1 Introduction
The genus Vachoniochactas was described by González-Sponga [1] to accommodate a new species, V. lasallei González-Sponga, 1978, collected in the ‘Cerro Venamo’ state of Bolívar in Venezuela. The genus remained monotypic until the discovery and description of a second species, Vachoniochactas amazonicus González-Sponga, 1991 [2] from the Venezuelan slopes of ‘Cerro de la Neblina’. Subsequently, Lourenço [3] described a third species belonging to this genus, Vachoniochactas ashleeae, this time from the Brazilian slopes of the ‘Pico da Neblina’ (= ‘Cerro de la Neblina’). Very recently, Flórez et al. [4] described one more new species, V. humboldti from the Vichada department of Colombia, region located in the extreme East of the country, in the border with Venezuela.
All the species of Vachoniochactas, so far described, have been collected in the ‘mountain’ formations defined as the Tepuys distributed mainly in Venezuela, Brazil, Guyana and Colombia. The Tepuys region corresponds largely to the Guayana, a floristic lowland province that has been delineated botanically [5].
In the present Note, another new species of Vachoniochactas is described from another Tepuys formation, ‘Mount Roraima’, located on the borders of Brazil, Guyana and Venezuela. The description of this new species brings further evidence of the biogeographic pattern of distribution of the genus Vachoniochactas which is confirmed as an endemic element present only in the Tepuys formations of South America.
2 The Tepuys formations and Mount Roraima
The Tepuys (or Tepuis) are table-top mountains (mesas) found only in the Guayana highlands of South America. They consist of rather isolated entities, and present few connected ranges. This geographical particularity makes the Tepuys outstanding endemic centres for both plants and animals. From a geological point of view the Tepuys are composed of sheer blocks of Precambrian sandstone and quartzite rocks. These ‘mesas’ are the remains of a large sandstone plateau that once covered the granite basement complex between what is today the northern border of the Amazon Basin and the Orinoco, between the Atlantic coast and the Rio Negro. Throughout the course of the Earth's geological history the plateau has been eroded and the Tepuys formed from the remaining inselbergs (monadnocks). The plateaux of these ‘mesas’ are completely isolated from the low-lying forest, making them ‘ecological islands’. However, the altitude engenders a different climate from that of lowland forest. The tops enjoy cool temperatures with frequent rainfall, while the bases of the mountains have a warm and humid tropical climate. The 2734-meter high Mount Roraima represents the triple border landmark of Brazil, Venezuela and Guyana. Its vertical scarps can exceed 500 meters in height and are formed of nearly 2 billion year old sandstones. Geologically, it represents a stratigraphic landmark of the Roraima Supergroup, a Paleoproterozoic age sedimentary basin of the Guyana Shield, north of the Amazonian Craton [6–8].
3 Biogeography of Tepuys; palaeoclimates refugia (endemic centres)
As explained in a recent publication [9], the unique floral biota of the Guayana Highlands was formerly considered to be the result either of vicariance after long evolution in isolation, or of dispersal by successive connection and disconnection of summit floras due to Pleistocene climatic changes. Accordingly, ‘dispersalists’ adopted the refuge hypothesis [10–12] as a mechanism for genetic differentiation. This hypothesis invokes the alternation of dry and wet climates due to the recurrence of glacial and interglacial phases, respectively. Glacial aridity would have determined the fragmentation of forests into refugia with wet, stable climates surrounded by dry forest or savannah formations. Interglacials on the other hand, would have been characterized by forest expansion and coalescence throughout the entire Neotropical lowlands. The associated diversification model suggests allopatric speciation within forest refugia which are still recognizable by their high biodiversity and endemism.
Rull [9] rejects, in part, the model of classical refugia and proposes instead one of diversification. This is based on the following observations from recent palynological data: (i) Vertical shifts and biotic interconnection among Tepuys summits triggered by glacial/interglacial alternation have indeed occurred. (ii) About half of the summits would have remained isolated, so both vicariance and dispersal are needed to account for the long-term origin and evolution of the Pantepuy biota. (iii) The Guayana highlands were not, however, a biotic refuge during the Pleistocene glaciations [13–15]. Neverless, Rull assumes that these ‘keystone’ hypotheses should be tested by further studies yet to be carried out.
