1 Introduction
It has been suggested that a wide variety of pollutants interfere with testis function, following chronic or sub-chronic exposure. Indeed, heavy metals (such as cadmium or lead), pesticides or insecticides modify gametogenesis and steroidogenesis [1–7]. Besides these chemicals, the testis is also sensitive to radionuclides (131I, 228Th, 228Ra, 90Sr) and external irradiation [8–12].
Following the accident at Chernobyl nuclear power plant, several radionuclides were released into the environment. Depending upon their half-life, radionuclides contaminated subjects for a short (such as 131I) or a long time. Twenty years later, the main radionuclide still present is 137Cs, because of its 30-year half-life. This artificial radionuclide is a beta-gamma radiation emitter of the alkaline group. The main route of contamination for individuals living in contaminated areas is food ingestion, due to soil-to-plant-to-human transfer [13]. Moreover, it was demonstrated that absorption of cesium following ingestion is approximately 100% [14,15]. Besides this main route of contamination, individuals were also exposed to external irradiation from radioactive material around them.
Persistence of 137Cs contamination raises questions regarding public health for people living in contaminated areas. Several studies reported various dysfunctions among these populations affecting the cardiovascular, immune or respiratory systems [16–18]. Reproductive disorders have also been described in contaminated regions. For example, increases in infertility, impotence, and modifications of sperm parameters were observed among clean-up workers and other men living in these regions, along with alterations in testosterone levels [19–21]. In order to understand if these effects were induced by a chronic contamination with low doses of 137Cs on adult testis steroidogenesis, a rat model with exposure only during adult life was developed [22]. In this previous study, a decrease in 17β-estradiol concentrations in blood was reported, as well as modification of gene expression concerning several transcription factors regulating testicular steroidogenesis.
Besides the effects of chronic contamination by 137Cs in adulthood, the question of the effects in developing animals, in utero or soon after birth was raised. Children are known to be more sensitive to pollutants [23,24], thus it could be postulated that young animals may be more affected by this exposure than adults. Likewise, 137Cs was shown to be easily transferred to the fetus through the placenta [25], or to infants via breast-feeding [26]. Increases in children's general morbidity were reported as well as rare illnesses [21], including perturbations of the endocrine system, delayed and precocious puberties [21,27]. However, no molecular mechanisms underlying these pathologies have been described thus far.
To answer this question, rats were contaminated via their drinking water at a dose similar to that ingested by the population living in contaminated areas immediately after the Chernobyl accident [13]. Animals were contaminated at different ages and throughout 9 months, which is the approximate equivalent to 20 years of human life [28] and male reproductive system examined.
2 Material and methods
2.1 Animals
All experimental procedures were approved by the Animal Care Committee of the Institute of Radioprotection and Nuclear Safety and complied with French regulations for animal experimentation (Ministry of Agriculture Act No. 87-848, October 19, 1987, modified May 29, 2001). Water and food were delivered ad libitum.
Sprague-Dawley pregnant rat females (Charles River, L’arbresle, France) were individually housed with a 12 h light/dark cycle (light on 08:00 h/20:00 h) and a temperature of 22 ± 1 °C, and divided into four groups: two control groups (non-contaminated) and two contaminated groups. After weaning, 13 male pups per group were kept until the age of nine months. For eight of them, the two testes were stored at −80 °C for PCR, enzyme activity and western blot. For the five other animals, one testis was fixed for histology and the controlateral was used for 137Cs measurement. Animals were killed by intracardiac puncture, under 5% isoflurane (Abbot France, Rungis, France) anesthetics.
2.2 Contamination procedures
The rats in the experimental group were exposed to 137 cesium chloride (CERCA, Pierrelatte, France) at a dose of 6500 Bq/L (approximately 610 Bq/Kg/day, specific activity 3.2 × 1012 Bq/g) in their drinking water.
2.2.1 Post-natal contamination
In this experiment (Fig. 1), contamination occurred from birth until 9 months of age. Until weaning, pups were exposed via dam's milk. Indeed, from delivery, dams received 137Cs contaminated water. After weaning, rats were exposed directly through their drinking water, until the age of 9 months.
