1 Introduction
The midgut of insects is the main organ of the digestive tract in which digestion and absorption take place, being the wall formed by a single layered digestive epithelium and by two muscle layers, the inner circular and the outer longitudinal. In the digestive epithelium there are three cell types: digestive, endocrine and regenerative cells [1–8].
In hematophagous Hemiptera, the midgut can be divided into three regions when considering its color and content after a blood meal. Thus, red midgut identifies absence of blood digestion, which is then stored in the midgut anterior region. The grey midgut content characterizes the occurrence of chemical digestion and it is found in median and posterior midgut regions [9]. However, there are different models for Hemiptera midgut division when considering morphological and functional features, which are due to the variation of feeding habits in these insects. Billingsley [10] divides the Hemiptera midgut into two regions, the anterior midgut or stomach and posterior midgut or simply intestine, which is segmented in anterior and posterior intestine. Gonçalves [11] divides the triatomines midgut in anterior midgut or chyliferous ventricle and post-midgut or intestine. Guedes et al. [12] reported that the midgut of a zoophytophagous Hemiptera is divided into a dilated anterior, a tubular median and a dilated posterior region.
The anterior midgut region is mainly involved with transport of ions and water, keeping the electrolytic balance; also, it is part of carbohydrate digestion and represents an important site for lipids storage. In the median midgut region occurs enzymes secretion, whereas in the posterior region there is nutrient digestion, absorption and storage [10].
The midgut wall of Hemiptera, and its cells, are similar to what is described for other insects. However, its lumen surface is lined by perimicrovillar membrane, which is placed between the lumen and plasma membrane of the microvilli [13]. The perimicrovillar membrane has a function similar to the peritrophic membrane of other insects [10,14].
Among Hemiptera, there are representatives of interest to man, such as those considered as agricultural pests and vectors of different diseases. Studies on the midgut, such as those that enzyme inhibition or perimicrovillar membrane proteins that play a role in digestion and pathogen adherence can contribute to the comprehension of the physiology of these insects, and provide support for their control, because the interference in these mechanisms can reflect in survival of the parasite and the vector, decreasing the success in invasion of anthropic areas, also leading to ecological benefits, when avoiding the long term use of chemical insecticides [15].
To date, available studies about the morphology of triatomines midgut are describing Rhodnius prolixus and Triatoma infestans, because of their importance as vectors for Chagas’ disease. However, recent findings have warned about the risk that domiciliating some wild species such as T. vitticeps represents for public health [16,17].
The present study describes the histology and ultrastructure of the T. vitticeps midgut submitted to different starvation periods, contributing for a better comprehension of the digestive processes in Hemiptera.
2 Material and methods
2.1 Animals
Adult insects of both sexes of T. vitticeps were obtained from the Instituto Renné Rachou – FIOCRUZ, Belo Horizonte, Minas Gerais, Brazil. The animals were kept in colonies in the Entomology Laboratory of the Centro Universitário de Caratinga, at 28 °C and 60% relative humidity. After 15 days of starvation, the insects were fed on anesthetized rats (Rattus norgevicus) (ethical commission agreement protocol n° CQB:0024/97) and 3, 7, 20 and 25 days after a blood meal they were dissected in 125 mM NaCl solution and the midgut, carefully pulled apart, was sectioned in anterior, median and posterior regions.
2.2 Histology
Anterior, median and posterior regions of the midgut were transferred to Zamboni's fixative solution [18] for 24 hours, dehydrated in a graded ethanol series and embedded in glycol methacrylate (Historesin-Leica). Sections 3 μm thick were stained with 1% borax/toluidine blue or Cazon's trichromic. Some sections were submitted to the following histochemical tests: PAS-Alcian blue to neutral polysaccharides and glycoconjugates, mercury bromophenol blues for identification of total proteins, and Nile blue sulfate for neutral and acidic lipids [19].
2.3 Morphometry
For morphometry, ten histological sections per insect were assessed, and in each section ten digestive cells of midgut epithelium were assessed, totaling 1000 cells/insect. Cell height and area, nucleus area and nucleus/cytoplasm ratio were determined with the aid of software Image Pro Plus 4.0 (Media Cybernetics Ltd.). Data were submitted to variance analysis and t-student test with 5% of significance level.
