1 Introduction
Plants are potential sources of natural antioxidants because they contain phenolic compounds such as phenolic acids, flavonoids, tannins, and phenolic diterpenes [1]. In recent years, considerable attention has been devoted to phenolic antioxidants from different types of plant materials, especially in the time-honored traditional Chinese medicines (TCMs) [2]. Numerous reports of antioxidant extracts from medicinal plants have appeared, strongly inspired by an increasing consumer interest in “natural” healthy diets [2,3].
The genus Phlomis, perennial herbs of the family Lamiaceae, consists of more than 100 species distributed in Africa, Asia and Europe. In China, 43 species have already been recorded, particularly in Sichuan and Yunnan [4]. A number of Phlomis species have medicinal characteristics. They are used for herbal teas in China as stimulants, tonics and diuretics, and they are also claimed to exhibit interesting biological properties for the treatment of ulcers and haemorrhoids [5–7]. Furthermore, there is evidence indicating various antimicrobial [5], anti-inflammatory, immunosuppressive [8] and free radical scavenging properties [9,10] for Phlomis species. Previous phytochemical investigations of the genus Phlomis have shown that they contain iridoids, flavonoids, phenylpropanoids, phenylethanoids, lignans, neolignans, diterpenoids, alkaloids and essential oils [11,12]. In regard to phenolic acids, a wide variety of caffeic acid derivatives, including acteoside and forsythoside B from some Phlomis species have been identified [7,10,13,14]. Some of the biological effects of this genus may be related to the presence of flavonoids and other phenolic compounds. The separation and determination of the active phenolic components in medicinal plant extracts represents a viable method to achieve standardization and quality control of TCMs. However, the research on the quantification of multifarious phenolic acids in complex extracts from Phlomis species has not been developed.
Phlomis umbrosa Turcz. and Phlomis megalantha Diels are two kinds of TCMs and grow abundantly in the wild within the Qinling Mountains of China. However, their antioxidant potentials and phenolic constituents have not been previously reported. As part of our studies on the biological activities of phenolic-rich TCMs from the Qinling Mountains, the objectives of this research project were to: (1) evaluate and compare the in vitro antioxidant activities of various extracts from P. umbrosa and P. megalantha by five classical assays (DPPH radical scavenging assay, superoxide radical scavenging assay, β-carotene-linoleic acid assay, ferric reducing/antioxidant power assay, and plasmid DNA damage protection potential); (2) identify and quantify major phenolic compounds present in the tested species by HPLC; (3) determine the correlation between antioxidant activities and phenolic compounds of the Phlomis species given here to confirm whether phenolic constituents are responsible for the antioxidant activities of the plants.
2 Materials and methods
2.1 Chemicals
Folin–Ciocalteau's phenol reagents, 2,2-diphenyl-1-picrylhydrazyl (DPPH), ascorbic acid (VC), α-tocopherol (VE), butylated hydroxytoluene (BHT), dihydronicotinamide adenine dinucleotide (NADH), phenazine methosulfate (PMS), nitroblue tetrazolium chloride (NBT), β-carotene, linoleic acid, and Tween 40 were purchased from Sigma-Aldrich (St. Louis, USA). Sodium percarbonate (Na2CO3), sodium nitrite (NaNO2), aluminium chloride (AlCl3), sodium hydroxide (NaOH), ferric trichloride (FeCl3), potassium ferricyanide (K3Fe(CN)6), trichloroacetic acid (TCA), 30% hydrogen peroxide (30% H2O2), trifluoroacetic acid (TFA) of analytical grade, and methanol of HPLC grade were purchased from the Tianjin Chemical Reagent Co., Ltd. (Tianjin, China). Milli-Q water (Millipore, Bedford, MA) was used in all work. Standards of gallic acid, protocatechic acid, chlorogenic acid, vanillic acid, caffeic acid, p-coumaric acid, ferulic acid, benzoic acid, salicylic acid, rosmarinic acid, cinnamic acid, -catechin, -epicatechin, and rutin were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). All standards were prepared as stock solutions in methanol. Stock working solutions of the standards were stored in darkness at . Agarose was purchased from Gene Tech. Co., Ltd. (Shanghai, China). Ethidium bromide (EtBr) was purchased from Runde Co., Ltd. (Xi'an, China). The Bio Spin Plasmid DNA Extraction kit from Bioer Technology Co., Ltd. (Hangzhou, China) was used. All other chemicals and solvents are of analytical grade and purchased from Xi'an Chemical Reagent Company (Xi'an, China).
2.2 Plant materials
Leaves of P. umbrosa (Voucher No. MPR 609) and P. megalantha (Voucher No. MPR 610) were collected from the North Slope of Taibai Mountain (the peak of the Qinling Mountains), Shaanxi Province of China, when flowering (July to August, 2006), respectively. Voucher specimens were identified by Professor Yi Ren and deposited in the Key Laboratory for Medicinal Plant Resource (MPR) and Natural Pharmaceutical Chemistry, Shaanxi Normal University, Ministry of Education, PR China.