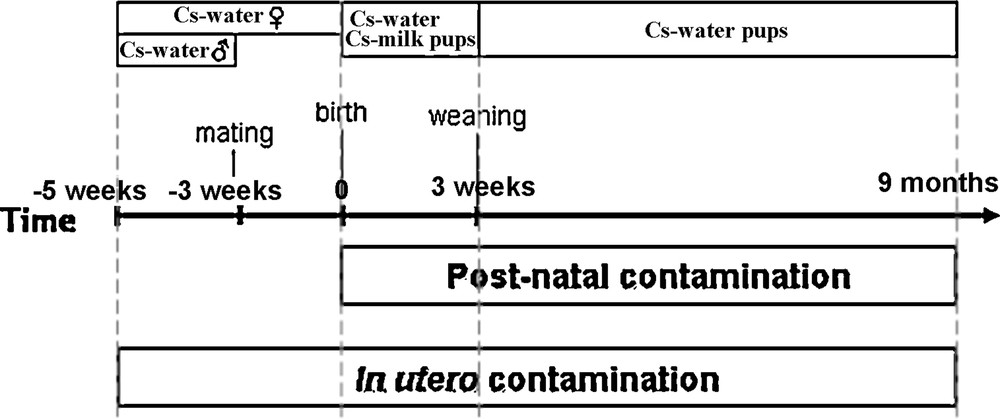
Scheme of contamination procedures.
2.2.2 In utero contamination
In this experiment (Fig. 1), parents were exposed to 137Cs at a dose of 6500 Bq/L in their drinking water 2 weeks before mating. Then females were contaminated during pregnancy, until weaning. After weaning, pups were directly exposed through their drinking water until 9 months of age.
2.3 Cesium measurement
Gamma spectrometry with a gamma counter (Cobra) was used to measure 137Cs; the whole testis was counted for 60 min, and the count related to its weight. The radiation dose received by the testis was calculated according to models developed for rodents [29].
2.4 Real-time PCR applied to testicular tissue
Testicular total RNA was prepared with the RNeasy total RNA isolation Kit (Qiagen, Courtabœuf, France) according to the manufacturer's instructions. The cDNA was produced from 1 μg of total RNA by reverse transcription with BD Sprint PowerScript PrePrimed 96 plate (BD Biosciences Clontech, Erembodegem, Belgium). Real-time PCR was performed on an Abi Prism 7900 Sequence detection system (Applied Biosystems, Courtabœuf, France) using 10 ng of template DNA for each reaction. Samples were normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT) [30]. Sequences for the primers are indicated in Table 1.
Primer information used for the qPCR analysis.
| Gene (accession number) | Primers | 5’-3’ sequence | Amplicon size (bp) | Reference |
| cyp11a1 (NM017286) | fw | CTCTGCAATGGAACCTTTATGAAAT | 66 | [22] |
| rev | CTTCAGCCCGCAGCATCT | |||
| cyp17a1 (NM012753) | fw | TGGCTTTCCTGGTGCACAATC | 90 | [22] |
| rev | TGAAAGTTGGTGTTCGGCTGAAG | |||
| cp19a1 (NM017085) | fw | ACCATCATGGTCCCGGAAA | 62 | [22] |
| rev | AGGCCCATGATCAGCAGAAG | |||
| 3β-HSD1 (NM001007719) | fw | CCCAGGCAGACCATCCTAGAT | 62 | [22] |
| rev | ACGCAGGCCTCCAATATGTTC | |||
| 17β-HSD3 (NM054007) | fw | TCGGGAAAGCCTATTCATTTGA | 63 | [22] |
| rev | TCCGGCTGATAAGTACAACATTGA | |||
| 5α-R1 (NM017070) | fw | TCCTGGTCACCTTTGTCTTGGC | 128 | [31] |
| rev | GTTTCCCCTGGTTTTCTCAGATTC | |||
| StAR (AB001349) | fw | TCAGAGTAGCAGCTCCCTTGTTTG | 106 | [32] |
| rev | CTCCAAATCCTGAAACGGGAATGC | |||
| apoD (NM012777) | fw | GATGGCGACCATGCTGTTG | 63 | [22] |
| rev | TGTCCTTCGGTTGTGGTGAA | |||
| AR (NM012502) | fw | GGGTTGGCGGTCCTTCA | 64 | Primer express |
| rev | GAAAACCAGGTCAGGTGCAAAG | |||
| RXRα (NM012805) | fw | CGCAAAGACCTGACCTACACC | 133 | [33] |
| rev | TCCTCCTGCACAGCTTCCC | |||
| LXRα (NM031627) | fw | AGCAACAGTGTAACAGGCGCT | 62 | [34] |
| rev | GTGCAATGGGCCAAGGC | |||
| LXRβ (NM031626) | fw | GATCCTCGAGTAAGATGACCACGATGTAGG | 170 | [34] |
| rev | GATCGGATCCATGTCTTCTTCCCCCACAAGTTC | |||
| FXR (U18374) | fw | TGACAAAGAAGCCGCGAAT | 98 | [22] |
| rev | TGTAATGGTACCCAGAGGCCC | |||
| SHP (NM057133) | fw | CCTGGAGCAGCCCTCGT | 64 | [35] |
