1 Introduction
In social biology, the spatial distribution of individuals is an important issue that determines the organization of groups. In many species, individuals form aggregates, increasing local population density much more than in the surrounding environment [1,2]. Forming such aggregates has many advantages both for the individual and the group as it provides easier access to food and mates as well as protection against predators [3,4]. It also presents disadvantages as forming aggregates means sharing food, mates and living space, and therefore may result in inter-individual conflicts [3]. Although group formation is of critical importance for the survival of many species, few studies have been done on the parameters modulating spatial distribution, especially in non-eusocial arthropods such as Tetranychus urticae [5,6].
T. urticae is considered as a major pest of many cultivated plants (more than 200 host-plants listed [7]). Consequently, research has focused mainly on the management and control of this species, whereas its behaviour, especially its social behaviour, has often been disregarded. However, T. urticae is a very good biological model with which to study group formation, as this species builds a collective web by weaving silk threads, cooperating in its enlargement and repair [8]. The web is used like a shield protecting mites from rain [9], pesticides [10] and predators [11], and it seems to be a prevailing factor inducing the formation of mite aggregates observed in the field from leaf to leaf or plant to plant [12,13].
Another crucial factor that modulates spatial distribution is the individual genetic background [1,2,14–18]. Aggregate with kin may help individuals avoid predators by diluting each individuals' risk of being attacked (dilution hypothesis [19]) [1,2,16], whereas kin avoidance can be a mechanism to avoid kin mating and so maintain a high level of genetic diversity in the population [15,17]. In insects [1,18,20–22] and arachnids [23], chemical cues such as specific cuticular hydrocarbon may mediate kin recognition. Many arthropod species have the ability to discriminate between kin and non-kin odors and often choose to form an aggregate with close relatives [1,18,21,24]. In T. urticae, females (sisters or non-sisters) coming from inbred and outbred population are able to modify their offspring sex-ratio according to the genetic relatedness of the group (Roeder [25]). Therefore, T. urticae mites have the ability to discriminate individuals differing by their original populations and environments. Do mites still display such discriminative abilities when they face very close relatives? In other words, are they able to perceive tiny genetic differences between close relatives coming from sib-mating lines? Such questions are very important for the study of T. urticae socio-ecology. Indeed, in the field, the probability of inbreeding is very high in groups living on the same plant due to the low mobility exhibited by mites (increasing the likelihood of interactions among kin) and to their mating systems (parthenogenesis and haplo-diploidy) [26,27].
How inbreeding influences the spatial distribution of mites is also unknown. Previous studies on this topic have mainly focused on insects [1,2,18,20,22] while mites have been disregarded. In order to address this question, we studied the spatial distribution within small groups of mites during the colonization of a new leaf through successive generations of inbred individuals. Our results will be discussed in the context of T. urticae ecology.
2 Materials and methods
We tested the relation between inbreeding and spatial distribution by using a red form of T. urticae provided by Dr. Lebdi Grissa (Institut National Agronomique, Tunis). The strain was reared on bean leaves placed on damp cotton (Phaseolus vulgaris) in Petri dishes [28]. Breeding stocks were maintained in a climate room at 26 °C, 50–60% RH, 16:8 (L:D).
2.1 Establishment of sib-mating lines
Founding sib-mating lines is a basic and essential tool for investigating the influence of inbreeding [29]. Three sib-mating lines were established; each was initiated from a virgin female isolated at the deutonymph stage and placed with three unrelated males on a bean leaf for 48 h. Mated females were then isolated on a new bean leaf. Their offspring was subsequently used for sib-mating for four generations to form a highly related line. In each line, three females of the first generation (G1) were fertilized by their brothers. The other females of this first generation were used in aggregation experiments and were called G1. Three females of the second generation were also fertilized by their brothers. Their sisters were also used for the test and were called G2. The above manipulations were repeated in order to obtain many individuals of known age for four generations (G1 to G4). Generation after generation, inbreeding increased. Data obtained using G3 are not presented in this paper because of a lack of replication.