2.3 Preparation of the extracts
The air-dried plant materials (50.00 g) from each taxon were ground to a fine powder in a mechanical grinder with a 2 mm diameter mesh and then successively extracted with petroleum ether, acetone, and methanol in a Soxhlet apparatus for 6 h. After filtration of each solvent, the organic phases were independently concentrated under a vacuum by evaporating to dryness. The acetone and methanol extracts were stored at for further analysis. The extraction efficiency (%) of acetone and methanol extracts are shown in Table 1. Each sample was extracted in triplicate.
Extraction efficiency (%), total phenolic (TP), total flavonoid (TF) and phenolic acid contents of extracts from two Phlomis species.a
| Acetone extract of P. umbrosa | Methanol extract of P. umbrosa | Acetone extract of P. megalantha | Methanol extract of P. megalantha | |
| Extraction efficiency (%)b | 4.22 ± 0.09ac | 8.66 ± 0.13b | 5.16 ± 0.12a | 9.09 ± 0.13b |
| TP (mg GAE/g extract) | 40.31 ± 0.28a | 39.43 ± 0.44a | 43.42 ± 1.09b | 55.20 ± 0.88c |
| TF (g EE/g extract) | 12.14 ± 1.58a | 17.12 ± 1.66b | 54.33 ± 1.26c | 35.91 ± 1.90d |
| Phenolic compounds (μg/mg) | ||||
| Gallic acid | 0.50 ± 0.01a | trd | 0.21 ± 0.02b | 0.15 ± 0.01c |
| Protocatechic acid | 1.05 ± 0.02a | 6.16 ± 0.07b | 1.51 ± 0.15c | 0.24 ± 0.02d |
| (+)-Catechin | 1.57 ± 0.04a | 5.65 ± 0.11b | 1.59 ± 0.08a | tr |
| Chlorogenic acid | 5.34 ± 0.09a | 20.71 ± 0.14b | 26.15 ± 0.20c | 77.06 ± 0.14d |
| Vanillic acid | tr | tr | 2.54 ± 0.12 | tr |
| Caffeic acid | 0.28 ± 0.06a | 0.75 ± 0.06bd | 0.97 ± 0.15cd | 0.21 ± 0.07a |
| (−)-Epicatechin | 12.84 ± 0.09a | 3.66 ± 0.07b | 4.21 ± 0.15c | 1.37 ± 0.08d |
| p-Coumaric acid | tr | tr | 0.19 ± 0.09 | tr |
| Ferulic acid | 10.79 ± 0.16a | 2.60 ± 0.22b | tr | tr |
| Benzoic acid | 5.48 ± 0.17a | 3.14 ± 0.20a | 6.00 ± 0.25a | 93.82 ± 2.52b |
| Rutin | 4.57 ± 0.15a | 7.63 ± 0.17b | 13.92 ± 0.29c | 9.38 ± 0.34d |
| Salicylic acid | 5.09 ± 0.70a | 2.44 ± 0.25b | 2.60 ± 0.32c | 0.65 ± 0.16d |
| Rosmarinic acid | 7.17 ± 0.46a | 19.04 ± 2.51b | 7.78 ± 0.42a | 4.89 ± 1.18a |
| Cinnamic acid | tr | tr | 0.11 ± 0.06 | tr |
a Each value is presented as mean ± standard error .
b The extraction efficiency is expressed as the % of acetone or methanol-extractible dry weight of plant material.
c Row wise values with same letter indicate no significant difference .
d tr (trace): concentration <0.10 μg/mg.
2.4 Determination of antioxidant activities
2.4.1 DPPH radical scavenging assay
This spectrophotometric assay used the stable radical DPPH as a free radical and was carried out according to the method of Brand-Williams et al. [15] with some modifications. Different dilutions of the extracts and five phenolic compounds (2 mL; 0.0009–0.1200 mg/mL) were added to 1 mL of a 0.008% methanol solution of DPPH. VC, VE and BHT were used as positive references. After a 30 min incubation period at room temperature, the absorbance was read against a blank at 517 nm. The inhibition of the DPPH free radical was calculated using the following equation:
| (1) |
2.4.2 Superoxide radical scavenging activity
The superoxide radical scavenging activities of the extracts were measured by the method of Robak and Gryglewski [16] with some modifications. An aliquot of 1 mL of each of the following solutions were prepared in 0.1 M phosphate buffer at pH 7.4: NBT (78 μM) and NADH (468 μM) with or without plant extracts (0.20–1.00 mg/mL). The reaction was started by adding 0.4 mL PMS (60 μM) to the mixture and the absorbance change was recorded at 560 nm after 1 min. VC and five phenolic compounds (0.02–0.10 mg/mL) were used as a standard antioxidant. The scavenging activity on superoxide radicals was expressed as follows:
| (2) |
2.4.3 β-Carotene-linoleic acid assay
This experiment was carried out according to the method of Socha et al. [3] with some modifications. A stock solution of β-carotene-linoleic acid mixture was prepared as follows: 1 mg of β-carotene was dissolved in 10 mL of chloroform (HPLC grade); 2 mL of the carotene–chloroform solution was pipetted into a boiling flask containing 25 μL linoleic acid and 200 μL Tween 40. The chloroform was removed using a rotary evaporator at 40 °C for 5 min and 100 mL of distilled water was added to the residue, slowly with vigorous agitation, to form an emulsion. Five milliliters of the emulsion were added to a tube containing 200 μL of 2 mg/mL concentrations of the extracts and five phenolic compounds in methanol. The absorbance was immediately measured at 470 nm against a blank, consisting of an emulsion without β-carotene. The tubes were placed in a water bath at 50 °C and the oxidation of the emulsion was monitored spectrophotometrically by measuring absorbance at 470 nm after sample preparation and at 20 min intervals until the end of the experiment. Control samples contained 200 μL of methanol instead of extracts. BHT and VE were used as positive references. The antioxidant activity was expressed as a percentage of inhibition with respect to the control according to the following equation:
| (3) |
| (4) |
2.4.4 Ferric reducing/antioxidant power assay
The ferric reducing/antioxidant power of the extracts were determined according to the method of Oyaizu [17]. A 2.5 mL aliquot of various concentrations and five phenolic compounds (0.01–0.5 mg/mL) of extracts were mixed with phosphate buffer (2.5 mL, 0.2 mol/L, pH 6.6) and K3Fe(CN)6 (2.5 mL, 1%). The mixture was incubated at 50 °C for 30 min. After 2.5 mL of 10% TCA was added, the mixture was centrifuged at 3000g at room temperature for 10 min. The upper layer of the solution (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1%) and the absorbance was measured at 700 nm. BHT and VC were used as positive references. An increase in the absorbance of the reaction mixture indicated increased reducing power.