2.4 Ultrastructure
Fragments of the three midgut regions were transferred to 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer pH 7.2 for 2 hours. After washing in buffer, samples were post-fixed in 1% osmium tetroxide in the same buffer for 2 hours. After a new washing in buffer, the material was contrasted en bloco in 2% aqueous uranyl acetate for 18 hours, dehydrated in graded acetone series and embedded in Spurr's resin. Ultrathin sections were stained with 2% uranyl acetate and with lead citrate [20] and examined in a Zeiss EM 109 transmission electron microscope operated at 80 kV.
3 Results
The midgut was lined by a simple epithelium whose histology allows the distinction of two cell types: (i) digestive cells characterized for presenting striated border, presence of granules with distinct staining in cytoplasm and nucleus with chromatin in different condensation levels; and (ii) regenerative cells isolated or forming nidi between the digestive cells base, which lie on the basal membrane (Fig. 1). Below the basal membrane, there were an internal circular muscle layer and an external longitudinal (Fig. 1).
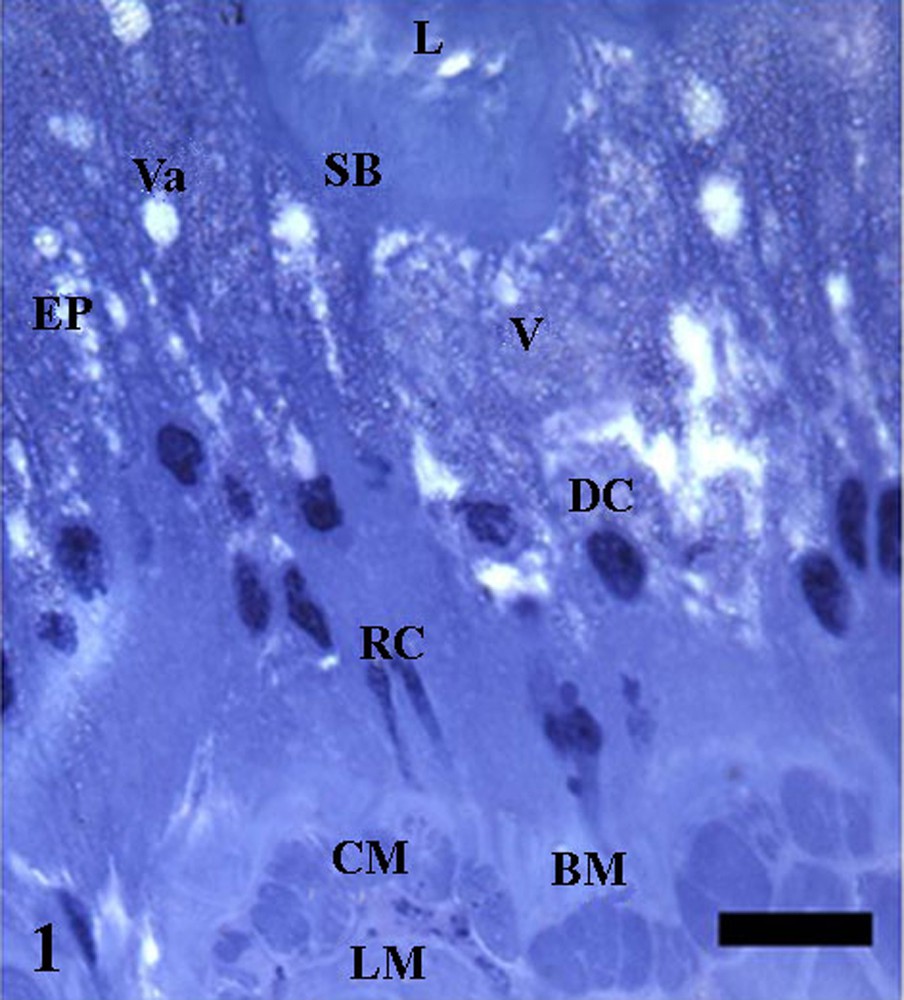
Anterior midgut sections of Triatoma vitticeps with 20 days of starvation showing the epithelium (EP) with digestive cells (DC) with folded apex and striated border (SB), cytoplasm full with stained (V) or not stained vacuoles (Va). Notice regenerative cells (RC) in nests in the epithelium base and inner circular (CM) and outer longitudinal (LM) muscle layers below the basal membrane (BM). L: lumen. Bar: 10 μm.