2.2 Spatial distribution tests
Experiments were performed in a climate room at 26 °C, 50–60% RH, 16:8 (L:D). Ten virgin females belonging to the same sib-mating line, of the same generation and less than 48 hours old (number of replicates: G1: ; G2: ; G4: ) were placed one by one in the centre of a bean leaf disc (). The time taken to placed the individuals never exceeded 8 min. Bean leaf discs were placed on damp cotton, to prevent mite escape. These bean leaves were freshly cut before the experiment and were chosen to minimize heterogeneity due to veins. A numeric camera (Panasonic WV-CP450/G) with a macroscopic lens (1:1.2/12.5–75) and a magnifying lens (1.2×) was focused on the leaf disc and recorded female behaviour.
We started recording 15 minutes after the last individual was placed on the leaf to avoid stress behaviour. One picture was taken every 30 seconds for 15 minutes. These pictures were analysed using a data processing program called Fourmilier V3.3 (software creator, Jean-Michel Hudry (jm.hudry@ifrance.com)) [30], which attributed spatial coordinates to each individual and thus allowed further analyses and quantification of the spatial distribution of individuals.
In order to link the inbreeding and the spatial distribution, three parameters were measured:
- (1) The angular distribution “s” is the angular position of individuals on the leaf (Fig. 1) [31,32]. It was measured for each mite group at the end of each session. The angular distribution determined whether individuals were closer than expected by chance alone. We compared the s experimental values to the reference value obtained for randomly distributed individuals (reference value degrees for ten individuals). The reference value H was obtained from 50 000 simulations of ten individuals randomly distributed on a circular area. A high value of s corresponds to a homogeneous distribution whereas a low value of this parameter would mean an aggregated distribution of individuals. This indicator is not a precise measure of the spatial distribution, as several individuals can stay in the same angle and go away from each other. That is why this indicator is used as a qualitative indicator of the spatial distribution to answer the question whether individuals are randomly distributed or not and so if they are attracted to a particular stimulus in its environment (individual, place, heterogeneity) or not.
- (2) The radial distribution is the radial position of mites on the leaf. Quantification of the radial distribution allows one to analyse the trend to follow the leaf border a kind of thigmotactism (also called the border effect). In other words, this parameter determined the position at which mites were numerous. The leaf was divided into five rings of 1.5 mm (0–1.5, 1.5–3, 3–4.5, 4.5–6 and 6–7.5), the external ring corresponds to the edge of the arena. We took into account the differences between the areas of all rings by comparing spider mite density per ring.
- (3) The mean distance between individuals is an indicator of the inter-individual spatial proximity [33]. The individual distance is the length between individuals at a given moment. This was drawn from the spatial coordinates assigned to each individual. The mean distance between individuals is calculated by adding all distances between an individual and an other one and by dividing the result by 45, the total number of such distances [34]. This certainly reflected the attraction to other individuals and/or to a same place.