2.4.5 DNA damage protection potential
DNA damage protection activities of the extracts were evaluated on pKannibal plasmid DNA, which was isolated by the Bio Spin Plasmid DNA Extraction Kit. Plasmid DNA was oxidized with 30% H2O2 + UV treatment in the presence of extracts and verified on 1% agarose gels according to Russo et al. [18] after some modifications. In brief, the experiments were performed in a volume of 10 μL in a microcentrifuge tube containing 3 μL pKannibal plasmid DNA (361 ng/μL), 1 μL of 30% H2O2, and either 5 μL (0.1 mg/mL) of extract and some phenolic compounds in water or water alone. The reactions were initiated by UV radiation and continued for 5 min on the surface of a UV transilluminator (Guohua Technologies and Instruments Co., Ltd., Shanghai, China) with an intensity of 8000 μW/cm2 at 302 nm at room temperature. After radiation, the reaction mixture (10 μL) was mixed with gel loading dye and loaded onto a 1% agarose gel for electrophoresis. Untreated pKannibal plasmid DNA was used as a control in each run of gel electrophoresis along with partially treated plasmid, i.e. only UV or only 30% H2O2 treatment. Gels were stained with EtBr and photographed with the Gel documentation system (Flour Chem™, Alpha Innotech Corp., San Leandro, CA, USA). VC and rutin were used as controls.
2.5 Phenolic compounds analysis
2.5.1 Determination of total phenolics (TP)
TP were measured using a modified colorimetric Folin–Ciocalteu method [2]. Aliquots of test samples (1.0 mL, 1.0 mg/mL) were mixed with 1.5 mL of 20% Na2CO3 and incubated at room temperature for 2 min. After the addition of 500 μL 50% Folin–Ciocalteau's phenol reagents and 7 mL water, the reaction tube was further incubated for 2 h at room temperature and finally the absorbance was read at 760 nm. Measurements were carried out in triplicate and calculations were based on a calibration curve obtained with gallic acid (8–48 μg/mL). The total phenolics were expressed as milligrams of gallic acid equivalents (GAE) per gram of extract. The calibration equation for gallic acid was .
2.5.2 Determination of total flavonoids (TF)
TF were determined following the procedure of Dewanto et al. [19]. One milliliter of 0.5 mg/mL test sample was placed in a 10 mL volumetric flask. 8 mL of 60% ethanol were added followed by 0.2 mL of 5% NaNO2. After 6 min, 0.2 mL of 10% AlCl3 was added. After another 6 min, 0.6 mL of 4% NaOH was added and then water to a volume of 10 mL. The solution was mixed and the absorbance was measured at 510 nm. Measurements were carried out in triplicate and calculations were based on a calibration curve obtained with -epicatechin (0.1–0.3 mg/mL). The total flavonoid content was expressed as grams of -epicatechin equivalents (EE) per gram of extract. The calibration equation for epicatechin was .
2.5.3 Analysis of individual phenolic compounds by analytical RP-HPLC
Dried samples from P. umbrosa and P. megalantha leaves were hydrolyzed according to the slightly modified method of Proestos et al. [20]. The qualitative and quantitative analyses of the phenolic compounds in the extracts were performed according to the following procedure. Different extracts were separated using a reversed-phase HPLC column on a SHIMADZU LC-2010A system equipped with an autoinjector, a UV detector, and LC-Solution software (SHIMADZU, Kyoto, Japan). Spectral data were detected at 280 nm during the entire run. A SHIMADZU (5 μm, ) column (SHIMADZU, Kyoto, Japan) was used for phenolic compounds separation at 25 °C. The mobile phase was composed of solvent (A), water containing 0.5% TFA, and solvent (B), methanol containing 0.5% TFA. The solvent gradient was as follows: 0–15 min from 15% B to 30% B; 15–17 min from 30% B to 37% B; 17–37 min from 37% B to 45% B; 37–52 min from 45% B to 80% B; 52–60 min from 80% B to 15% B. A flow rate of 1.0 mL/min was used, and 10 μL of 10 mg/mL extracts in methanol were injected. Samples and mobile phases were filtered through a 0.22 μm filter prior to HPLC injection. Each extract was analyzed in triplicate.
