1 Introduction
Global warming is one of the principal challenges confronting insects worldwide. The Intergovernmental Panel on Climate Change [1] has predicted an increase in air temperature of 1.1 to 6.4 °C by 2100, due largely to the effects of greenhouse gases, including atmospheric carbon dioxide (CO2; forecast increase in atmospheric concentration of 40 to 110% by 2030) and tropospheric ozone (O3). These climatic changes affect the biological and ecological characteristics of insect species, through direct effects on the physiology of organisms and through indirect effects on their habitat [2–4]. Some of the biological features particular to aphids render these insects especially sensitive to these changes.
1.1 The key effects of temperature on aphids
The aphid group includes some 4400 species worldwide, which have preferentially developed in the temperate regions of the northern hemisphere [5]. Temperature is one of the key factors underlying this geographic distribution. Aphids are particularly well adapted to regions with a cold winter, during which they survive in the form of eggs having a high level of cold hardiness [6]. These insects multiply only within a certain range of temperatures. The minimum temperature at which aphid development occurs is generally around 4 °C, but this figure varies within and between species. For example, estimates for Acyrthosiphon pisum (Harris, 1776) range between 2.3 and 6.3 °C [7–9]. Optimal temperatures and upper limits are also variable but usually in the range of 20 to 25 °C and 25 to 30 °C, respectively (reviewed by [10]). Thus, the rate of development in aphids is directly dependent on temperature. A female aphid requires a certain number of degree-days above the developmental threshold to reach adulthood (reviewed by [10]). Again this figure is variable with different studies showing it to range from 99 to 147 in A. pisum [11]. This is a particularly short generation time even among insects. In France, with mean temperatures of between 10 °C in the north and 15 °C in the south, aphids are mostly living in suboptimal temperature conditions. Global warming should therefore, in principle, favour the development of aphid populations. This effect is likely to be even greater than for other insects, due to the very short generation time of aphids and their extremely large reproductive capacity [12]. Thus, an increase in temperature of only 2 °C would allow the number of generations produced per year to increase from 18 to 23 in the UK [13] with a potentially huge increase in population size.
Other biological functions influenced by temperature include dispersal and reproduction. Aphid species disperse principally through the production of large numbers of winged individuals capable of travelling considerable distances, both to move to a new plant when experiencing decreasing nutritional quality and for the transfer from winter to summer host plants in the case of species with seasonal alternation between different host plant species. Both the number of winged individuals produced and their flying capacity depend on temperature, with increasing temperatures favouring mobility. Lower temperature thresholds for flight are generally around 13–16 °C and upper thresholds around 31 °C (reviewed by [14]).
Aphids reproduce in two ways (see “Evolutionary and functional insights into reproductive strategies of aphids” by JC Simon et al. in this issue). Some species are entirely parthenogenetic throughout the year. In such species, there is a mean of 18 generations each year in UK for instance [13]. In other species, year-round parthenogenetic reproduction is interrupted in the autumn by the establishment of an amphigonic generation producing eggs. The production of this latter generation is triggered by an increase in night length, but is also regulated by temperature. Thus, an increase in temperatures might delay or even totally prevent sexual reproduction, if temperatures remain above 20 °C [15]. Higher temperatures favour parthenogenetic reproduction and the survival of active individuals throughout the year.
1.2 The effects on aphids of increasing CO2 and O3 concentrations
Although temperature is a key factor governing insect life in general and that of aphids in particular, aphids are also affected by the environment through the host plants. Increases in concentrations of CO2 and O3 are of particular significance. Indeed, increases in CO2 concentration stimulate plant growth, but decrease the nutritional quality of plants for phytophagous insects [16]. By contrast, ozone tends to inhibit plant growth by decreasing carbon fixation through negative effects on the rate of photosynthesis [17]. The responses of aphids to high concentrations of CO2, O3 or both gases are, however, highly variable. Depending on the aphid species considered, development and fertility rates may increase [18–20], decrease [21] or remain unaffected by such atmospheric changes [22,23]. A single aphid clone may display different responses to high CO2 content according to the host plant [18,20]. Consequently, despite the many studies carried out on this subject, it is not possible to establish general rules or to predict whether all aphid populations will be affected by global warming [24,25].