Regardless of the starvation period, the three midgut regions could be differentiated by the aspect of epithelium apical folds and the muscle layer thickening. Therefore, anterior and posterior midgut regions showed a folded epithelium when compared to the median region (Figs. 2–7). In addition, the muscle layer was more developed in the anterior region in relation to what was found in the other two regions of the T. vitticeps midgut.

Histological sections of midgut of Triatoma vitticeps. 2 – Anterior midgut of insect starved for three days. 3 – Anterior midgut of insect starved for 20 days. 4 – Median midgut region of insect starved for three days. 5 – Median midgut of insect starved for 20 days. 6 – Posterior midgut of insect starved for three days. 7 – Posterior midgut of insect starved for 20 days. AF: folds in the apical surface of epithelium; ba: basophilic substance; BM: basal membrane; L: lumen; M: muscle; n: nucleus of digestive cell; V: stained vesicles; Va: vacuole; SB: striated border. Bars = 10 μm.
In insects three days after the meal, the anterior midgut region showed epithelium with projections in its apical surface and basal folds, whose digestive cells have stained and clear cytoplasmic granules and nucleus with predominance of decondensed chromatin (Fig. 2). Twenty days after the blood meal, vesicles with different staining features and folds of apical surface of the epithelium were more frequent when compared with than found in insects starved for three days (Figs. 3–7).
Median and posterior midgut regions of T. vitticeps after different starvation periods also had epithelium with folds in the apical portion and cytoplasmic vesicles with different aspects, which are more evident and numerous in those insects 20 days after the meal in comparison with insects with a shorter starvation period (Figs. 4–7). The posterior midgut region of insects three days after the blood meal had digestive cells with strong basophilic regions.
Morphometric data (Table 1) showed that there was no difference in height and area of cells, in the nuclei area and in nucleus/cytoplasm ratio of midgut digestive cells of T. vitticeps, when different midgut regions and different starvation periods were compared.
Mean values of height and width, nuclei area and nucleus/cytoplasm ratio in the digestive cells of the different midgut regions of Triatoma vitticeps in different starvation periods.
| Midgut region | Starvation period | |||||||
| Three daysa | Twenty daysa | |||||||
| CH | CA | NA | NCR | CH | CA | NA | NCR | |
| Anterior | 24 | 170.3 | 20.2 | 0.2 | 41.1 | 238.1 | 20.3 | 0.1 |
| Median | 36.2 | 402.1 | 41.4 | 0.1 | 39.2 | 352.9 | 34.5 | 0.1 |
| Posterior | 29.2 | 301.6 | 37.5 | 0.2 | 39.7 | 314.3 | 31.1 | 0.1 |
a Not significative by the t test (P = 0.05).
Histochemical tests in the three midgut regions in the different starvation periods showed that neutral glycoconjugates occurred mainly in the epithelial apex, basal membrane and cytoplasmic granules of digestive cells, regardless of the starvation period (Table 2). However, three days after the blood meal, there was an increase of PAS-positive regions in the epithelial apex and and in the basal membrane of anterior and median midgut regions and cytoplasmic granules in digestive cells in the anterior midgut region (Table 2). In insects starved for 20 days, in the three midgut regions, the epithelial apex remains PAS-positive, as well as the cytoplasmic granules seem to increase in quantity.
Summary of histochemical tests of the three midgut regions of Triatoma vitticeps subjected to different periods of starvation.
| Cell region | Starvation periods | ||||||||||||||
| 3 days | 20 days | ||||||||||||||
| AMG | MMG | PMG | AMG | MMG | PMG | ||||||||||
| PAS | BB | NB | PAS | BB | NB | PAS | PAS | BB | NB | PAS | BB | NB | PAS | BB | |
| Granules | ++ | nd | ++ | + | nd | + | + | +++ | ++ | +++ | ++ | + | ++ | ± | +++ |
| Apical | +++ | +++ | ++ | +++ | + | ++ | ++ | +++ | ++ | ± | +++ | ± | +++ | ± | ± |
| Basal | +++ | ++ | ++ | +++ | + | ± | + | ++ | ++ | ± | +++ | ± | ± | ± | ++ |
Mercury bromophenol blue test showed that three days after the meal the epithelial apex of the anterior and median midgut regions had a positive reaction for proteins, with scarce cytoplasmic granules protein positive (Table 2). In T. vitticeps 20 days after the blood meal, throughout the midgut, epithelial apex, cytoplasmic granules and basal membrane had more protein than in those insects starved for three days (Table 2).