| rev | AACACTGTATGCAAACCGAGGA | |||
| SF-1 (AB009575) | fw | TGCTTACCAGACCTTGGGATGT | 65 | [22] |
| rev | GGTGCTCGTGTGGAAATGG | |||
| DAX-1 (NM053317) | fw | CCGATGTTGTCACTGAACTCTTTT | 81 | [22] |
| rev | CACAGAGCATCTCCAGCATCAT | |||
| GATA-4 (NM144730) | fw | GATGGGACAGGACACTACCTATGC | 88 | [22] |
| rev | GGCGCTGAGGCTTGATGA | |||
| hprt (NM012583) | fw | GCTCGAGATGTCATGAAGGAGA | 108 | [30] |
| rev | TCAGCGCTTTAATGTAATCCAGC |
2.5 Western Blot
Proteins were extracted from testis using the Mammalian cell lysis kit (Sigma, St Quentin-Fallavier, France), following the manufacturer's instructions. Protein concentrations were measured using the BioRad protein assay kit (BioRad Laboratories, Marnes-la-Coquette, France). Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes (Invitrogen, Cergy-Pontoise, France). Blots were incubated with anti-SF-1 (Steroidogenic Factor-1) (1/800), anti-CYP19 (cytochrome P450 19A1) (1/800) or anti-FXR (Farnesoid X-Receptor) (1/100) antibodies (Tebu-bio, Le Perray-en-Yvelines, France) at 4 °C overnight. A glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Tebu-bio) was used as an internal control for equal protein loading. The immunoreactive bands were detected by enhanced chemiluminescence and quantified using the LAS-3000 system (Raytest, Courbevoie, France) and multi Gauge Software (Fujifilm).
2.6 Microsome preparation
One gram of testis was homogenized in ice-cold buffer (KH2PO4 50 mM; sucrose 300 mM; dithiothreitol 0.5 mM; EDTA 10 mM; NaCl 50 mM pH7.4) [36]. The homogenate was centrifuged for 20 min at 20,000 × g and the supernatant centrifuged at 100,000 × g for 1 h. The microsomal pellet was homogenized in buffer, collected and stored at −80 °C until required.
2.7 Aromatase activity assay
Aromatase activity was measured according to [37] with minor modifications. Microsomal protein (500 μg) was incubated with 5.3 μM 4-androstene-3,17-dione (Sigma), 1.3 μM [1β-3H(N)]-androst-4-ene-3,17-dione (Perkin Elmer, Courtabœuf, France), 0.3 mM NADPH, in 1 mL of 0.05 M TRIS maleate pH 7.4 for 1 h at 37 °C with shaking. The reaction was stopped with 1 mL chloroform. Tubes were centrifuged at 2700 × g for 5 min. Five hundred μL of 7% activated charcoal-1.5% dextran in solution were added to the aqueous phase and centrifuged at 2700 × g for 15 min. The enzyme activity was assayed by measuring the radioactivity of the 3H2O released in the supernatant, and of the substrate in the organic phase, in a scintillation counter for one min.
2.8 Hormone assays
Plasma testosterone, 17β-estradiol (DSL, Cergy-Pontoise, France), Follicle-Stimulating Hormone (FSH) (Amersham Pharmacia, Orsay, France) and Luteinizing Hormone (LH) (Biocode Hycel, Pouilly en Auxois, France) were measured by RIA, according to the manufacturer's instructions.
