1 Introduction
The name Gomphocerinae (Orthoptera, Caelifera, Acrididae) comes from the Greek “gomphos”, which means “inflated” and “ceros”, the horns or antennae, because of the club shape at the distal part of the antennae in the males of certain species. This feature has been recognized for a long time as diagnostic in systematics (for example, the Gomphocerini tribe in the sense of Harz [1]), but it was recently revealed as the probable result of parallel evolution from molecular phylogeny analyses [2].
It has been reported that most species bearing clubbed antennae use to move them backward over the head during the courtship [3]. So the particular shape of the antenna tip reinforces the visual signal displayed by the males and is often associated to other visual elements, such as hind leg strokes, as in Myrmeleotettix maculatus [4], Gomphocerus sibiricus [5], Gomphocerripus rufus [6,7], Chorthippus oschei [8] and several species in the genus Stenobothrus [3,9,10]. The relationship between the strokes of antennae and hind legs is not strict, as the antenna movement is the only visual display observed in Myrmeleotettix antennatus [11]. Moreover, the link between the optical display of antenna stroke and apical thickening is not absolute since during courtship, Chorthippus oschei moves its antennae, which are not clubbed. It is the same for C. lacustris in which the filiform antennae harbour white tips [9]. These visual elements of complex courtship have developed in independent lineages of the Gomphocerinae [12], in addition to the fundamental acoustical component, produced by a femoral-tegmina mechanism, shared by all the members of the tribe.
In Western Europe, the taxa bearing clubbed antennae live in various habitats, as mountain grasslands (Aeropedellus variegatus, G. sibiricus), herbaceous stratum under clear woods (G. rufus) or various herbaceous places, from dunes to heathlands (M. maculatus) [13].
As there is no obvious link between courtship visual component and an ecological context, we suspect that the significance of the club shape of antenna could be enlightened by the study of its sensory function. The sensilla composition of antennae in Caelifera is involved in sexual recognition, or plant odour sensitivity, or both [14,15]. We shall follow here the nomenclature adopted by Yamamoto-Kihara et al. [16] who identified in grasshoppers large and small basiconic sensilla, trichoid sensilla (TS) and coeloconic sensilla (CS) (Table 1). Within the last category, Bernays and Chapman [17] distinguished two sub-categories [18]. Chen et al. [19] studied the relation between the antennal sensilla repertoire, the sex, the systematic position and the food mode in various subfamilies of Acrididae of Mongolia. They found that the graminivorous species have more sensilla and in particular more coeloconic sensilla (CS) than the other studied species, not graminivorous. The authors showed that these differences are especially due to the variations in the abundance of the type A basiconic sensilla (BAS) and coeloconic, which are also known to be olfactive sensilla. Moreover, other factors were shown to act on the number of sensilla, as the microhabitat. Otherwise, mechanoreceptors, such as campaniform sensilla, are recorded in the antenna of several insect orders [20] and are able to detect a deformation of the cuticule [21].
Nomenclature of the different sensilla types on the grasshopper antennae.
| Abbreviations | Yamamoto-Kihara et al. [16] | Bernays and Chapman [17] | Chen et al. [19] | Description | Attributed functions |
| TS | Trichoid | Trichoid | Long Basiconic | Basal insertion in the cuticule Single pore at the end Important size |
Contact chemorecepors [17] |
| CS | Coeloconic | Coeloconic | Coeloconic | Very short hair size in a more or less circular depression of cuticule Grooves on the cuticular structure With wall pores Grooves on the cuticular structure With wall pores |
Olfactory receptor [17] |
| Without wall pores | Hygro- or cold-sensitive [18] |
||||
| BAS | Basiconic type A | Large Basiconic | Short Basiconic | Thin-walled and short hair size Placed in a cuticular depression Numerous pores |
Olfactory receptor [17] |
| BBS | Basiconic type B | Small Basiconic | Short Slender Basiconic | Thin-walled and short hair size Diameter smaller than in BAS Placed in a cuticular depression Lower number of pores than in BAS |
Olfactory receptor [17] |
| DS | Campaniform | Circular establishment in the cuticule: DS | Mechanoreceptor, sensitive to the deformation of cuticule [21] |
In Gomphocerinae and Oedipodinae (Acrididae), there is also a link between the gustatory sensilla of the inner face of the labrum (conical contact chemoreceptors type A) and the food mode (El Ghadraoui et al. [22]). The authors showed that the polyphagous gomphocerine Dociostaurus maroccanus harbour more sensilla (particularly for the sensilla A10), than the graminivorous species of the same subfamily. These results were supported by the study undertaken on other polyphagous species, in particular in Oedipodinae (El Ghadraoui, personal communication). Thus, there is an increase in the number of sensilla when the food mode is diversified. The polyphagous grasshoppers need a greater number of chemoreceptors to recognize the secondary metabolites associated with the leaves of dicotyledones, more varied than those met in the leaves of Poaceae.