Regarding lipids, there was strong positive reaction in the epithelial apex and base, as well as in the cytoplasmic granules of digestive cells in the anterior midgut region in insects starved for three days, whereas in the median midgut region lipids occurred in less amount (Table 2). However, at 20 starvation days, reactions in cytoplasmic granules of digestive cells become more evident both in the anterior and median midgut regions, occurring the presence of neutral lipids near the epithelial apex and in the basal membrane, in addition to the increase of acid and neutral lipids in the cytoplasmic granules of digestive cells in the anterior midgut region (Table 2).
Digestive cells of different midgut regions of T. vitticeps showed apex with microvilli supported by filamentous material correlated to the actin cytoskeleton; also, the microvilli surface were lined by an additional membrane corresponding to the perimicrovillar membrane (Figs. 8–10). Septate junctions were found in the lateral plasma membranes of adjacent digestive cells (Plate 4Fig. 12).
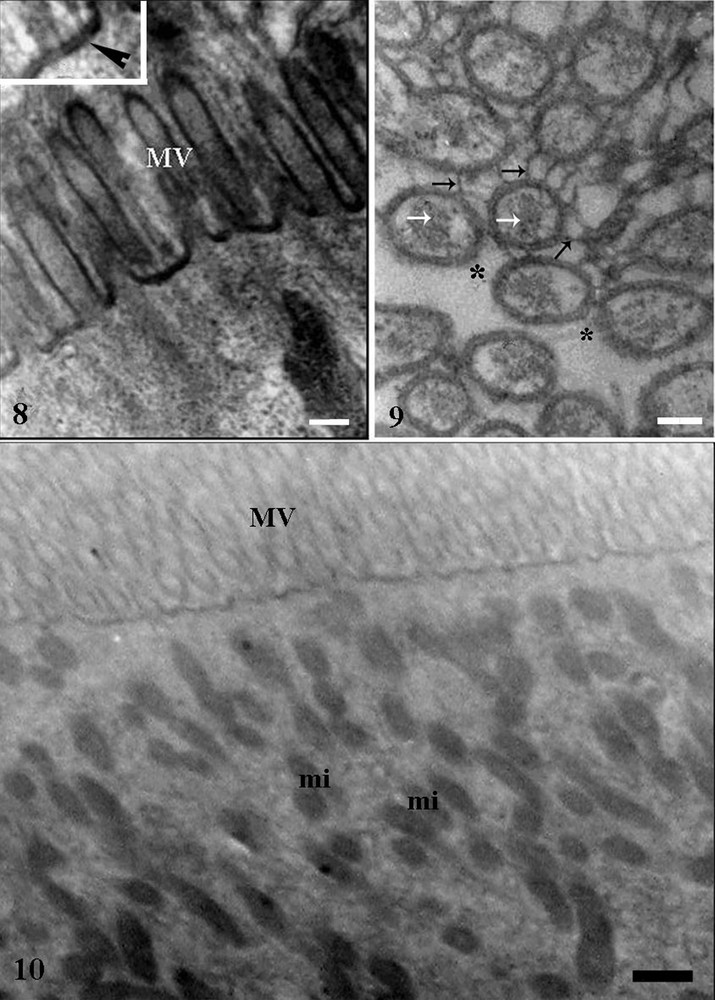
Transmission electronic micrographs of midgut epithelium of Triatoma vitticeps starved for 25 days. 8 – Apical region of digestive cell in the anterior midgut showing microvilli (MV). Bar = 0.5 μm. Inset: microvilli lined by double membrane (arrowhead). 9 – Microvilli supported by actin-like filaments (white arroes) covered externally by perimicrovillar membrane (black arrows) with some areas without perimicrovillar membrane (asterisk). Bar = 0.5 μm. 10 – Apical region of the digestive cell of the posterior midgut showing many mitochondria (mi). Bar = 1 μm.