2.9 Histological study
Testes were fixed in a modified Davidson's solution (4% formaldehyde [2/9 (v/v)]; absolute ethanol [1/3 (v/v)]; glacial acetic acid [1/9 (v/v)]; distilled water [1/3 (v/v)]) for 3 days. Tissues were then dehydrated in a Tissue-Tek VIP (Sakura, Villeneuve d’Ascq, France) with formaldehyde solution, increasing concentrations of alcohol, and xylene, before being embedded in paraffin. Tissues sections of 5 μm were then stained with hematoxylin/eosin/saffron solution. Presences of all stages of spermatogenesis, as well as vacuoles were examined.
2.10 Statistical analysis
Results are reported as mean ± SE. Statistical analyses were performed with Student's t or Mann-Whitney tests. The Mann-Whitney test was used for non-parametric values. Differences were considered significant when P < 0.05.
3 Results
3.1 General health status of male rats
The chronic 9-month contamination of rats did not affect food or water intake, weight gain, or general health status (data not shown). Pre-mating contamination did not modify litter sizes or sex-ratio.
3.2 Study of testis
At the time of sacrifice, testis weight was identical between control and contaminated groups for both protocols (data not shown). Following contamination, 137Cs levels were measured in testis. As expected, tissue from control groups (post-natal and in utero contamination experiments) did not show detectable levels of 137Cs. In the post-natal contamination group, the average amount of 137Cs was 2.19 ± 0.09 Bq/g, whereas it was 5.02 ± 0.41 Bq/g for the in utero contamination (corresponding approximately to respective doses of 2 and 4 mGy).
Hematoxylin-eosin-saffron staining showed no gross histological modification in either model (data not shown). In both control and contaminated groups, interstitial Leydig cells were present and tubules appeared identical as shown by some Sertoli cells and the various stages of germ cells: spermatogonia, spermatocytes and spermatids. No modification of tubule size was observed.
3.3 Hormonal status
To study the endocrine function of testis, the hormonal levels of testosterone and 17β-estradiol were measured in blood, as well as LH as the main regulator of the steroidogenesis. LH values did not significantly change in any contamination process (data not shown).
The 17β-estradiol level was also identical in the four groups, ranging from 2 to 3 pg/mL. Reflecting the absence of any LH variation in blood, testosterone did not differ between contaminated and control groups. Moreover, the level of FSH, which is involved in gamete maturation, showed no significant alterations in either group (data not shown).
3.4 Gene expression
The molecular effects of contamination were studied by analyzing the content of various mRNA involved in testicular steroidogenesis: the transporters StAR and apoD, and the enzymes 3β-HSD1 and 17β-HSD3, 5α-R1, CYP11A1 and CYP17A1. No significant variation was observed in both protocols of Cs-exposure (Fig. 3). The CYP19A1 encoding aromatase was modified by Cs-exposure: a significant 2.3-fold increase was observed in the post-natal exposed group whereas in utero contamination induced a 28% decrease compared to the control group (Fig. 2).
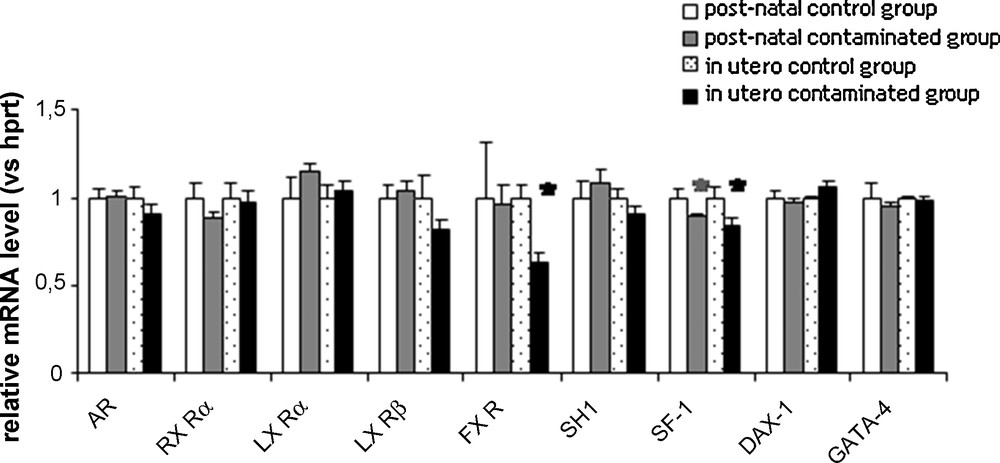
. Basal relative expression of genes encoding some transcription factors involved in the regulation of the steroidogenic genes in rats exposed to chronic contamination with cesium.