To our knowledge, no study on sensilla was devoted to the special case of clubbed antenna in Gomphocerinae. As this feature is always more developed in males than in females, the variations in the sensilla repertoire are expected to be related to sexual recognition. This work proposes to understand which types of sensilla are associated to the antennal thickening present in certain gomphocerine species. With the aim of enriching our information on the roles of the various types of sensilla, their numerical variations will also be tested according to the food modes. Indeed, the majority of Gomphocerinae are oligophagous and consume plants belonging to the Poaceae family, and it is very likely to be the case for the ancestors of the tribe [22]. A few species became polyphagous (mixture of dicotyledones and monocotyledones) during evolution, as in various subspecies of Glyptobothrus binotatus [23] or in Dociostaurus maroccanus [24], and even monophagous (only one plant genus consumed), such as for example in the type subspecies of Glyptobothrus binotatus [25,26]. As a consequence, this approach will help to understand the link between clubbed antennae, diet and courtship.
2 Material and methods
2.1 Sampling
The antennae were taken on individuals of the collection of zoology of the University of Limoges. Sampling includes 15 gomphocerine species (Table 2). The antennae were dissected from two different male individuals. The phylogenetic position of each species was given by the comparison of cytochrome oxydase I (COI) sequences available in GenBank (Table 2). The analysis was conducted with MEGA 4.0. [27], using minimum evolution and pairwise deletion options, and the robustness of branches estimated by 2025 bootstrap replicates (Fig. 1A). A synthetic tree (Fig. 1B) including G. brunneus was constructed using the phylogeny produced by Contreras and Chapco [2] who analyzed two gene sequences of mitochondrial DNA, the deshydrogenase V (NADH5) and COI. We completed this synthetic tree by the introduction of Myrmeleotettix maculatus, a genus close to Omocestus-Stenobothrus, in reason of a common epiphallus structure and the loss of the bulge at the anterior margin of the fore wings. This position was retrieved by Vedenina [28] using the analysis of COI sequences in a wide range of gomphocerine species. We also added Glyptobothrus binotatus subsp. saulcyi as a sister species of the nominal subspecies [7].
Studied grasshopper species and antennal characteristics.