2.6 Statistical analysis
IC50 values from the in vitro data were calculated by regression analysis. Each experiment was repeated three times. The data were expressed as means ± standard error (SE) and analyzed by SPSS (version 13.0). One-way analysis of variance (ANOVA) and Scheffe multiple comparisons were carried out to test for any significant differences between the means. Differences between the means at the 5% confidence level were considered significant. Correlation coefficients (r) to determine the relationship between variables were calculated using the Bivariate correlation statistical function.
3 Results and discussion
3.1 Antioxidant activities of the extracts
3.1.1 DPPH radical scavenging activities
As shown in Fig. 1A, DPPH scavenging activities of the extracts were similar at the beginning of the reaction (2.20–9.16%) and diverged with the increase in reaction time until they stabilized at 30 min (78.64–92.18%). All the extracts showed a strong hydrogen-donating capacity and can efficiently scavenge DPPH radicals. IC50 values in scavenging abilities were in the order of for the acetone extract of P. megalantha, the methanol extract of P. megalantha, the methanol extract of P. umbrosa, and the acetone extract of P. umbrosa, respectively (Table 2). It seems that the acetone extract of P. megalantha was superior to all the extracts tested and similar to VE with regard to scavenging abilities. On the other hand, none of the samples showed activity as strong as BHT and VC . With regard to the five pure phenolic compounds (protocatechic, chlorogenic, benzoic, rosmarinic acid, and rutin), protocatechic acid had the highest radical scavenging ability, followed by rosmarinic acid, chlorogenic acid and rutin, whereas benzoic acid did not work effectively.
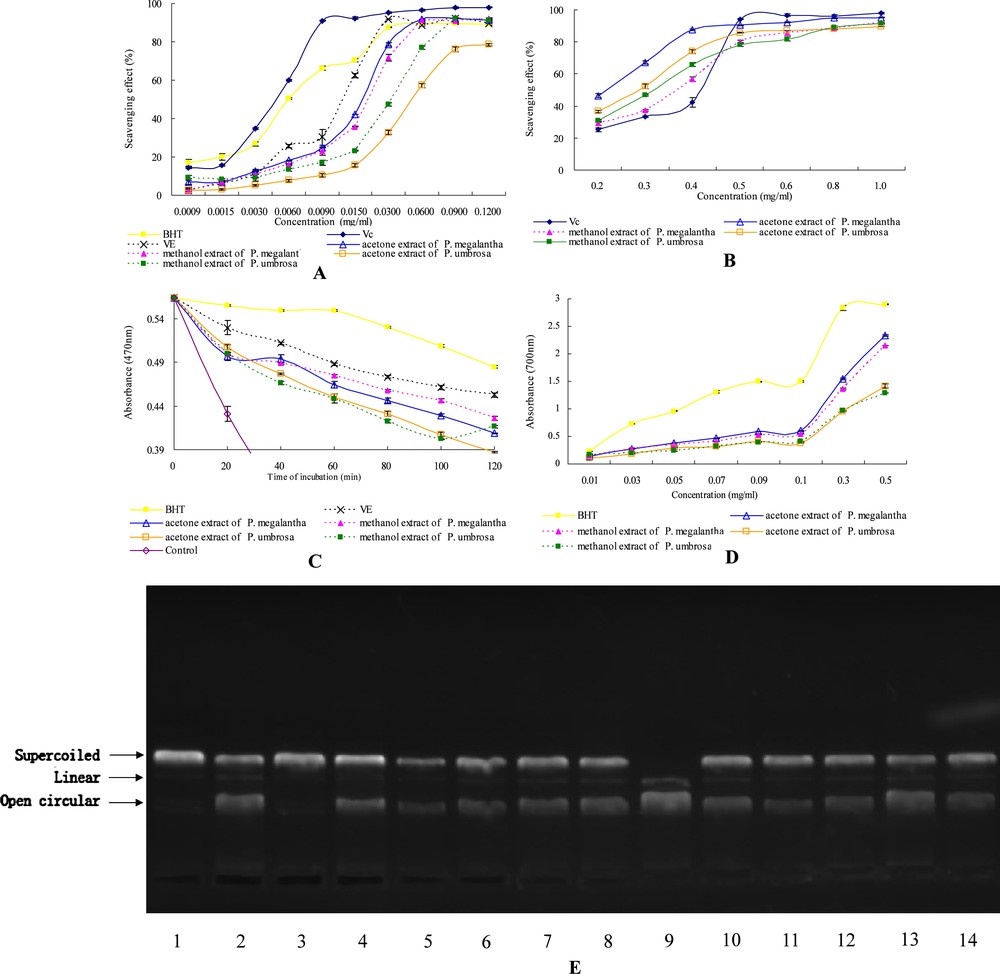
Antioxidant activities of extracts of two Phlomis species. Each value is presented as mean ± standard error (n = 3). The vertical bars indicate standard errors, where they exceeded the symbol size. (A) The DPPH radical scavenging activity of positive references (VC, VE and BHT) and extracts (0.0009–0.1200 mg/mL); (B) The superoxide radical scavenging activity of VC (0.02–0.10 mg/mL) and extracts (0.20–1.00 mg/mL); (C) Antioxidant activity of positive references (VE and BHT), control and extracts (2 mg/mL) obtained using β-Carotene-linoleic acid assay; (D) Reducing power of BHT and extracts (0.01–0.50 mg/mL); (E) Electrophoretic pattern of the pKannibal plasmid DNA after treatment with UV and 30% H2O2 in the absence or presence of the extracts. Lane 1: untreated control; lane 2: UV + 30% H2O2 combination treatment; lane 3: UV only treatment; lane 4: 30% H2O2 only treatment; lane 5: the acetone extract of P. megalantha; lane 6: the methanol extract of P. megalantha; lane 7: the acetone extract of P. umbrosa; lane 8: the methanol extract of P. umbrosa; lane 9: VC; lane 10: rutin; lane 11: rosmarinic acid; lane 12: protocatechic acid; lane 13: benzoic acid and lane 14: chlorogenic acid.