Relative levels of messengers were determined by qPCR. Levels in the non-contaminated control rats were arbitrarily set at 1. Number of rats per groups, n = 6–8. Hprt was used as an internal control. Histograms are indicated as mean ± SEM. *, p < 0.05 compared with the respective control groups. RXR: retinoid X receptor; LXR: liver X receptor; FXR: farnesoid X receptor; SHP: small heterodimeric partner; SF-1: steroidogenic factor-1; DAX-1: dosage-sensitive adrenal hypoplasia congenita on the X-chromosome, locus 1; GATA-4: GATA-binding protein 4.
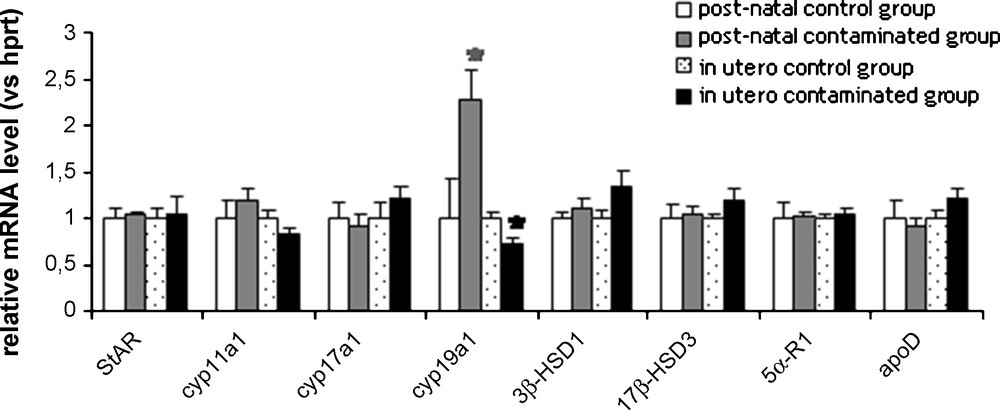
Basal relative expression of genes encoding steroidogenic proteins in rats exposed to chronic contamination with cesium.
Relative levels of messengers were determined by qPCR. Levels in the non-contaminated control rats were arbitrarily set at 1. Number of rats per groups, n = 7–8. Histograms are indicated as mean ± SEM. *, p < 0.05 compared with the respective control groups. StAR: steroid acute regulatory protein; cyp11a1: cytochrome P450 side chain cleavage; cyp17a1: cytochrome P450 17α-hydroxylase; cyp19a1: cytochrome P450 aromatase; 3β-HSD1: 3β-hydroxysteroid dehydrogenase type 1; 17β-HSD3: 17β-hydroxysteroid dehydrogenase type 3; 5α-R1: 5α-reductase type 1; apoD: apolipoprotein D. Hprt was used as the internal control.
Messenger RNA described to control steroidogenesis was analyzed. As shown in Fig. 3, a significant decrease in SF-1 levels was detected for both exposure procedures. Moreover, a 37% significant decrease in FXR messenger expression was measured following in utero exposure. There was no variation of LXRα or LXRβ.
3.5 Protein expression and enzyme activity
In order to test whether messenger variations were reflected at protein level, accumulation of SF1, FXR and aromatase (CYP19A1) was examined and aromatase activity measured. No significant modification of protein accumulation was noted in the contamination protocols (Fig. 4), or in testicular aromatase activity (Fig. 5).