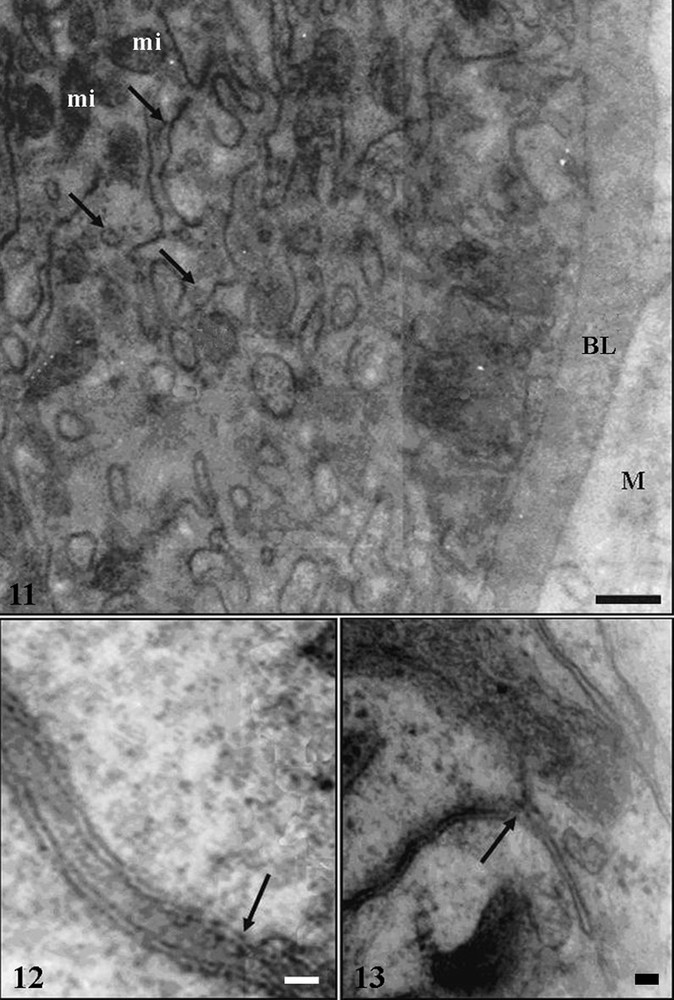
Transmission electronic micrographs of midgut epithelium of Triatoma vitticeps starved for 25 days. 11 – Cells resting on basal lamina (BL) following muscle layer (M). Notice basal plasma membrane with many infoldings (arrows) associated to mitochondria (mi). Bar = 4 μm. 12 – Septate junctions (arrow) between the lateral membranes of adjacent cells in the anterior midgut. Bar = 6 nm. 13 – Detail of the basal plasma membrane infoldings (arrow) forming a basal labyrinth in the anterior midgut, whose membranes are very tight. Bar = 20 nm.
Perimicrovillar membrane (Fig. 9) covered the microvilli, isolating them from the lumen, and in some points of the midgut, in the insect starved for 25 days, they were absent. The apical cytoplasm of digestive cells of the posterior midgut region had mitochondria that were small and with many ridges (Fig. 10).
The basal plasma membrane of digestive cells, in the three midgut regions, had infoldings forming a well developed basal labyrinth associated to mitochondria (Fig. 11). Extracellular spaces of the basal labyrinth were more widened in the anterior midgut region in insects starved for seven in comparison with those of insects with others starvation periods (Fig. 13).
Digestive cells in the three midgut regions showed a nucleus with decondensed chromatin and some small areas with condensed chromatin, being the perinuclear cytoplasm rich in Golgi complex region, rough endoplasmic reticulum and mitochondria (Figs. 14, 15), in addition to vesicles with varied electron-densities and size.