Antioxidant activities of extracts from two Phlomis species and positive references measured by different assays.a
| Plant extracts | IC50 in DPPH⋅radical (μg/mL) | IC50 in superoxide radical (μg/mL) | Inhibition of linoleic acid oxidation (%) | Reducing powerb |
| Acetone extract of P. umbrosa | 35.3 ± 0.33ac | 270.8 ± 1.32ab | 82.4 ± 0.07ac | 1.4 ± 0.07a |
| Methanol extract of P. umbrosa | 20.2 ± 0.66b | 304.1 ± 0.48a | 88.6 ± 0.41ab | 1.3 ± 0.01a |
| Acetone extract of P. megalantha | 13.8 ± 0.20cg | 193.2 ± 1.30bde | 95.5 ± 1.46b | 2.3 ± 0.03b |
| Methanol extract of P. megalantha | 15.7 ± 0.28c | 340.5 ± 1.64a | 87.8 ± 0.10bc | 2.2 ± 0.01c |
| Positive reference | ||||
| BHT | 7.2 ± 0.35d | –d | 93.3 ± 0.26b | 2.9 ± 0.02d |
| VC | 4.9 ± 0.25fi | 32.5 ± 0.58c | – | – |
| VE | 12.1 ± 0.26g | – | 88.1 ± 0.18ab | – |
| Phenolic compounds | ||||
| Protocatechic acid | 2.0 ± 0.16h | 49.9 ± 0.66c | 47.2 ± 0.16df | 3.5 ± 0.08e |
| Chlorogenic acid | 3.9 ± 0.33hi | 76.7 ± 0.82c | 37.0 ± 0.22e | 3.3 ± 0.02f |
| Benzoic acid | n.a.e | 49.3 ± 0.48df | 46.5 ± 0.34d | 0.1 ± 0.01g |
| Rutin | 5.9 ± 0.22di | 166.7 ± 1.12eg | 40.9 ± 0.32de | 3.5 ± 0.02e |
| Rosmarinic acid | 2.9 ± 0.12fh | 85.8 ± 0.96cfg | 56.2 ± 0.62f | 3.2 ± 0.01f |
a Each value is presented as mean ± standard error .
b Absorbance at 700 nm (0.5 mg/mL).
c Column wise values with same letter indicate no significant difference .
d –, data is not analyzed.
e n.a., data is not available.
3.1.2 Superoxide radical scavenging activities
The scavenging abilities of VC and four extracts on superoxide radicals are exhibited in a dose-dependent manner. All the extracts showed good superoxide radical scavenging activities (89.63–95.10%) at a concentration of 1.0 mg/mL in the reaction mixture (Fig. 1B). As can be seen in Table 2, the acetone extract of P. megalantha showed the highest scavenging activity among all the extracts with an IC50 value of , and corroborated well with the results of the DPPH assay. However, the scavenging activities of all the extracts were found to be low when compared to VC. For the five phenolic compounds, benzoic acid possessed the highest superoxide radical scavenging ability, which is significantly better than VC , whereas rutin showed the lowest; the difference was almost four-fold.
3.1.3 β-Carotene-linoleic acid assay
As shown in Fig. 1C, the absorbance of the control at 470 nm decreased to a minimal value of 0.124 after 120 min, while the extracts decreased to a range of 0.387–0.426. These results indicate that all the extracts can significantly inhibit oxidation of linoleic acid, thus implying that the extracts of Phlomis species are powerful natural antioxidants. The calculated % inhibition capacity of the extracts are given in Table 2 and decreased in this order: the acetone extract of P. megalantha > BHT > the methanol extract of P. umbrosa > VE > the methanol extract of P. megalantha > the acetone extract of P. umbrosa. The results indicate that both the acetone extract of P. megalantha and the methanol extract of P. umbrosa exerted marked effects on inhibition of linoleic acid oxidation, which were as strong as the positive references, BHT and VE (no significant difference, ). However, the inhibition capacities of the five phenolic compounds were all significantly lower , only about half that of the extracts. According to the β-carotene bleaching data, the extracts as a mixture are preponderant in a complex heterogeneous medium compared to the pure phenolic compounds. This suggests that the extracts of Phlomis species may show a potential for use as antioxidants in emulsion-type systems.