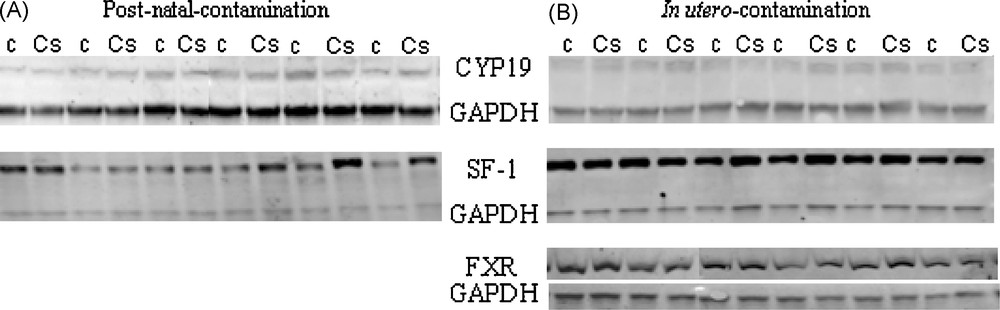
Protein accumulation of CYP19, SF-1 and FXR in testes of rats exposed to chronic contamination with cesium.
Western blot analysis was performed on testes following post-natal or in utero contamination for CYP19A1, SF-1 and FXR. GAPDH was used for normalization of sample loading. Cs: contaminated group; c: control group.
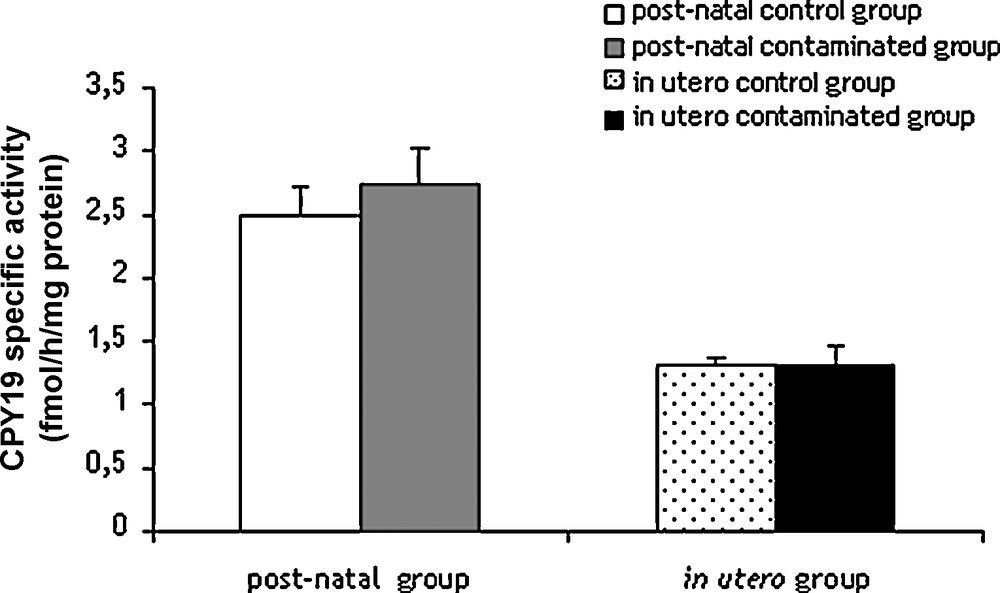
Determination of aromatase activity of testes of rats exposed to chronic contamination with cesium.
Specific activity of CYP19 enzyme was determined in rat testes as described in Material and Methods. Histograms are indicated as mean ± SEM and expressed in fmol.h−1.mg proteins−1. Number of rats per groups, n = 6.
4 Discussion
This study mimics children exposure to 137Cs, in areas contaminated following Chernobyl nuclear power plant accident. Two models of exposure were used: from birth and since uterine life. These experiments demonstrate that chronic ingestion of post-accidental dose of 137Cs only modified a few gene expressions, without affecting protein expression or activity, testis morphology, or blood hormonal levels, despite distribution of 137Cs in testis in both experiments.
Surprisingly, these results are in contrast with those obtained following 137Cs chronic exposure from adulthood [22]. Indeed following adulthood exposure, a decrease in estradiol circulating level was observed. In the two studies, animals were exposed to the same dose (6500 Bq/L, through drinking water) and concentration (1.48 × 10−11 Bq/g cesium chloride) of 137Cs, during 9 months (from 3 to 12 months of age for adults animals, from birth or conception until the age of 9 months for post-natal and in utero groups). Thus, data suggest that in our experimental conditions animals exposed during pre- or neonatal period are less sensitive to 137Cs than animals exposed from adulthood. These results are not in agreement with general acceptance, considering children more sensitive than adults [24,38]. However, it was shown that age-sensitivity depends on toxicity as well as dose and duration of exposure [39–41]. In the present study on growth models, the lack of toxicity may be explained by the initiation of systems regulating hormonal metabolism to limit adverse effects that lead to failure of development. It might be postulated that adaptive response of growing organisms is more efficient than adults. The small amount of 137Cs found in testis may also account for these results.