| Species | Diet | Number of segments | COI sequence a. number | Apical thickening |
| Aeropedellus variegatus (F. von Waldheim, 1846) | Graminivorous | 22 | DQ230712 | Segments 15 to 22 |
| Arcyptera fusca (Pallas, 1773) | Graminivorous | 23 | AY738368 | – |
| Chorthippus parallelus (Zetterstedt, 1821) | Graminivorous | 22 | AY738355 | – |
| Dociostaurus jagoi Soltani, 1978 | Polyphagous | 24 | DQ230734 | – |
| Dociostaurus maroccanus (Thunberg, 1815) | Polyphagous | 24 | DQ230714 | – |
| Glyptobothrus biguttulus (Linnaeus, 1758) | Graminivorous | 24 | AY738449 | – |
| Glyptobothrus b. binotatus (Charpentier, 1825) | Monophagous | 24 | DQ230724 | – |
| Glyptobothrus binotaus saulcyi (Krauss, 1888) | Polyphagous | 24 | – | – |
| Glyptobothrus brunneus (Thunberg, 1815) | Graminivorous | 24 | – | – |
| Gomphocerippus rufus (Linnaeus, 1758) | Graminivorous | 24 | DQ230733 | Segments 14 to 21 |
| Gomphocerus sibiricus (Linnaeus, 1767) | Graminivorous | 23 | AY738358 | Segments 16 to 22 |
| Myrmeleotettix maculatus (Thunberg, 1815) | Graminivorous | 23 | – | Segments 18 to 22 |
| Omocestus rufipes (Zetterstedt, 1821) | Graminivorous | 23 | FJ555544 | – |
| Stenobothrus lineatus (Panzer, 1796) | Graminivorous | 25 | FJ555549 | – |
| Stauroderus scalaris (F. von Waldheim, 1846) | Graminivorous | 23 | AY738360 | – |
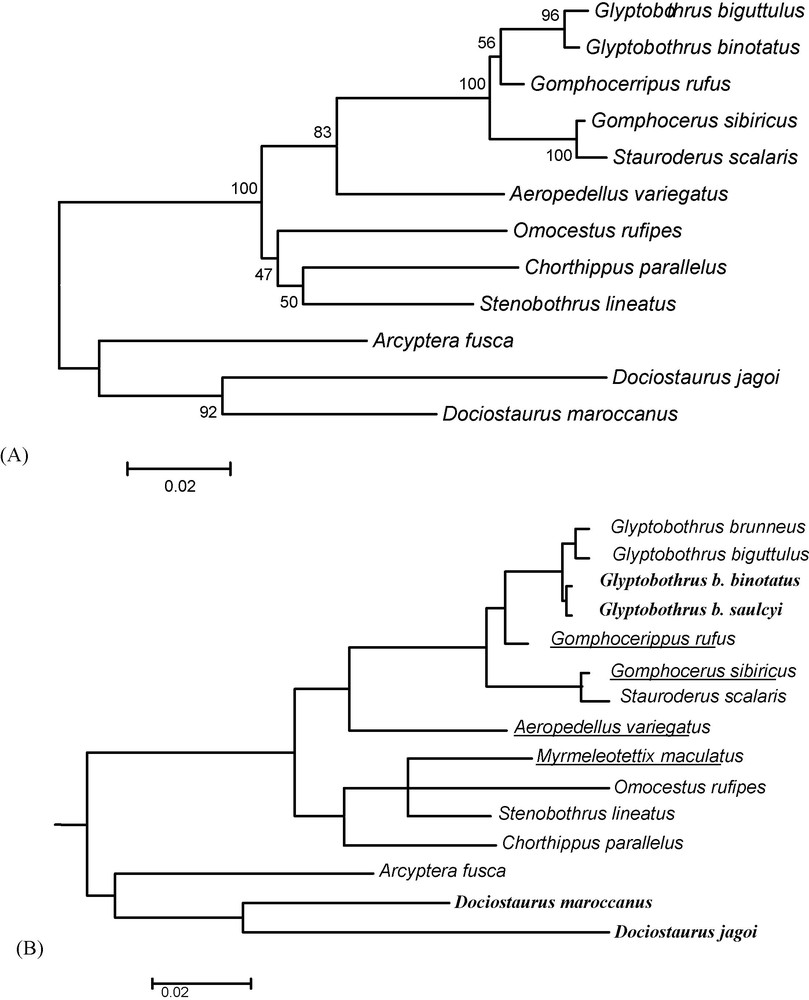
Phylogenetic tree for the 15 studied species. A. Species relationships constructed with MEGA 4.0 [27], using pairwise deletion and minimum evolution options, for COI sequences (540 pb). The confidence was estimated by bootstrap percentages calculated from 2025 replicates. B. Synthetic tree taking into account the work of Contreras and Chapco [2] and systematics information given in Ragge and Reynolds [7]. The non-graminivorous species are in bold, and the species with clubbed antennae underlined.