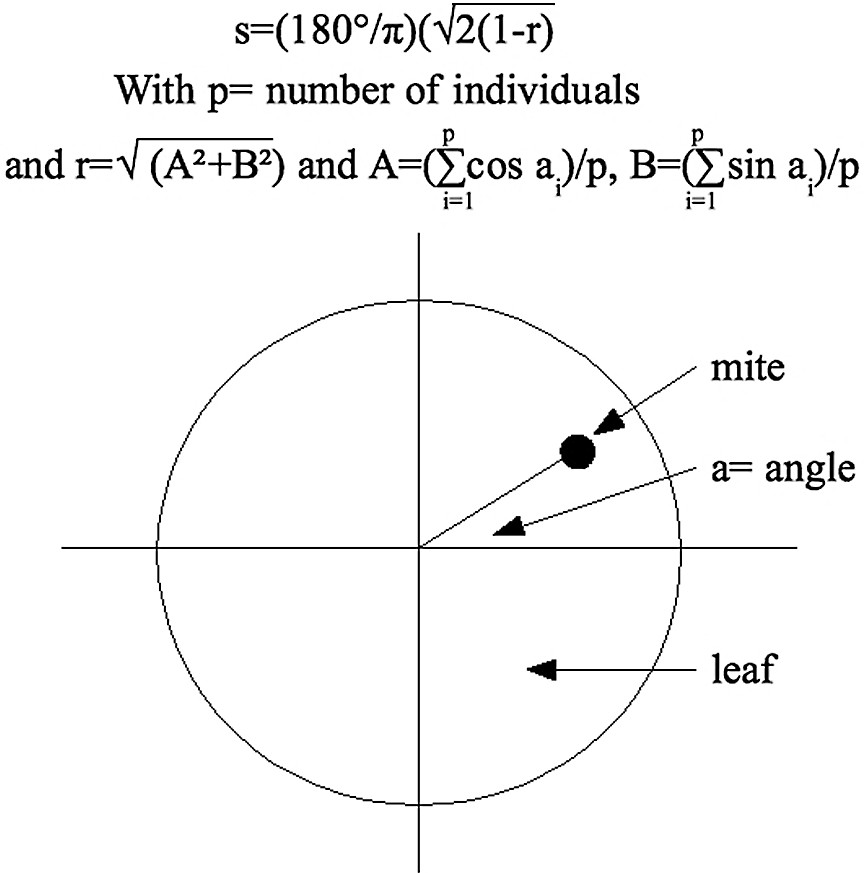
Calculation of the mean angular deviation.
These three parameters were measured for each individual, for each generation and each sib-mating line.
2.3 Statistical tests
Friedman tests were used to test whether the three measured indicators (the angular distribution, the radial distribution, the mean distance between individuals) changed according to time. Kruskal Wallis tests of variance (KW) and Dunn post test were also used to compare changes in angular distribution, radial distribution and distances between individuals and between generations. Tests were performed using GraphPad Prism version 5.01 for Windows, GraphPad Software, San Diego, California, USA (http://www.graphpad.com). All tests were applied under two-tailed hypotheses and the significance level p was set at 0.05.
3 Results
The three measured indicators (the angular distribution, the radial distribution, the mean distance between individuals) did not change according to time since the beginning of the recording in any tested generation (Friedman test for the angular distribution: G1: , ; G2: , ; G4: , ; for the radial distribution, G1: , ; G2: , ; G4: , ; the mean distances between individuals: G1: , ; G2: , ; G4: , ). That is why the results presented here focused on the final state of the mite group (31st picture).
3.1 The mean angular distribution “s”
The mean s (°) values for G1, G2, and G4 were not statistically different but (G1 ± Standard Error , , ; , ; ; Dunn: G1 vs. G2: , G1 vs. G4: , G2 vs. G4: ) (Fig. 2) for each generation, s was statistically different from the reference value i.e. the expected H values of randomly distributed individuals (, , ; Dunn: G1 vs. H: , G2 vs. H: , G4 vs. H: ) (Fig. 2). This result showed that mites were non-randomly distributed at the end of the experiments.