Moreover, in our study, levels of the pituitary hormones (FSH and LH) were unchanged for both models. Thus 137Cs appeared not to affect the hypothalamo-pituitary-gonadal axis, in relatively low doses of irradiation. Indeed, irradiation of 2.8 Gy (which depleted the spermatogonial cells) did not alter concentrations of FSH, confirming that the production of inhibin by Sertoli cells remained unaltered [42]. Similarly, serum levels of LH were not changed even by a dose of 4.7 Gy X-ray irradiation, indicating normal functioning of Leydig cells. Thus ionizing radiation induced by our chronic exposure procedure (in the range of mGy) did not appear to affect hypothalamo-pituitary-gonadal axis.
Following the Chernobyl accident, studies concerning male children and adults living in contaminated territories showed hormonal modifications as well as testis morphological and spermatogenesis disturbances [20,43,44]. Exposure with 137Cs did not induce histological modification of testis in terms of structural, steroidogenic and gamete cells. However, following the Chernobyl accident, individuals were exposed to several radionuclides and non radioactive toxics spread in environment as well as to external irradiation. Thus it appears that effects observed among young and adults were not strictly due to 137Cs exposure, but also to other radionuclides, as well as external irradiation. Indeed, the testis is one of the most radiosensitive tissues, since ionizing radiation in the range of 0.7–7 cGy induced by chronic consumption of drinking water containing radionuclides of Chernobyl area affected rat male gametes [45]. A direct radiation dose as low as 0.15 Gy produced a significant depression in male sperm count and temporary azoospermia after doses of 0.3 Gy [10]. Moreover, irradiation during uterine life induced alterations of testis in adults. Irradiation of pregnant rats (2.1 Gy) lead to irreversible damage of spermatogenesis and androgen production when observed at 70 days after birth [46]. Thus, in our experiment, ionizing radiation doses induced by ingestion of post-accidental dose of 137Cs (2–4 mGy) are insufficient to modify testis morphology.
Data showed that modification was induced at the molecular level, without affecting plasma levels of hormones [22]. In our growth models, the expressions of three genes were modified: cyp19a1, fxr and sf-1. These results are in agreement with those observed following adult exposure, identifying these genes as 137Cs targets. In another study [47], rats were exposed to depleted uranium (heavy metal presenting almost no radioactive properties) versus enriched uranium (presenting both metal and radiological properties). It was found that aromatase gene was targeted by enriched but not by depleted uranium. Thus it appears that radionuclides (of which are 137Cs and uranium) affect metabolism/regulation of aromatase (CYP19A1) in testis. It may be postulated that aromatase is a particular target of chronic internal radiation exposure. However, the modifications induced do not occur concurrently with protein alterations, suggesting that an adaptive response at the post-translational level prevents dysregulation of CYP19, FXR and SF-1 at the protein level.
In conclusion, chronic exposure of growing rats with 137Cs at doses found in the Chernobyl area exerted few effects on testicular steroidogenesis, despite presence of 137Cs in the organ. Testis histology as well as steroid hormone levels and hypothalamo-pituitary-gonadal axis were not modified. Some gene expressions were changed, without modification at the protein level (expression or activity). Thus, surprisingly, growing organisms appear less sensitive to 137Cs exposure than adults, suggesting a more efficient adaptive response. This phenomenon is not in agreement with Chernobyl population outcomes, suggesting that 137Cs may not be the main factor inducing perturbations among these populations.
Acknowledgements
The authors thank E. Tourlonias for 137 cesium measurement, E. Blanchardon and D. Broggio for their scientific help and T. Loiseau, F. Voyer and C. Baudelin for their assistance during animal treatment and experimentation. This study was part of the ENVIRHOM research program supported by the Institute for Radioprotection and Nuclear Safety (IRSN), and by Electricité de France (EDF).