2.2 Protocol
After section at the base, the antennae were put in a concentrated KOH solution until discolouration. They were then rinsed with water and preserved in glycerol-coated gelatine of Kaiser (Merck) between two glass blades. The sensilla were observed and counted under Olympus cx41 optical microscope. The surface of each segment was measured from photographs taken with a Nikon Coolpix p1 numerical camera. We measured the length (L) and the width (W) of each segment, and then calculated the surface of a half cylinder by multiplying L, W and Pi/2. For each species, only the sensilla of the upper half-cylinder of each segment were counted.
2.3 Statistical processing
Statistical analyses of correlation were carried out with PAST 1.81 [29]. The General Linear Model (GLM), conducted with SYSTAT 12.0 for Windows [30], was preferred to two-ways ANOVA because in our sampling, all the species bearing clubbed antennae are graminivorous. Using this GLM, the overall variance is well split in what is related to the food mode and in what is associated to the antennal thickening. Two tests were conducted, one with the observed number of sensilla per segment and per species, i.e. 9 × 15 values (135), and the other with the mean number of sensilla per segment and by species, i.e. 15 values. The second test allowed avoiding the problem of a possible too important weight brought by a very divergent species for a given category.
3 Results
3.1 Global analysis of the sensilla
The global analyses of the number of sensilla on the antennae in all the studied species showed that the species, which have a clubbed antenna, have less TS (Kruskal-Wallis, n = 15, p = 0.016) than the other species (Fig. 2). However, the density of these sensilla per segment surface did not differ between the two groups of species (Kruskal-Wallis, n = 15, p = 0.185). As for BAS ones, there is only a tendency of a lower number in the species having thickened antenna tip (Kruskal-Wallis, N = 15, p = 0.068).

Variations in the total number of trichoid sensilla. The species with clubbed antennae are in white boxes.
3.2 Relation between the number of sensilla and segment surface
In the species with filiform antennae, the surface of the segments is peaking in the medium part of the antenna, for example between segments 8 and 16 for D. maroccanus (Fig. 3A). In contrast, in the species having a thickened tip, the segments with the largest surface are situated at the distal end (Fig. 3B): in G. rufus, the bulge extends between segments 14 and 21. Moreover, it appears that there is a better parallelism between the segment surface variations and the numbers of TS than the other types of sensilla.
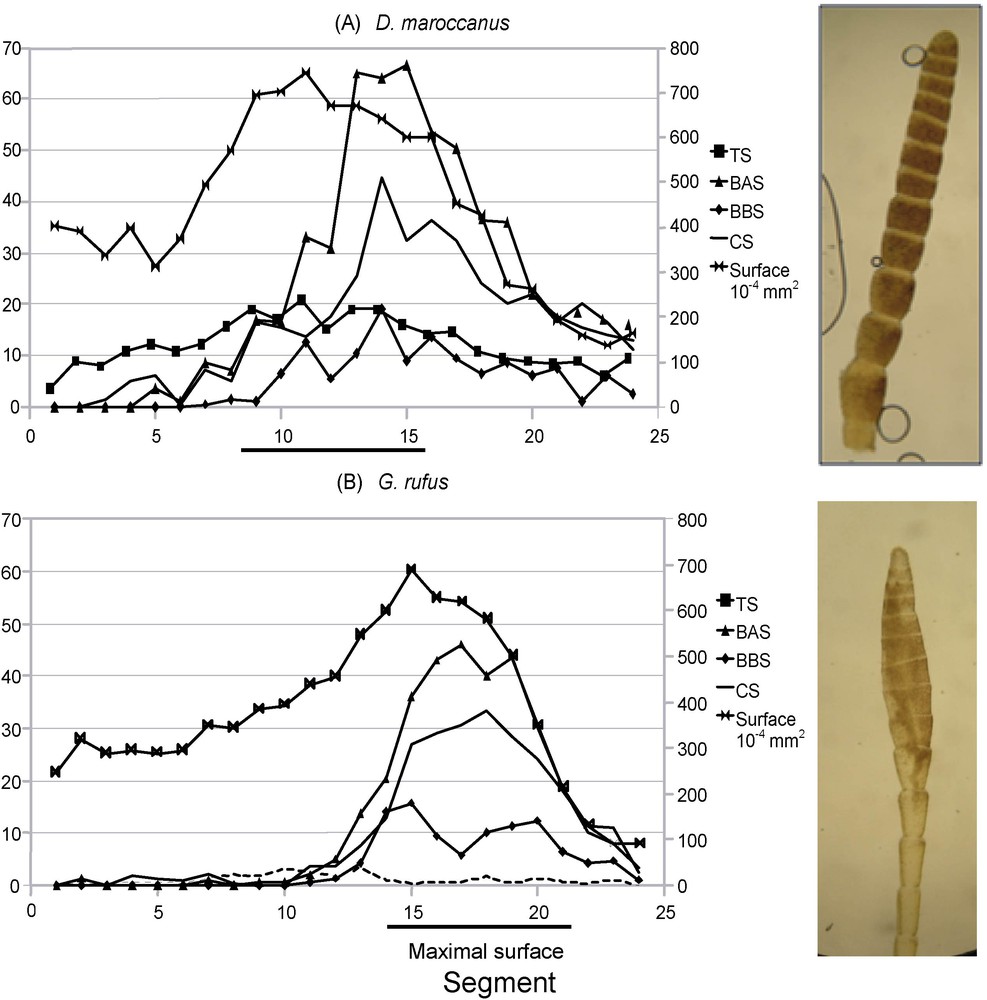
Variations in the number of the different sensilla types according to antenna segments. At the right, photograph of antenna tips. A. Dociostaurus maroccanus. B. Gomphocerus rufus. TS: trichoid sensilla; BAS: basiconic A sensilla; BBS: basiconic B sensilla; CS: soeloconic sensilla; DS: dome sensilla.