Aphids are affected not only by increases in temperature and greenhouse gas levels, but also by changes in the landscape due to agricultural policies and by atmospheric pollution. The effects of these factors, considered separately, are gradually becoming clearer, but it remains difficult to appreciate the overall effects of these factors, as there are many interactions between them. The individual effects discussed above translate, at population level, into phenological effects (that is, effects on the dates at which certain events occur in the annual cycle, such as egg hatching at the end of winter, the recommencement of activity and spring migration and the initiation of sexual reproduction in the autumn), and effects on the geographic distribution and abundance of species [26]. These effects may in turn disturb the interactions between aphids and their host plants and natural enemies.
The effects of climate change on aphid populations have been evident for several years [10]. We focus here on the effects on the diversity and phenology of aphid populations and the interactions of these populations with their natural enemies. We do this through a literature review and with the help of original findings from a long series of data from monitoring networks.
2 Effects on aphid biodiversity
For more than 40 years, European scientists have been following aphid populations and assessing changes in these populations (biodiversity, phenology and abundance) through a permanent observatory, the EXAMINE network (Fig. 1) [27]. This network provides daily information on the flight activity of several hundred species, from 58 sites throughout Europe. It is an ideal tool for evaluating changes in insect communities at a vast geographic scale.
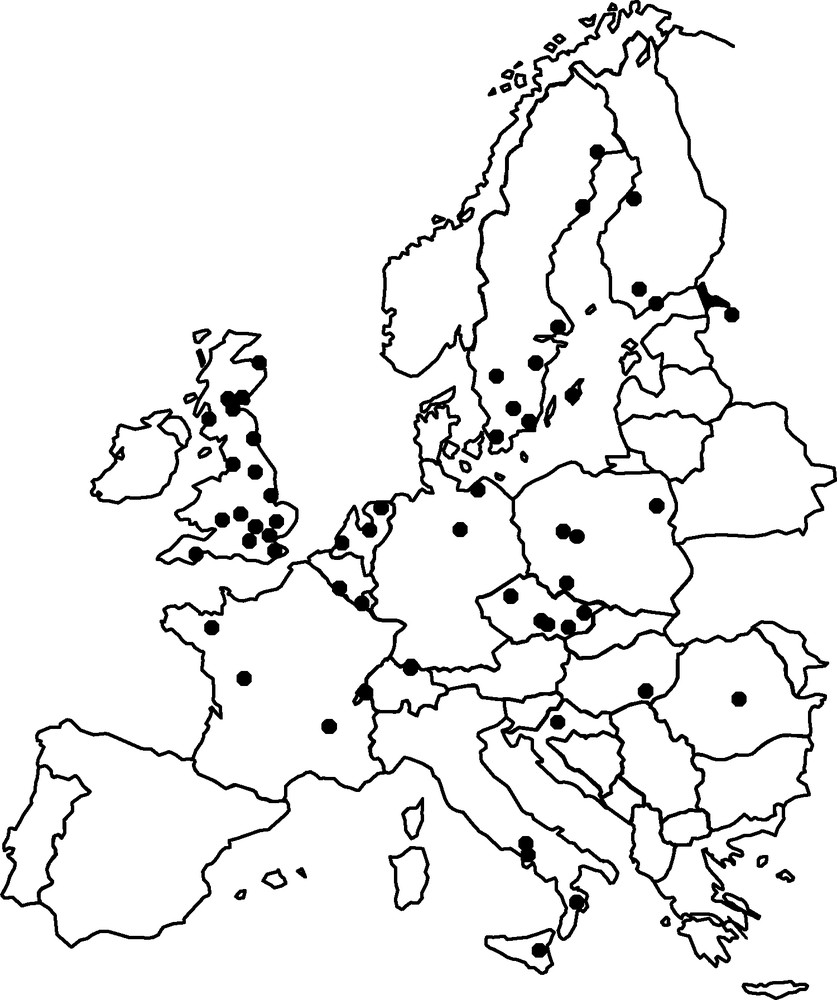
EXAMINE, a European network of 58 sites for studies of the impact of global climate change on aphids.