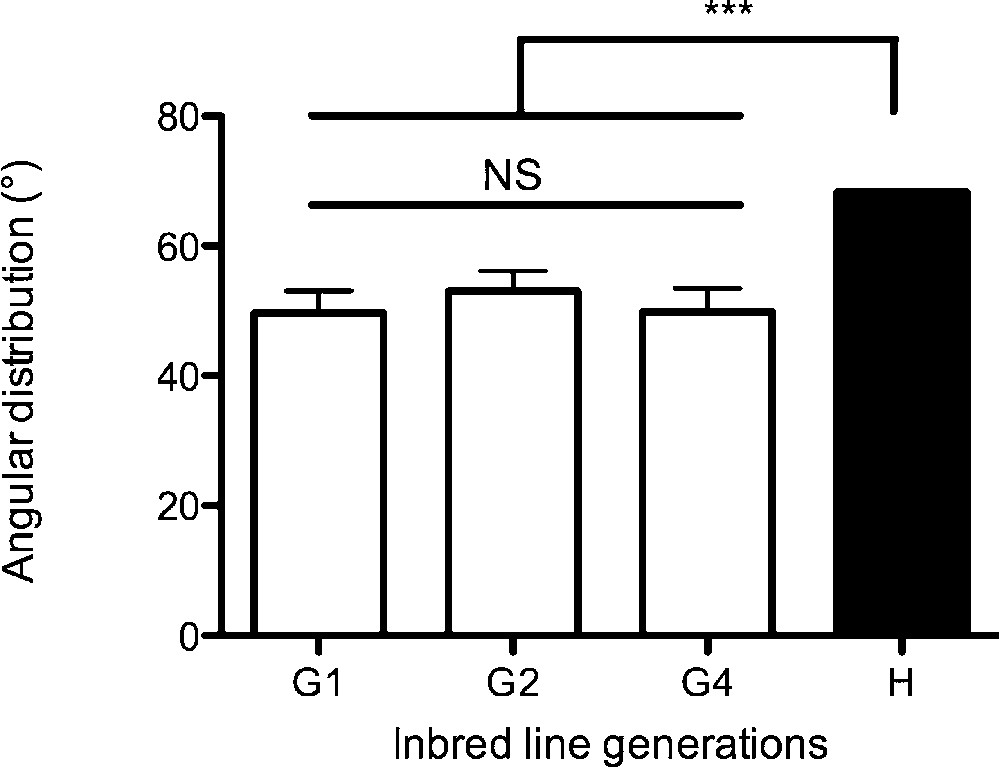
Mean angular distribution “s” (°) of individuals for each generation (G1, n = 14; G2, n = 13; G4, n = 25) ± S.E. at the end of experiments (30 min). H is the reference for ten randomly distributed individuals, and was obtained from 50 000 simulations of ten individuals that were randomly distributed in a circular area.
3.2 The radial distribution of the mites
The mean values of the radial distribution differed between successive generations: In the central ring, the highest density of mites was observed at G1 () and G2 (), whereas the lowest density was observed at G4 (). The difference was statistically significant (, ; ; Dunn: G1 vs. G2: , G1 vs. G4: , G2 vs. G4: ) (Fig. 3). In terms of mite population fraction, 7% of G1 individuals and 0.4% of G4 individuals were found in the central ring.
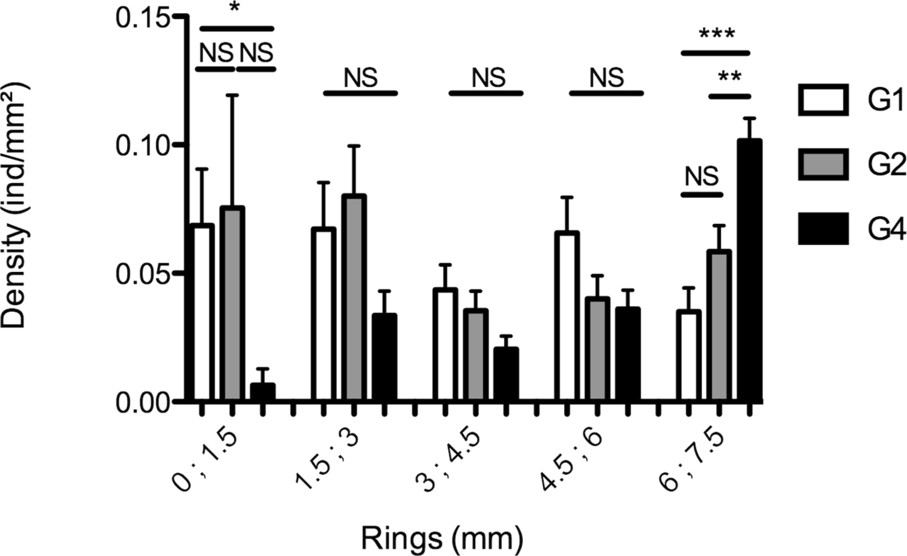
Mean radial distribution of individuals: density of individuals for each generation (G1, n = 14; G2, n = 13; G4, n = 25) (mean number of ) in the five rings (0 represents the centre of the leaf disc) at the end of the experiment (30 min).