3.1.4 Ferric reducing/antioxidant power assay
The reducing power of all samples was proportional to the concentrations used (Fig. 1D). They are similar at the concentration of 0.01 mg/mL (0.111–0.172) and diverged with the increase in reaction concentration to 0.5 mg/mL (2.153–2.335). According to the results of Table 2, extracts of P. megalantha were found to be better radical reducers than P. umbrosa extracts in this system, suggesting that they have strong electron-donating capacities. However, they are still slightly less effective than that of BHT, a widely used commercial antioxidant (Table 2). With regard to the five pure phenolic compounds, protocatechic, chlorogenic, rosmarinic acid and rutin are remarkably potent in donating electrons to reactive free radicals, converting them into more stable non-reactive species and terminating the free radical chain reaction, whereas benzoic acid did not work effectively; the results corroborated well with the DPPH assay.
3.1.5 DNA damage protection potential
It is now recognized that the hydroxyl radicals derived from superoxide radicals and hydrogen peroxide are the most potent reactive oxygen radicals; these cause DNA damage by converting guanine into 8-hydroxyguanine [21]. In order to find new compounds to control oxidative DNA damage, which has been particularly implicated in carcinogenesis [22], the effects of extracts from Phlomis species on DNA cleavage were investigated. The protective effects of extracts and controls (VC, protocatechic, chlorogenic, benzoic and rosmarinic acids, and rutin) on 30% H2O2 + UV-induced damage were investigated using the pKannibal plasmid DNA. Fig. 1E shows the electrophoretic pattern of DNA after UV-photolysis of 30% H2O2 in the presence or absence of extracts. DNA derived from the pKannibal plasmid produced three bands on an agarose gel (lane 1): the faster migrating prominent band corresponded to the native supercoiled circular DNA, whereas the slower migrating and very faint band represented the linear DNA and the slowest migrating band represented the open circular form. The UV radiation of DNA in the presence of 30% H2O2 (lane 2) resulted in the cleavage of some supercoiled circular DNA to a faint linear DNA and some open circular DNA, indicating that the hydroxyl radicals generated from UV-photolysis of 30% H2O2 produced DNA strand scission. It was noted that UV treatment alone (lane 3) could not induce damage as strong as 30% H2O2 treatment alone (lane 4) or the combined treatment (lane 2); this indicates that hydroxyl radicals derived from H2O2 were the major contributor to the damage of supercoiled circular DNA. This damage can be reduced in the presence of the five phenolic compounds (rutin, rosmarinic, protocatechic, benzoic, and chlorogenic acids) as shown in lanes 10 to 14, while VC did not work effectively (lane 9). The addition of extracts of two Phlomis species to the reaction mixture of 30% H2O2 conferred significant protection to the damage of native supercoiled circular DNA (lanes 5–8). The DNA damage protection activities of extracts corresponded to their antioxidant potentials. It is interesting that the damage could not be reduced in the presence of the natural antioxidant, VC. On the contrary, it seems that VC accelerated the DNA strand scission and induced degradation of almost all supercoiled circular DNA to linear and open circular forms (lane 9). Zhu et al. [23] have reported that the reactive oxygen species (hydroxyl radicals, superoxide radicals, and hydrogen peroxide) can oxidize VC (ascorbic acid) to the ascorbic acid radical, suggesting that DNA strand scission may be accelerated by the presence of both hydroxyl radicals and the ascorbic acid radical in our study.
As far as our literature survey could ascertain, data on the antioxidant activities of Phlomis species are scarce. Only Kyriakopoulo et al. [10] reported that the active flavonoids isolated from the methanol extract of Phlomis samia exhibited remarkable DPPH radical scavenging activity, indicating that the Phlomis species could be potential sources for free radical scavenging extracts. Our study can fulfill the literature deficiency and the investigated genus could be further developed as phenolic antioxidants in food and pharmaceutical industries.
In many in vitro studies, phenolic compounds demonstrated higher antioxidant activities than vitamins and synthetic antioxidants [2,3,20]. Our data is consistent with the previous studies. Among the five pure phenolic compounds, protocatechic acid exhibited the highest activity in DPPH and FRAP array, whereas benzoic acid did not work effectively. Their antioxidant effectiveness is mainly attributed to the different structural conformation, as well as the number and location of phenolic hydroxyl group of the compounds [24]. Structure–activity relationships have been used as a theoretical method for predicting antioxidant activity and are studied by numerous researchers [25]. It was postulated that the polymeric polyphenols with lower bond dissociation energy values (BDE) were more efficient antioxidants compared to monomeric phenolics that had higher BDE values [26]. Therefore, it can be presumed that the inefficacy of benzoic acid compared to the other four phenolic compounds (protocatechic, chlorogenic, rosmarinic acid, and rutin) could result from the lack of phenolic hydroxyl group. The antioxidant activity also depends on the polarity of the test system and substrate to be protected by the antioxidants [25]. Thus, lower inhibition capacities of the five phenolic compounds compared to VE and BHT in β-carotene-linoleic acid assay of the present study could be the result of their polar characteristics (water-solubility).