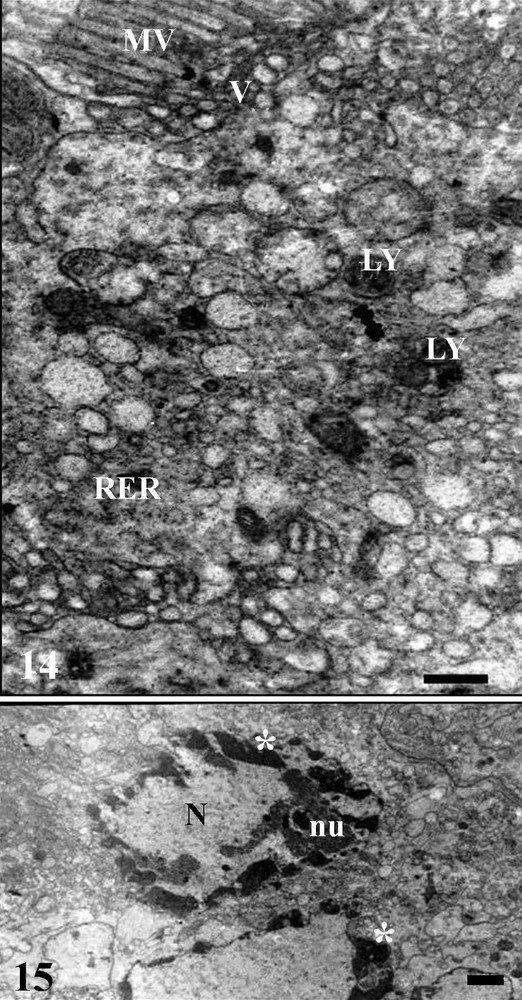
Transmission electronic micrographs of median midgut region of Triatoma vitticeps starved for 7 days. 14 – Apical region of the digestive cell showing the microvilli (MV), rough endoplasmic reticulum (RER), lysosome (LY) and vesicles (V) scattered in the cytosol and associated to microvilli (MV). Bar = 5 μm. 15 – digestive cell with decondensed chromatin and in some regions forming cloths (asterisk) in the nucleus (N). nu - nucleolus. Bar = 0.6 μm.
Some features of vesicles and granules in the cytoplasm enabled differ the digestive cells of the three midgut regions of T. vitticeps. Thus, in the anterior midgut region the digestive cells had glycogen granules, lipid droplets and spherocrystals. Some spherocrystals with an aspect of degradation by lysosomal action were found in this midgut region. In the median midgut region the digestive cells had accumulations of granules and vesicles with different electron-density (Figs. 14, 15), which were found being released to the midgut lumen. In the posterior midgut region, digestive cells had vesicles with various aspects, and they might be disperse throughout cytoplasm, as well as being released among microvilli (Figs. 16, 17) and lipid droplets and hemozoin granules that are characterized by strong density to electrons (Fig. 19). Cells in degeneration process, characterized by microvilli disorganization and cytoplasmatic vacuolation, were found in this midgut region of insects 25 days after blood meal (Fig. 18).
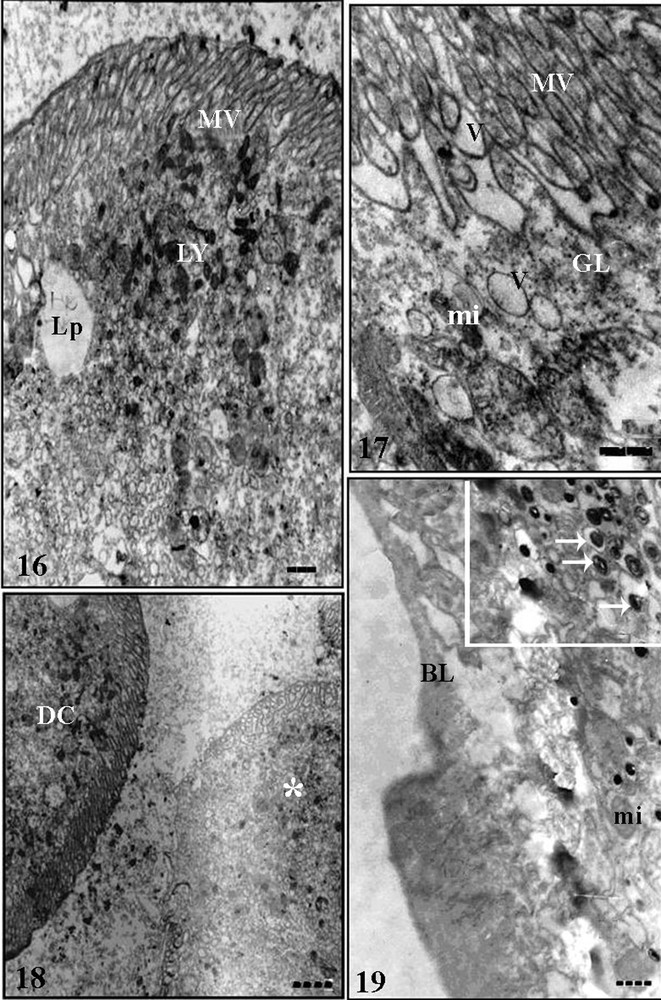
Transmission electronic micrographs of posterior midgut epithelium of Triatoma vitticeps starved for 25 days. 16 – Apical cytoplasm of digestive cells with lysosomes (LY) and lipid droplet (Lp). MV: microvilli. Bar = 1.5 μm. 17 – Apical region of the digestive cells showing microvilli (MV), electron-lucent vesicles (V), mitochondria (mi) and glycogen granules (GL). Bar = 2 μm. 18 – Apices of digestive cells (DC) and a cell in degenerating process (asterisk). Bar = 1 μm. 19 – Basal region of the digestive cell showing the basal lamina (BL) and mitochondria (mi). Inset: details of hemozoin granules (arrows). Bar = 1 μm.
4 Discussion
The midgut of T. vitticeps has a wall formed by a simple columnar epithelium that lies on a basal membrane followed by external longitudinal and internal circular muscle fibers. The epithelium is constituted of two basic cell types, digestive and regenerative. Although it is accepted that endocrine cells can also be found in the midgut epithelium, its safe identification would only be possible with immunocytochemical techniques. Our findings indicate that the midgut of this Hemiptera is similar to that of other insects [1–8]. Triatoma vitticeps as well as other hematophagous Hemiptera, such as R. prolixus [21] and T. infestans [22] have perimicrovillar membrane lining the surface of microvilli in digestive cells, which is a structure commonly found in Hemiptera [14,23,24].