On the other hand, in the external ring, the highest mite density was observed at G4 (), whereas the lowest density was observed at G1 (). The difference was statistically significant (, ; ; Dunn: G1 vs. G2: , G1 vs. G4: , G2 vs. G4: ) (Fig. 3). In other words, 23% of G1 individuals and 68% of G4 individuals were found in the external ring.
No difference across generations was found in the other rings (1.5; 3: , , ; 3; 4.5: , , ; 4.5; 6: , , ).
3.3 The mean distance between individuals
The mean distance between individuals (mm) increased significantly across generations from in G1 to in G4 (, ; ; Dunn: G1 vs. G2: , G1 vs. G4: , G2 vs. G4: ) (Fig. 4). After four inbred generations, the mean distance between individuals increased by about 1.6 mm. This result showed that inbreeding lowered mutual attraction among spider mites. The higher the inbreeding level, the higher the inter-individual distances.
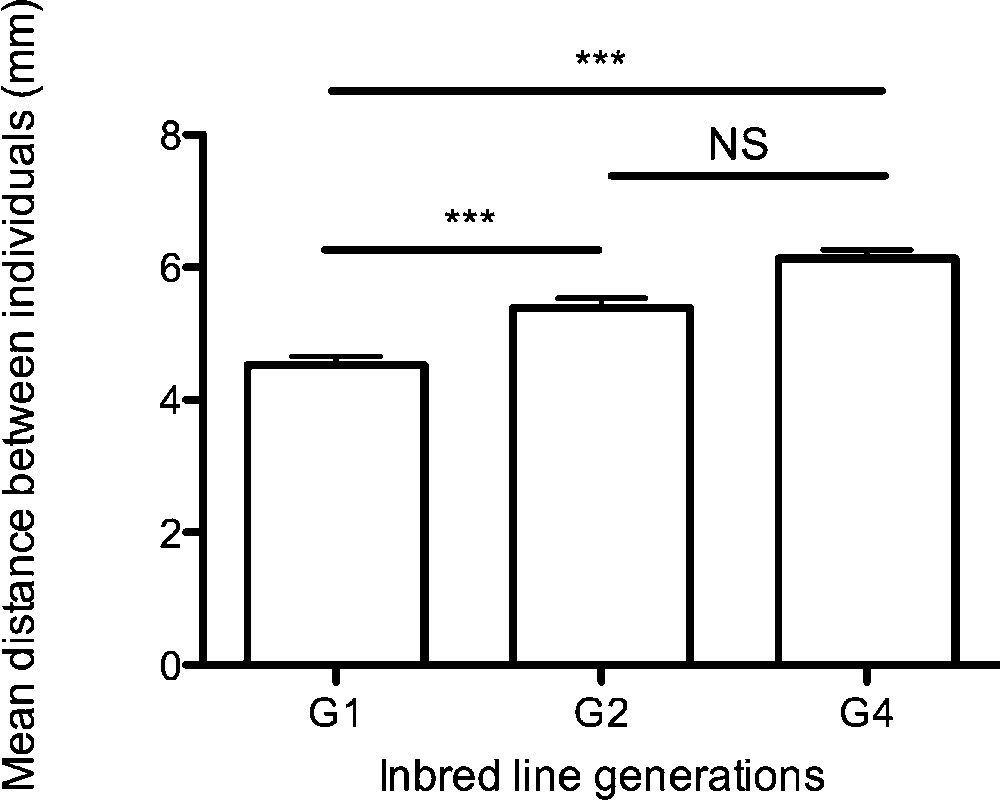
Mean distance (mm) between individuals for each generation (G1, n = 14; G2, n = 13; G4, n = 25) ± S.E. at the end of experiments (30 min).