The patterns of distribution and differentiation of scorpions have been used to define refugia, or at least endemic centres in the neotropics [16,17]. The refugia of scorpions correspond closely with those proposed for other groups of plants and animals in particular with those outlined by Prance [11,12] for woody plants. The species of Vachoniochactas show a pattern of biogeographical distribution over several Tepuys of the Guayana Highlands. V. amazonicus, V. ashleeae and V. humboldti are found on the ‘Imeri’ endemic centre, which ranges from Brazil through Venezuela into Colombia. The first two species are distributed precisely on the ‘Neblina-São Gabriel’ subcentre of ‘Imeri’. V. lasallei and V. roraima sp. n., are distributed more to the East, on the ‘Imataca’ endemic centre. This includes both ‘Venamo’ and ‘Roraima’ Tepuys.
In the Guayana floristic province [5], major endemic areas defined as having the highest priority for conservation based on scorpion biodiversity, include ‘Imataca’ and ‘West Guiana’ [17]. The inventory for scorpions in the Guayana Highlands is far from complete. This can be attested by the fact that, during the period of classical studies on scorpions, only two species of Broteochactas Pocock have been described from Mt. Roraima [18]. Consequently, one can expect new species of Vachoniochactas and other genera as well, to be found in different Tepuys.
4 Methods
Measurements and illustrations were made using a Wild M5 stereo-microscope with a drawing tube (camera lucida) and an ocular micrometer. Measurements follow those of Stahnke [19] and are given in mm. Trichobothrial notations are those developed by Vachon [20] and the morphological terminology mostly follows that of Hjelle [21].
Taxonomic treatment
Family Chactidae Pocock, 1893
Genus Vachoniochactas González-Sponga, 1978
In a reanalysis of several genera of chactids, the genus Vachoniochactas was redefined, by Lourenço [16], as a group of species within the genus Broteochactas Pocock. Subsequently, it was reestablished as a valid genus by Sissom [22], a decision acknowledged by Lourenço [3]. Flórez et al. [4] proposed a more complete diagnosis for Vachoniochactas. This diagnosis is adopted in the present study. For further details refer to these authors.
Vachoniochactas roraima sp. n. (Figs. 1, 2)

Vachoniochactas roraima sp. n. 1. Carapace. 2. Chelicerae. 3. Metasomal segment V and telson, lateral aspect (male holotype). 4–5. Sternum, genital operculum and pectines (male holotype and female paratype). 6–7. Leg IV (male holotype and female paratype).
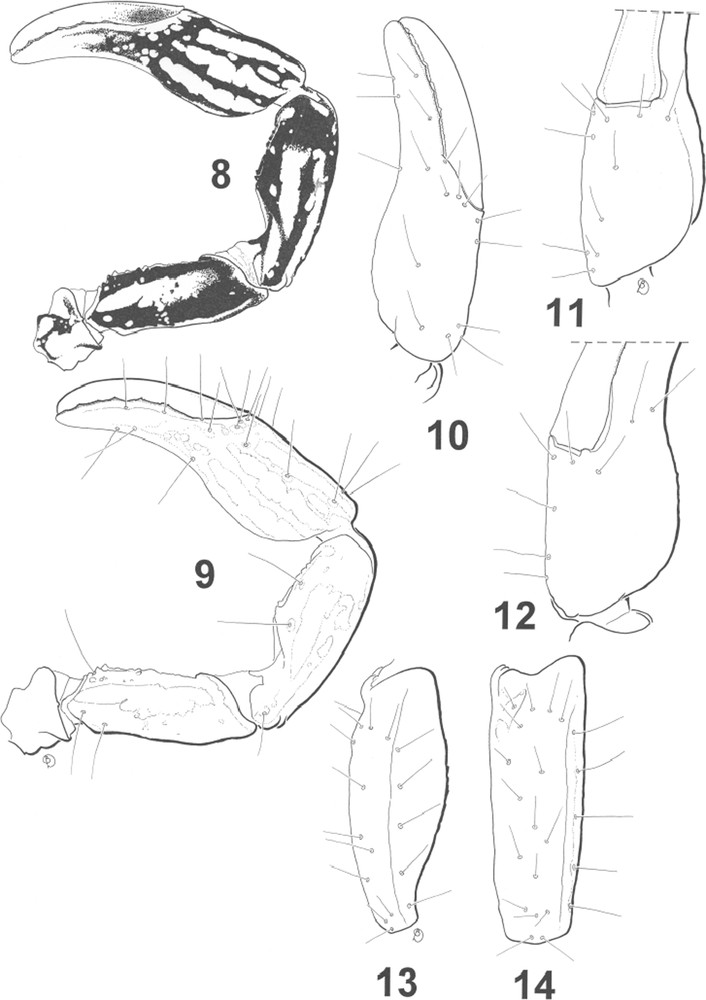
Vachoniochactas roraima sp. n., male holotype. 8. Pedipalp, dorsal aspect, showing pigmentation pattern. 9. Idem, showing trichobothria. 10–14. Trichobothrial pattern. 10–12. Chela, dorso-external, ventral and internal aspects. 13–14. Patella, ventral and external aspects.