Nucleus/cytoplasm ratio of digestive cells of T. vitticeps in different starvation periods indicates that starvation does not change this parameter and that cells remain active during all feeding cycle, corroborating data obtained for R. prolixus [25]. On the other hand, in the cockroach Periplaneta americana after four weeks of starvation there was decrease in the midgut epithelium size [26], what was not found by us, maybe due to differences in the omnivorous feeding habits of cockroaches and hematophagous of T. vitticeps.
The histological, histochemical and ultrastructural results showed aspects of morphology and physiology of digestive cells in the different midgut regions that modify according to the starvation period. The presence of vesicles of different sizes and staining in the digestive cells indicates that these cell types may be involved in secretory and absorptive functions. In this sense, small and electron-dense vesicles are associated with secretory functions, whereas large and electron-lucid vesicles are associated with the absorption [27,28]. In the midgut of phytophagous Hemiptera Lygus hesperus digestive cells can also be distinguished by size and electron-density of vesicles in their cytoplasm [29].
The presence of lipid droplets and glycogen granules in the cytoplasm of digestive cells suggests that the anterior midgut region of T. vitticeps play a role in the metabolism of lipids and carbohydrates. In addition, the occurrence of the basal labyrinth more developed in the digestive cells of this region and the presence of spherocrystals suggest its role in transport and storage of ions and water. These functions for the anterior midgut region are reported for other Hemiptera, as well as hematophagous [10,23,30,31], omnivores [7] and predators [12,24].
Higher positivity to bromophenol blue in the apical and basal portions of the anterior midgut of T. vitticeps may be due the high concentration of membrane transporters proteins. Electrolytes exchanges between cells and extracellular space are performed by membrane proteins in the microvilli and basolateral domains of digestive cells [14]. A countercurrent fluid flux occurs in the insect midgut, by means of fluid secretion in the posterior region and its absorption in the anterior region [32].
Lipid droplets in the anterior midgut region of T. vitticeps starved for 20 days, support the role of this region in lipid storage corroborating data obtained for R. prolixus [33].
The role of median midgut region of T. vitticeps in secretion, digestion and absorption was evidenced by the decondensed aspect of nuclear chromatin, presence of rough endoplasmic reticulum, Golgi complex region, lysosomes, vesicles and cytoplasmic inclusions in the digestive cells. The median midgut of Hemiptera has been pointed out as the main region for digestion and absorption [10,12,21,24,34–36].
