum; Fig. 4). They may also attach their nests to the long, thin thorns situated along the central vein under the leaves of juvenile Astrocaryum sciophylum palm trees. (2) Apoica pallens nests were noted on numerous plants, none of them sheltering an ant colony, and were attached to horizontal branches up to 2 cm in diameter. It is noteworthy that 10 nests out of the 28 recorded (35.7%) were constructed on branches that stick out over streams running just at the forest edge. (3) On the contrary, the three remaining wasp species were mostly or always associated with arboreal ants. All but one of the 23 Angiopolybia pallens nests occurred on a myrmecophyte (i.e., Tococa guianensis; Cordia nodosa and Hirtella physophora) or on plants sheltering dominant arboreal ants (once with Dolichoderus bidens on a Vismia sessilifolia; the other cases with Azteca chartifex on several tree species). One Polybia rejecta nest was not associated with ants, another was associated with a Dolichoderus bidens colony nesting in a Vismia sessilifolia foliage, while all 33 others were associated with large Azteca chartifex colonies associated with different plant species. Finally, all Protopolybia emortualis nests were associated with Dolichoderus bidens colonies, most of them nesting under Vismia sessilifolia leaves, explaining the apparent specificity of this wasp species vis-à-vis V. sessilifolia.
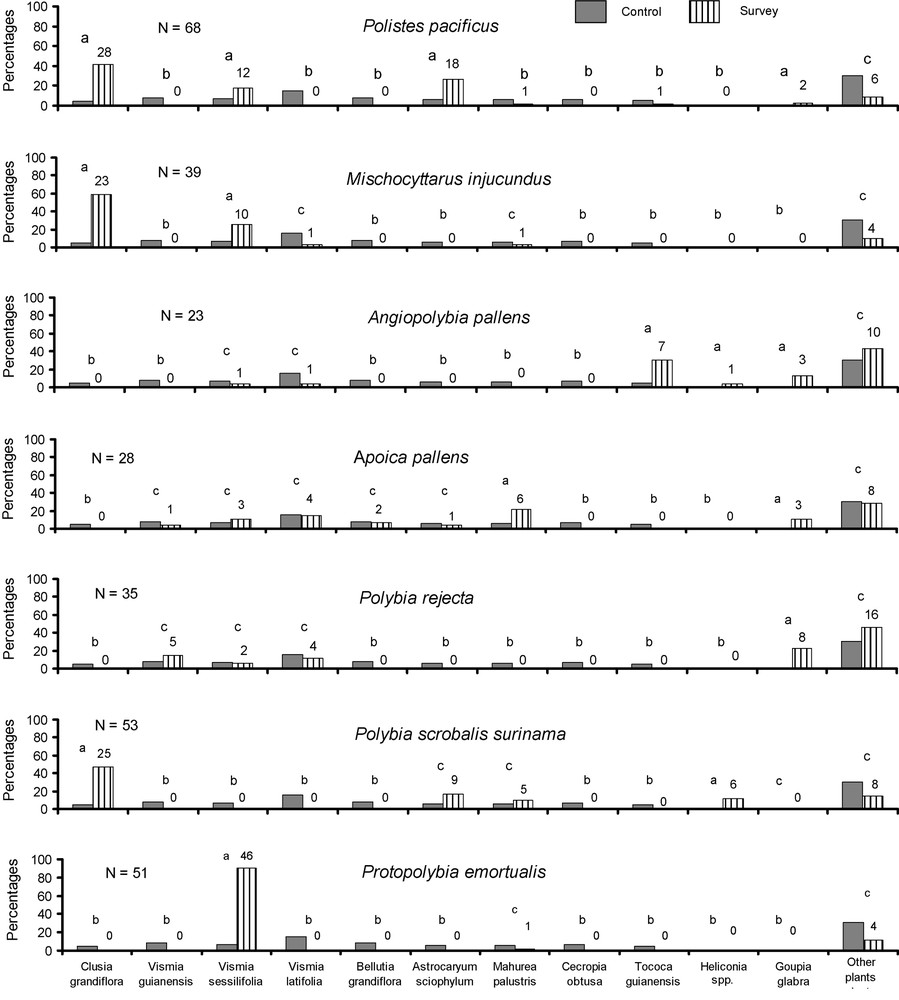
Plant species sheltering the wasp nests of seven targeted wasp species recorded between 1998 and 2005; comparison in each case being with the reference sample from Fig. 3. The numbers shown above the experimental plots correspond to the numbers of cases. All Tococa guianensis (a myrmecophyte) sheltered Azteca bequaerti. For Angiopolybia pallens, not associated with ants in only one case when installed under a Heliconia sp. leaf, the plot corresponding to “other plants” was composed of two myrmecophytes: Cordia nodosa sheltering Azteca sp. (five cases) and Hirtella physophora sheltering Allomerus decemarticulaticulatus (five cases). Vismia sessilifolia sheltered Dolichoderus bidens, while all other trees sheltered Azteca chartifex. Statistical comparisons (see Table 1). The legend of Fig. 3 provides explanations concerning letters.