Table 3 exposes the relation that exists, species by species, between the sensilla and segment surfaces on the level of the thickening (segments 14 to 22). The TS number is strongly correlated to the surface of the segments because the lowest correlation coefficient found is equal to 0.63 (p = 0.001) in C. parallelus, but up to 0.90 (p = 2.10 × 10−9) in G. b. saulcyi. This confirms the results shown in Fig. 3. For the BAS type, 11 results out of 15 are significant, and the average coefficient of correlation is equal to 0.50. In addition, for the B basiconic sensilla (BBS), seven results out of 15 are significant, and the average coefficient of correlation is of 0.27. For the CS type, only two results out of 15 are significant, with a mean correlation coefficient equal to 0.26. Lastly, no result is significant for the dome sensilla type (DS).
Pearson correlation between the number of each type of sensilla and the surface of each half-segment for the various studied species. The p-values are indicated between brackets.
| Species | Trichoid | Basiconic A | Basiconic B | Coeloconic | Dome |
| A. variegatus | 0.75 (0.0001) | 0.50 (0.017) | 0.45 (0.034) | 0.22 (0.331) | – |
| A. fusca | 0.84 (0.47 × 10−6) | 0.42 (0.05) | 0.29 (0.173) | 0.27 (0.214) | |
| C. parallelus | 0.63 (0.001) | 0.41 (0.062) | 0.01 (0.979) | 0.28 (0.207) | – |
| D. jagoi | 0.72 (5.45 × 10−5) | 0.67 (3.51 × 10−4) | 0.35 (0.003) | −0.04 (0.097) | −0.03 (0.859) |
| D. maroccanus | 0.86 (8.09 × 10−8) | 0.45 (0.03) | 0.40 (0.050) | 0.28 (0.190) | – |
| G. biguttulus | 0.81 (1.79 × 10−6) | 0.59 (0.002) | 0.18 (0.404) | 0.19 (0.380) | 0.08 (0.712) |
| G. b. binotatus | 0.76 (1.49 × 10−5) | 0.68 (2.580 × 10−4) | 0.46 (0.023) | 0.50 (0.013) | 0.30 (0.149) |
| G. b. saulcyi | 0.9 (2.10 × 10−9) | 0.47 (0.022) | 0.46 (0.026) | 0.34 (0.106) | 0.32 (0.126) |
| G. brunneus | 0.7 (0.015) | 0.49 (0.419) | 0.17 (0.325) | 0.21 (0.105) | – |
| G. rufus | 0.65 (6.10 × 10−4) | 0.53 (0.007) | 0.45 (0.026) | 0.36 (0.080) | 0.39 (0.063) |
| G. sibiricus | 0.66 (5.53 × 10−4) | 0.39 (0.064) | 0.25 (0.254) | 0.15 (0.483) | – |
| M. maculatus | 0.66 (3.70 × 10−4) | 0.80 (3.7 × 10−4) | 0.50 (0.016) | 0.60 (2.4 × 10−3) | 0.18 (0.424) |
| O. rufipes | 0.83 (0.49 × 10−6) | 0.55 (0.005) | 0.22 (0.303) | 0.18 (0.401) | 0.47 (0.020) |
| S. lineatus | 0.83 (0.22 × 10−6) | 0.30 (0.141) | 0.15 (0.485) | 0.17 (0.430) | – |
| S. scalaris | 0.82 (1.87 × 10−6) | 0.38 (0.076) | −0.30 (0.162) | 0.28 (0.202) | – |
3.3 Variation in the sensilla number among species