3.2 Analysis of phenolic compounds
3.2.1 Total phenolic (TP) and total flavonoid (TF) contents
In this study, the results of the TP and TF contents are presented in Table 1. The amounts of TP and TF extracted from different Phlomis species using different solvents varied widely, ranging from 39.43 to 55.20 mg of GAE/g and from 12.14 to 54.33 g of EE/g of extracts, respectively. A high content of total phenolics was observed in the methanol and acetone extracts from P. megalantha in comparison with P. umbrosa . The content of flavonoids in the acetone extract of P. megalantha was about 4.5-fold that of the acetone extract of P. umbrosa.
3.2.2 Qualitative and quantitative analyses of phenolic compounds
In the present study, the data from the qualitative and quantitative analyses of the acetone and methanol extracts from P. umbrosa and P. megalantha using HPLC is presented in Table 1, while the chromatogram with detector responses at 280 nm is shown in Fig. 2. It is noted that satisfactory separation with good resolution can be achieved within 60 min. A total of 14 phenolic compounds (gallic acid, protocatechic acid, chlorogenic acid, vanillic acid, caffeic acid, p-coumaric acid, ferulic acid, benzoic acid, salicylic acid, rosmarinic acid, cinnamic acid, -catechin, -epicatechin, and rutin) were identified by comparisons to the retention times and UV spectra of authentic standards analyzed under identical analytical conditions, while the quantitative data were calculated from their respective calibration curves (Table 1). The HPLC assay for phenolic compounds was found to be linear in the range of 0.01–1.00 mg/mL with high (0.9918–0.9999) correlation coefficients and the equation of linear regression was determined using the least-squares linear regression analysis method (Table 3).
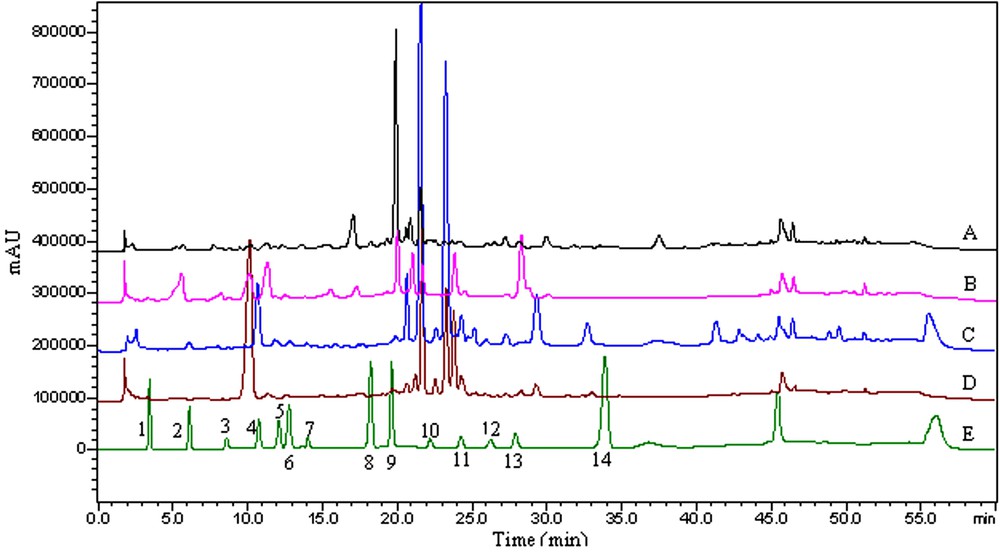
HPLC chromatograms of (A) the acetone extract of P. umbrosa; (B) the methanol extract of P. umbrosa; (C) the acetone extract of P. megalantha; (D) the methanol extract of P. megalantha and (E) a mixture of 14 standard phenolic compounds at 280 nm. Peaks' numbers are according to Table 3.
Linear calibration curves for the HPLC-UV analysis of the phenolic compounds of extracts from two Phlomis species.