4 Discussion
In group-living species spatial distribution results from responses to environmental heterogeneity [35–37] and/or to mutual interactions between individuals [38]. During this study, we showed that the level of inbreeding and environmental heterogeneity influence mite distribution during the colonization of a new leaf.
Indeed the results of mean angular distribution are about 50° for all generation of sib-mating and each of them is different from the reference value for ten individuals randomly distributed. Therefore individuals are not randomly distributed in our experiments, they are so attracted each other or/and to a same place. For each generation our results showed a difference of about 27% between empirical and theoretical distribution of s. In our experimental set up, leaf disc has been chosen to minimize heterogeneities due to vein. We can thus imagine that in more natural condition there would be more unevenness like veins to initiate web spinning (personal observation) and aggregation, and so we would have a lower level of angular distribution, and a higher level of aggregation.
We also observed that after 4 generations of sib-mating, the mean distance between T. urticae mites increased of about 1.6 mm which means three times the size of an individual. These results confirm that mites have the capability to discriminate their kin [25,39,40] and to perceive tiny differences between very close relatives.
These differences between generations suggest that inbreeding should increase the tendency to leave the patch or, at least, increase the inter-individual distance. What could be the benefit of such avoidance of close relatives? Increasing inter-individual distance may decrease competition for food, mates and space between relatives [41,42]. This may also decrease the probability of mating with relatives [17,43] and thereby may prevent, at least partly, inbreeding depression and its negative physiological consequences [44].
In addition, the spatial distribution of mites is influenced by environmental heterogeneity such as the borders of the experimental set-up. The trend to follow the leaf border increases with inbred generations. This suggests that individuals has a higher probability of leaving the leaf disc but are limited to it. That demonstrated link between radial distribution and inbreeding emphasizes the role of inbreeding in the spatial distribution of T. urticae.
Previous papers showed that spatial distribution results from social interactions mediated by visual, auditory and tactile stimuli [1,4]. The web in combination with social interactions mediated by pheromones could favor the initiation of clustering in a particular site or reinforce gregariousness [45]. Like many species of gregarious arthropods, spider mites are probably able to produce aggregative pheromones [4,46] that may be contact pheromones that are adsorbed on the cuticle [47,48], deposited on the leaf surface and/or on the silk [49,50]. They might also exert a remote aggregating effect as volatile pheromones attracting at short or long distances [1,51]. No aggregation pheromone has yet been found in T. urticae but such a pheromone has clearly been identified in families other than Tetranychidae [52,53]. These studies suggest the possible existence of an aggregation pheromone in this mite.
Our work contributes to the understanding of spatial organization in spider mites during the colonization of a new leaf. This study shows that with 10 individuals, the spatial distribution is influenced by the genetic background of the group. The following step in this study should be to test with different numbers and density of individuals, different stases, with a higher level of inbreeding. Identifying aggregative pheromones and studying their interplay with the web structure could also help to draw a coherent picture of the mechanisms involved in the spatial distribution of mites and to open new applied perspectives in the control of this worldwide pest.
Acknowledgements
We are very grateful to Dr. Lebdi Grissa Kaouthar who supplied the red form of Tetranychus urticae used in our experiments and to George van Impe for the useful discussions about Tetranychus urticae. We would like to thank Marie Pairon, Gregory Sempo and all the team of the “Unité d'écologie et biogeographie” for their help in data analysis. The authors are also indebted to the National Fund for Scientific Research (FNRS, Belgium) for funding through the Fund for Fundamental and Collective Research (FRFC, convention 2.4622.06). A.C. Mailleux is financially supported by IRSIB (Institut d'encouragement de la Recherche Scientifique et de l'Innovation de Bruxelles). This publication is number BRC140 of the Biodiversity Research Center.