3.4 Survey on isolated trees
We recorded 66 nests belonging to only nine wasp species on the 880 isolated trees that we monitored (see Appendix 2; two additional species found during this survey resulting in a total of 63 wasp species). Moreover, Brachygastra smithii and Polybia bistriata seemed particularly to seek out isolated trees growing more than 5 m from the forest edge, and both of them nested mostly on the same tree species as in the baseline survey (i.e., Vismia guianensis and Clusia grandiflora, respectively), showing constancy in their nest site selection.
4 Discussion
Although we conducted a relatively large-scale survey, our wasp inventory was not complete. Based on our estimates, we recorded up to 73% of the wasp species present locally. One third of the species were only recorded once or twice probably, because the forest edge is an ecotone where wasp species from both open and dense habitats can shelter. For example, we recorded two additional species on isolated trees pushing the total from 61 species during the baseline study to 63 species. The estimated number of 83 to 94 species would seem to be appropriate as 112 species of Polistinae have been recorded in French Guiana, including nine species newly recorded during the present study (Carpenter, unpubl. data). Also, by using traps plus actively searching for wasp nests in different ecotones (e.g., forest, secondary vegetation, forest edges and buildings) in Amazonian Brazil, Silveira [12] recorded 79 wasp species and there, too, the species accumulation curve did not plateau.
Nest site selection by Neotropical social wasps has been examined in several studies, but each focused on a limited number of species or nest sites. Taken as a whole, they do not reflect the entire reality of the situation. Moreover, associations with arboreal ants, although noted again in this study, have evidently been overrepresented in the literature. By looking at the broader picture, we found in the baseline survey that only seven wasp species out of 61 (11.5%) live in close proximity to arboreal ants – two of them only occasionally – or 55 nests out of 424 (13%; Appendix 1). In other words, most of the wasp species recorded (i.e., 54 out of 61; 88.5%) selected nest sites devoid of ants, although most plants at the forest edge in this area are occupied by arboreal ants [20]. This avoidance can be explained by the fact that most of these ants are good predators (Dejean, pers. obs.).
This study shows that there is a continuum in how attracted arboreal ants are to trees in forest edges, with obvious repercussions for nest site selection by wasps. First, some plant species (e.g., Astrocaryum sciophylum; Heliconia spp.; and Clusia grandiflora) never shelter arboreal ant nests and others (e.g., Vismia guianensis, V. latifolia and Bellutia grandiflora) only rarely. Second, numerous species are “intermediate” (the majority of them are grouped in “other plants” in Appendix 1 and Figs. 2–4). Third, the last series of plant species (especially Vismia sessilifolia; Dejean, pers. obs.) frequently shelter arboreal ant nests or, in the case of myrmecophytes, always shelter arboreal ant nests.
Wasps mirror this progression by selecting: (1) plants not attractive to ants and that furnish them with shelter under large leaves (e.g., Astrocaryum sciophylum; Heliconia spp.; and Clusia grandiflora); (2) a horizontal twig on a tree devoid of ants (i.e., Vismia guianensis, V. latifolia, Bellutia grandiflora), typically Apoica spp. (see also [21]) and Brachygastra spp. on isolated trees; or (3) a tree sheltering arboreal ants. Among the latter, one can distinguish wasp species (a) associated with ants only occasionally (Polistes pacificus and P. testaceicolor); (b) frequently associated with arboreal ants belonging to several species (Mischocyttarus lecointei and Angiopolybia pallens in this study; see [2] for other species); (c) always associated with ants belonging to several species, such as Synoeca virginea and Agelaia myrmecophila as cited by Jeanne [2]; and (d) specialized in their association such as Protopolybia emortualis (and P. duckei to a lesser degree [22]) whose nests resemble Dolichoderus bidens nests found under the leaves of host trees. Unsurprisingly, 33 Polybia rejecta nests out of 35 were associated with Azteca chartifex in this study; this wasp species is known to nest in association with several Dolichoderus, Azteca and Pseudomyrmex species [2,10]. The specificity noted here probably corresponds to a kind of “local tradition”, a phenomenon previously noted in wasps [10,13,23].
In conclusion, we noted a continuum in nest site selection by wasps ranging from species avoiding nesting on any plant to species nesting on plants, but avoiding those with arboreal ants, and, finally, wasps nesting in association with ants (even nesting in myrmecophytes), including in a specific association. This study makes clear that most wasp species nest on plants but avoid arboreal ants, with certain plant species, especially Clusia grandiflora, being selected particularly frequently because arboreal ants avoid nesting in them.
Acknowledgements
We are grateful to Pascal Petronelli (CIRAD) for the identification of the plants, Jacques H.C. Delabie (Laboratório de Mirmecologia, Itabuna, Brazil) for the identification of the ants and Andrea Dejean for proofreading early versions of the manuscript. We wish to thank the Laboratoire Environnement de Petit Saut (HYDRECO) that furnished logistical help. This work was supported by the Projet Amazonie from the CNRS-Guyane.