Analyses by GLM were carried out in order to study the variation in the numbers of sensilla compared to the presence or not of an antennal thickening and to the food mode. These GLM were made on the distal part of antennae between the segments 14 to 22, but also in the medium part between the segments 5 to 13 (Table 4). The results concerning segments 5 to 13 showed a strong relation between the number of TS (F = 67.590, p < 10−3), BAS (F = 29.599, p < 10−3), CS (F = 10.363, p < 10−3) and DS (F = 7.413, p = 0.007) and the presence or not of thickening (Fig. 4). This results in a number of these sensilla largely lower in the species with clubbed antennae. In contrast, there is a lower number of DS in the species with filiform antennae. With regard to the BBS, their number is not significantly different between the species with or without a thickening at the level of segments 5 to 13 (F = 1.515, p = 0.219). At the level of the distal part of the antenna (segment 14 to 22), only the numbers of TS and DS are significantly related to the presence of thickening (F = 6.824, p < 10−3 and F = 8.118, p = 0.005, respectively).
Analyses of global General Linear model (n = 135) and GLM per species (n = 15). Only p-values are given.
| Segments | Trichoid | Basiconic A | Basiconic B | Coeloconic | Dome | ||||||
| Global | Per sp. | Global | Per sp. | Global | Per sp. | Global | Per sp. | Global | Per sp. | ||
| 5–13 | Diet | 0.446 | 0.894 | 0.036 | 0.403 | 0.471 | 0.483 | < 10−3 | 0.448 | 0.850 | 0.737 |
| Thickening | < 10−3 | 0.008 | < 10−3 | 0.02 | 0.219 | 0.608 | 0.001 | 0.241 | 0.007 | 0.276 | |
| 14–22 | Diet | 4.122 | 0.531 | 0.102 | 0.855 | 0.08 | 0.933 | < 10−3 | 0.346 | 0.03 | 0.509 |
| Thickening | 0.009 | 0.237 | 0.068 | 0.358 | 0.93 | 0.243 | 0.228 | 0.751 | 0.005 | 0.474 |

Variations in the number of different sensilla types at the medium part of antenna (segments 5–13) or at the tip (segments 14–22). 0. Species without clubbed antennae. 1. Species with clubbed antennae. N.S.: non-significant variations. The significance was given by General Linear Model.
It was necessary, to carry out the GLM on the DS, to withdraw G. rufus because this species presents a largely higher number of DS than the other compared species. In this species, the number of these sensilla per segment peaks in the medium part of antenna (Fig. 3B).
The relations between the food mode and the number of sensilla are shown in Table 4 and Fig. 5. The numbers of TS, BBS and DS are not significantly different according to the diet from the segments 5 to 13 of the antenna. In contrast, the number of BAS and CS are different according to the food mode (F = 3.361, p = 0.036 and F = 14.403, p < 10−3, respectively). That results in an increase in sensilla BA and a reduction in sensilla C in the graminivores for segments 5 to 13. At the level of segments 14 to 22, the difference in the number of sensilla between the species graminivores and non-graminivores remains significant only for the CS (F = 30.770, p < 10−3) and becomes significant for the TS (F = 4.122, p = 0.017) and DS (F = 3.558, p = 0.030).