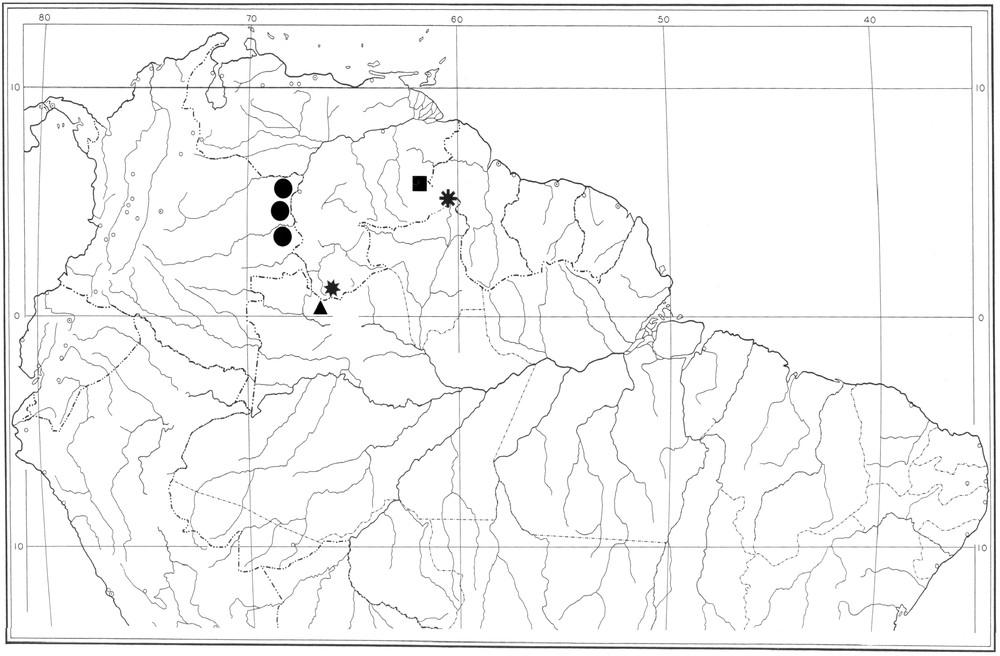
Map of Northern South America showing the distribution of Vachoniochactas species in the Guayana Highlands. V. lasallei (black square), V. amazonicus (black star), V. ashleeae (black triangle), V. humboldti (black circle) and V. roraima sp. n. (black asterisk).
Material. Frontier between Brazil, Guyana and Venezuela, Mt. Roraima (1380 m), 15/V/1977, collected by local Indians (Summer Institute leg.). Male holotype, male and female paratypes. Type material deposited in the Muséum national d'Histoire naturelle, Paris.
Etymology. The specific name is placed in apposition to the generic name and refers to Mt. Roraima, the location in which the new species was collected.
Diagnose. Small scorpions, 18 mm in total length, including telson (the species is the smallest ever described). Coloration reddish-yellow to reddish-brown, with pedipalps, legs and ventral aspect of female densely spotted. Body and appendages weakly granulated or smooth; carapace and tergites with minute punctations on male, smooth on female; metasomal segments and telson weakly granulated on male, strongly granulated on female; ventral aspect of metasomal segments IV–V granulated on both sexes. Pectines with 9 teeth on males and 7 on females. Telson with 2–3 small granules under the aculeus. Dentate margins on fixed and movable fingers of pedipalps with 6 almost linear rows of granules; Trichobothrial pattern type C, ‘majored’ neobothriotaxy. Tarsi of legs with long thin setae.