| No. | Phenolic compounds | Retention time (min)a | Equation of linear regression (peak area – concentration) | Linearity range (mg/mL) | |
| 1 | Gallic acid | 3.49 ± 0.07 | y = 4E − 08x − 0.0007 | 0.9934 | 0.01–1 |
| 2 | Protocatechic acid | 6.05 ± 0.18 | y = 3E − 08x + 0.0003 | 0.9929 | 0.01–1 |
| 3 | (+)-Catechin | 8.30 ± 0.10 | y = 1E − 07x − 0.0065 | 0.9996 | 0.01–1 |
| 4 | Chlorogenic acid | 10.85 ± 0.21 | y = 1E − 07x − 0.0065 | 0.9996 | 0.01–1 |
| 5 | Vanillic acid | 12.06 ± 0.09 | y = 5E − 08x − 0.0251 | 0.9941 | 0.01–1 |
| 6 | Caffeic acid | 12.73 ± 0.07 | y = 1E − 08x + 0.001 | 0.9997 | 0.01–1 |
| 7 | (−)-Epicatechin | 14.56 ± 0.23 | y = 1E − 07x + 0.0112 | 0.9979 | 0.01–1 |
| 8 | p-Coumaric acid | 18.28 ± 0.07 | y = 2E − 08x − 0.0523 | 0.9936 | 0.01–1 |
| 9 | Ferulic acid | 19.85 ± 0.07 | y = 3E − 08x − 0.0421 | 0.9918 | 0.01–1 |
| 10 | Benzoic acid | 22.16 ± 0.14 | y = 2E − 07x − 0.0178 | 0.9997 | 0.01–1 |
| 11 | Rutin | 24.35 ± 0.04 | y = 1E − 07x − 0.0012 | 0.9997 | 0.01–1 |
| 12 | Salicylic acid | 26.51 ± 0.10 | y = 1E − 07x − 0.0044 | 0.9999 | 0.01–1 |
| 13 | Rosmarinic acid | 28.29 ± 0.10 | y = 6E − 08x + 0.0247 | 0.9985 | 0.01–1 |
| 14 | Cinnamic acid | 34.46 ± 0.17 | y = 2E − 08x − 0.0523 | 0.9936 | 0.01–1 |
a Each value is presented as mean ± standard error .
The phenolic components of P. umbrosa and P. megalantha have never been reported previously. The main constituents are as follows: in the acetone extract of P. umbrosa, -epicatechin , ferulic acid , rosmarinic acid , benzoic acid , chlorogenic acid and salicylic acid were found to be the major components, whereas in the methanol extract of P. umbrosa, chlorogenic acid , rosmarinic acid , rutin , protocatechic acid and -catechin were the most abundant. In the acetone extract P. megalantha, chlorogenic acid , rutin , rosmarinic acid and benzoic acid were the primary phenolics, and in the methanol extract of P. megalantha, benzoic acid , chlorogenic acid and rutin were the most abundant (Table 1). The HPLC analysis of phenolic compounds in the acetone and methanol extracts from P. umbrosa and P. megalantha showed that protocatechic acid, chlorogenic acid, caffeic acid, -epicatechin, benzoic acid, rutin, salicylic acid, and rosmarinic acid were present in all samples. Also, traces of gallic acid, vanillic acid, p-coumaric acid, and cinnamic acid were detected (Fig. 2 and Table 1). There were some compounds which could not be identified; however, based on their chromatographic behaviors and UV spectra, their chemical class may correspond to unknown phenolic compounds.
3.3 Correlations between phenolic compound contents and antioxidant function
The results of TP, TF, HPLC analysis and the different antioxidant assays used in the present investigation were compared and correlated with each other. Correlation between the results of different assays is shown in the e-component.
There are linear regressions and a significant relationship between free radical scavenging capacity and % inhibition of linoleic acid oxidation or reducing power in all extracts. A positive correlation was further observed between the % inhibition of linoleic acid oxidation and the reducing power (, ). The noticeable correlation among different tests showed that the antioxidant assays selected in the present investigation are feasible and complementary to evaluate the antioxidant activities of extracts from P. umbrosa and P. megalantha. The contents of chlorogenic acid, benzoic acid, and TP correlated extraordinarily well (, for chlorogenic acid vs. TP; , for benzoic acid vs. TP). Moreover, all samples showed a linear correlation between the content of rutin and TF (, ). These results are consistent with the data shown in Table 1, and suggest that chlorogenic acid, benzoic acid, and rutin may be the predominant components within the full array of phenolic and flavonoid compounds. A strong correlation between two specific phenolic compounds (protocatechic and rosmarinic acids) and antioxidant assays was verified (, for protocatechic acid vs. DPPH radical; , for protocatechic acid vs. superoxide radical; , for protocatechic acid vs. reducing power; , for rosmarinic acid vs. DPPH radical; , for rosmarinic acid vs. superoxide radical; , for rosmarinic acid vs. reducing power). The results indicate that protocatechic and rosmarinic acids are the major contributors to the antioxidant activities of extracts from P. umbrosa and P. megalantha, and highlighted the importance of phenolic compounds in the antioxidant behavior of plant extracts.
4 Conclusion
This study is the first report on the antioxidant activities and phenolic composition of P. umbrosa and P. megalantha, and the results suggest that the investigated Phlomis taxa could be considered as potential natural antioxidant sources for medicinal and food applications.
Acknowledgements
We would like to acknowledge the support of the 10–11th “five-year-technique-project” by the Ministry of Technique and Science (2006BAI06A12-04), PR China. This work also benefited from financial support from the “Foundation for Excellent Doctor Degree Dissertation” (S2008YB04) and “Innovation Foundation of Graduate” (2008CXB014) of Shaanxi Normal University, PR China. We thank Dr. Zhongmin Dong (Department of Biology, St. Mary's University) for English language improving of this manuscript.