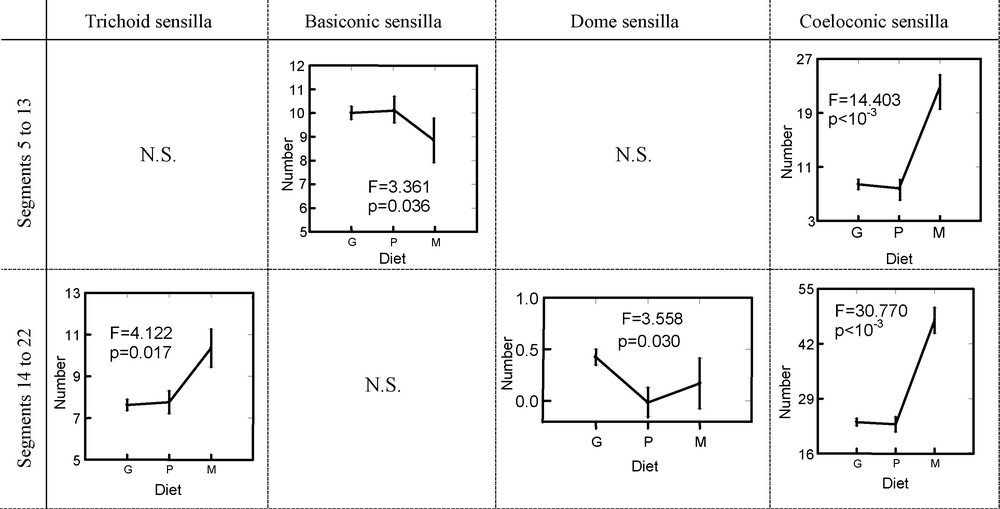
Variations in the number of different sensilla types at the medium part of antenna (segments 5–13) or at the tip (segments 14–22). G: graminivore species; P: polyphagous species; M: monophagous species; N.S.: non-significant variations. The significance was given by General Linear Model.
4 Discussion
4.1 Link between sensilla number and club shape
There are several reasons that support an absence of link between the shape of antennae and food mode. First, in all the examined species, the grasshoppers bearing clubbed antennae are graminivorous. Second, the thickening of antenna tips is more pronounced in male than in females, in spite of a comparable source of food in both sexes. Third, the presence or not of an antennal thickening has more impact on the number of sensilla than the food mode and this is even stronger on the median part of the antenna (segment 5 to 13). There is thus at this level of the antenna a significant difference in the number of sensilla (p < 0.007 for the TS, BAS, CS and DS) between the species, which have an antennal thickening and those, which have not. Among the seven gomphocerine species investigated by Chen et al. [19], both sexes of Myrmeleotettix palpalis, the only studied species with clubbed antennae, have the lowest number of sensilla, whatever the category. Although they did not separate in their analyses the median and subterminal parts of antennae, their results support our conclusions. As a consequence, it seems more appropriate to interpret sensilla number variations in the context of a link between clubbed antennae and courtship, as shown in the introduction.
4.2 Food mode and sensilla number
Although the number of sensilla is less influenced by the food mode than by the thickening, several sensilla categories vary significantly according to the diet. We could expect that a polyphagous grasshopper may encounter more secondary metabolites that an oligophagous one and a fortiori than a monophagous one, and thus would have more olfactive and gustative sensilla used to their detection. This prediction is partially retrieved for the BA sensilla, which are olfactive sensilla, where the monophagous species is poorer than the other species on the level of segments 5 to 13 (p = 0.036). However, when we consider the whole data set, the other sensilla follow a counter-intuitive tendency. The monophagous species feeding on Ulex is richer in TS (gustative) and in CS (olfactory) than the oligophagous or polyphagous ones. We can deduce that the transition from an oligophagous diet, based on the consumption of Poaceae, to a polyphagous one, extended to Dicotyledones and Monocotyledones as food source, has lead to weak changes in antenna sensilla repertoire.