Vachoniochactas roraima sp. n. can be distinguished from the other species in the genus Vachoniochactas, and in particular from Vachoniochactas lasallei which occurs in the nearby region of the ‘Cerro Venamo’ in Venezuela, by the following features: (i) smaller dimensions, with male and female identical in size, (ii) absence of tubercles under the aculeus; only 2–3 granules can be observed, (iii) ventral aspect of metasomal segments III to V with granulations.
Description (based on male holotype and male and female paratypes). Measurements follow the description.
Coloration. Basically reddish-yellow to reddish-brown, female densely spotted on pedipalps, legs and ventral aspect. Prosoma: Carapace reddish-brown, darker in male. Tergites reddish-brown, as for the carapace, with two longitudinal yellowish strips. Metasomal segments reddish-brown in male, reddish-yellow in female, with darker zones over carinae; vesicle reddish-yellow to reddish-brown. Chelicerae yellowish with diffuse variegated blackish spots only at the base of fingers; fingers yellowish with reddish teeth. Pedipalps reddish-yellow with dark spots over carinae. Legs yellowish with transverse dark spots. Venter and sternites reddish-yellow, paler on female; variegated dark spots over coxapophysis, sternum, genital operculum and sternites; pectines pale yellow.
Morphology. Carapace punctuated in male, lustrous in female; carinae vestigial with some sparse granulations; furrows shallow; median eyes anterior to the centre of the carapace; two pairs of lateral eyes. Sternum pentagonal, wider than long. Tergites acarinate, with only minute punctuations in male, smooth and shiny with some granulations in female. Pectinal tooth count 9–9 in males (7–7 in female), fulcra absent. Sternites smooth and shiny; spiracles small, almost rounded. Metasomal segments I to IV wider than long; metasomal tegument punctuated and with some granulations in male; strongly granulated in female. Vesicle punctuated in male, granulated in female. Ventral carinae absent from segments I to V in male, weakly marked on segments IV–V in female; dorsal carinae on segments I–IV with one stronger spinoid granule. Pedipalps: Femur with dorsal internal, dorsal external and ventral internal carinae moderately marked; ventral external carina vestigial; dorsal and ventral faces with minute granulations; internal face weakly granular. Patella smooth and lustrous; dorsal internal, ventral internal, ventral external and external carinae moderately marked; other carinae vestigial. Chela smooth and lustrous; carinae weakly marked to vestigial. Dentate margins on movable and fixed fingers composed of 6 almost linear rows of granules. Chelicerae with the dentition typical of the family Chactidae [23], and with intense setation ventrally and internally. Trichobothriotaxy type C; ‘majored’ neobothriotaxy [20]. Legs, tarsi with long and thin setae.
Morphometric values (in mm) of the male holotype and female paratype. Total length, 18.5/18.4 (including telson). Carapace: length, 2.6/2.9; anterior width, 1.7/1.9; posterior width, 2.7/3.1. Mesosoma length, 4.8/5.2. Metasomal segments. I: length, 1.0/0.8; width, 1.7/2.0; II: length, 1.1/1.0; width, 1.7/1.9; III: length, 1.2/1.3; width, 1.6/1.8; IV: length, 1.8/1.7; width, 1.6/1.8; V: length, 2.8/2.6; width, 1.6/1.7; depth, 1.3/1.5. Vesicle: length, 3.2/2.9; width, 1.4/1.4; depth, 1.0/1.0. Pedipalp: femur length, 2.1/2.1, width, 0.8/0.9; patella length, 2.4/2.4, width, 0.9/1.0; chela length, 3.5/4.1, width, 0.9/1.1, depth, 0.7/1.0; movable finger length, 2.1/2.3.
Acknowledgements
We are most grateful to Prof. John L. Cloudsley-Thompson, London, for useful comments to the manuscript, and to Elise-Anne Leguin, Museum, Paris, for the help with the preparation of the plates.