In the posterior midgut region, digestive cells have cytoplasm with granules similar to hemozoin, vesicles with protein content and lipid droplets, indicating the possibility of functioning in digestion, absorption, and storage processes, similarly to found in R. prolixus [25,33,35].
Our findings suggest expressive function in ion transport through the anterior region and nutrient digestion and/or absorption by the median and posterior midgut regions of T. vitticeps, similarly to that reported for other insects [12,23,24,35,37,38].
The higher number and types of cytoplasmic granules in the digestive cells in all midgut regions of T. vitticeps starved for 20 days suggest a link between the morphophysiological aspects of this cell and the time after blood meal corroborating data for other hematophagous insects [31,39].
Our results indicate an increase in the number of vesicles in the digestive cells of insects subject to long starvation period, which may be related to the fact that hematophagous insects have no feeding sources available constantly as occurs with phytophagous insects. Therefore, hematophagous need an enzymes reserve, probably stored in the electron-dense vesicles, which are promptly used when finding the blood source, as found in predatory Hemiptera [24] and Coleoptera [40], and in newly-emerged adult holometabolous [41].
The perimicrovillar membrane was found in all midgut regions of T. vitticeps, with discontinuous areas 25 days after blood meal, suggesting that this membrane is degenerated according starvation increases, similarly to what was found in R. prolixus [25] and Cimex hemipterus [23]. Perimicrovillar membrane of R. prolixus lines completely the apical surface of digestive cells 10 days after blood meal [42]. We found similar results, since in T. vitticeps with 7 days of starvation, microvilli are not yet completely lined by perimicrovillar membrane. In the predator B. tabidus, the presence of perimicrovillar membrane lining the microvilli of digestive cells throughout the midgut is constitutive and has no influence of diet or starvation period [24]. In this light, hematophagous and predatory Hemiptera may have developed different mechanisms for controlling the perimicrovillar membrane synthesis.
Apoptotic cells in the posterior midgut region of T. vitticeps starved for 25 days can be related to the physiological processes. Degenerative processes in the midgut cells of insects are described in different physiological events such as the metamorphosis [43] and nutrients that release toxic substances [7,44]. In all these events, the presence of digestive cells with apoptotic and/or necrotic aspect is described, which are released to the midgut lumen, being replaced by cells differentiated from regenerative cells [4,24,27,44–46].
Morphological features of the different midgut regions of T. vitticeps here presented, and the similarity with other hematophagous Hemiptera as T. infestans [22], C. hemipterus [23] and R. prolixus [35], sap sucking L. hesperus [29], predator B. tabidus [24], and omnivore Cenocorixa bifida [7], suggest the origin from a common ancestor for these insects. Among the Hemimetabole insects, Hemiptera represent one of the most numerous groups, comprising three suborders: Sternorrhryncha and Auchenorrhyncha, all herbivores, being most of them sap suckers, and Heteroptera that include hematophagous, predatory and phytophagous insects [15]. The Hemiptera ancestor was probably a sap sucking insect similar to Sternorrhyncha, since the loss of the peritrophic membrane in this insect order would be related to the adaptation of their ancestor to phloem feeding [14,47]. The perimicrovillar membrane probably emerged in Condylognatha, which is the common ancestor of Thysanoptera and Hemiptera, and it would have lost the peritrophic membrane and digestive enzymes for initial and intermediate digestion [14,48].
The midgut of T. vitticeps has differences in the digestive cells associated to starvation. Microvilli, perimicrovillar membrane and basal labyrinth are present in the digestive cells throughout the midgut, and can present variation due to specific function of each region and period after blood meal. From data here obtained, we suggest that the anterior midgut region stores substances, transports fluids and ions, and play a role in hemolysis and carbohydrates digestion, whereas median and posterior midgut regions have function in the enzymes secretion, although the posterior region act in blood digestion and nutrients absorption.
Acknowledgements
Authors are grateful to Nucleus of Microscopy and Microanalysis from Federal University of Viçosa, MG, Brazil for technical assistance and Brazilian research agencies CNPq and FAPEMIG for support this study.