The results obtained on the sensilla of the labrum in relation to food mode are somewhat different [22], as the polyphagous Acrididae are richer in A sensilla than oligophagous ones. Thus it seems that the sensilla repertoire shows a quicker adaptive response to diet changes at the labrum level than at antennae level. As for the particular case of G. b. binotatus, its specialised diet concerns different species of Ulex (Fabaceae) [23], a genus very rich in secondary metabolites, and especially alkaloids [26]. Observations made on labrum sensilla absolutely support our present results on antenna. In this case, we hypothesize that during the exploration of its environment, the insect is first sensitive to secondary metabolites emitted by the plant using its antennal olfactive repertoire, gets a confirmation by antennal contact sensilla and further by the taste of the juice extracted from the chewed leaves with labrum sensilla.
4.3 Interpretation of sensilla modifications in the context of courtship
The thickening of antenna tips is clearly associated to low values of TS, BAS and CS, obviously more pronounced in the median than in the terminal part of antenna. In contrast, the species with clubbed antennae are rich in DS, especially in G. rufus. These features are in close relation to the courtship of the males, which is generally complex, including a visual component.
During courtship of most species bearing clubbed antennae, the male projects its antennae backwards and the thickened tips reinforce the visual signal, as described in the introduction. There must be a control of these movements, as we observe a high density of DS, which are certainly campaniform sensilla, in the medium part of antenna. These sensilla would be involved in the transmission of cuticule deformation [31] caused by antenna stroke, and would not play a role as a contact receptor with the female during mating.
What are the consequences of antennal tip thickening in terms of sensilla repertoire? As the number of TS is proportional to segment surfaces, the thickening restricted to the male antennae makes it possible to increase their number and then becomes an asset for the reacting to cuticular pheromone, as demonstrated in cricket and grasshoppers [32–34]. The TS are contact chemoreceptors [17], which would allow the males to select the females at the time of the nuptial parade. More precisely, the antennae are in almost continual contact during copulation, but Ritchie [35] considered that prior the mating, this contact is a more important for the sexual recognition in C. p. subsp. erythropus than the song structure, which is prevalent in C. p. subsp. parallelus. As a by-product, the antennal thickening would increase the number of sensilla involved in mating success by the detection of volatile molecules. In this view, it may be that the CS and BAS specialized in odour detection would also be used to detect volatile pheromones emitted by the females. Although probably rare in grasshoppers, this possibility has been demonstrated by Siddiqi and Khan [36] in Hieroglyphus nigrorepletus (Hemiacridinae). The lower number of BAS in the females than in males [19] supports this view.
However, absolute values of sensilla numbers show that the species having clubbed antennae are poor in olfactive and contact sensilla. The thickening is sufficient to compensate the low level of CS but not for BAS and trichoid in the distal part of antennae. Contact chemoreceptors could allow the males to study a state of the female receptivity. It could be more critical to learn this state as soon as possible for the non-courting males and we suggest that species with filiform antennae bear more TS for this reason. In contrast, in the males with complex visual display, it seems to be not so critical, since long courtship could change the state of a female from semi-receptive to fully receptive stage [37].
5 Conclusion
The results show that the species having clubbed antennae are overall low in sensilla, in spite of the apical thickening. As the courtship of these species is complex and includes movements of antennae, the chance that a female is in a receptive state is high, so the need for numerous contact sensilla is less important for appreciating the receptivity of the female. In contrast, we hypothesize that these species have a better control on the antenna strokes, owing their numerous antennal mechanoreceptors. Moreover, our results indicate that the polyphagous species have no more olfactive sensilla than graminivore species. Instead, the change of diet from oligophagy to monophagy on Ulex bushes is linked to a dramatic increase in contact and olfactive sensilla, probably for detecting specific alkaloids, but this should be tested.
Acknowledgements
This study, belonging to “Globalbiodiv” program, was financially supported by Tassili 08MDU726, in a PAI/CMEP cooperation. The technical assistance of Florence Vallet was highly appreciated. We want to thank anonymous reviewers for their helpful remarks.