The EXAMINE network has revealed an increase in the number of species captured. This increase was studied at eight sites distributed across Western Europe, along a geographic axis extending over 2000 km, from northern Scotland to the southern Mediterranean (Fig. 2). The number of species found has increased by about 20% over the last 30 years. Over the same period, very few species have ceased to be captured, and the overall picture is one of increasing diversity. This considerable increase in the number of species closely follows the increase in mean temperature, by more than 1 °C, over the same period. The observations correspond to between two and 15 additional species per degree Celsius (°C), depending on the site considered. This evident increase in diversity may be due to the introduction of new species [28] or an increase in the numbers or activity of rare species that now reach aerial densities high enough for detection in the traps of the network. The combined effects of global warming, the increase in international movements (transport of foodstuffs, ornamental plants and travellers) and habitat modification effectively increase the probability of introducing new species [29,30]. For example, 261 (18%) of the 1415 aphid species present in North America are considered to be exotic. Most (90%) originate from palearctic areas. Europe is the leading source of such introductions (63%), whereas tropical and subtropical aphid species currently make up a minority of the exotic species present in North America. Among the 261 exotic species in North America, 43% are considered to be potential pests, with 18% being considered highly damaging pests [31]. More than 8% of the species present in Europe are considered to be exotic, with 10% of these exotic species originating from tropical and subtropical areas. This information has emerged from the Delivering alien invasive inventories (DAISIE for Europe; http://www.daisie.se) programme, which aims to identify all the exotic animal and plant species present in Europe. Some of these species were introduced a long time ago, but have recently escaped from long confinement in greenhouses to establish populations in natural conditions. One such species is Cerataphis brasiliensis (Hempel, 1901), which was first identified in greenhouses in the Loir-et-Cher region in 1998 and was subsequently found on outdoor palm trees growing in the Var in 2004 [32]. Some of these invading species are crop plant pests. This is the case for Toxoptera citricidus (Kirkaldy, 1907), the principal vector of citrus tristeza virus, which causes a serious disease of citrus crops. This aphid, which has become a major pest in the United States, was recently introduced into Portugal and Spain [33], where it currently presents a serious threat to citrus crops. Other species, such as Greenidea ficicola (Takahashi, 1921) and Reticulaphis distylli (van der Goot, 1917), two tropical Asian aphid species introduced with their host plants (ornamental figs), are now well established in southern Europe [34]. Mediterranean and sheltered regions serve as multiplication sites for tropical species, the populations of which are likely to expand throughout the rest of Europe under the influence of global warming.
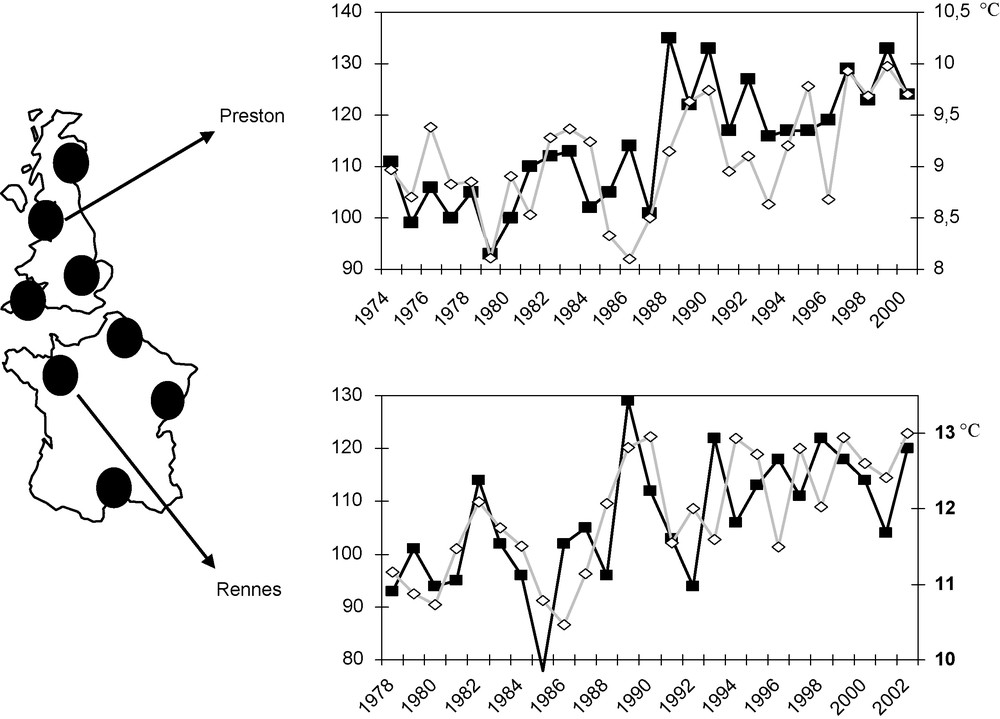
The eight study sites focusing on biodiversity and changes in species richness (number of species captured per year; black) and mean annual temperature (grey) from 1974 to 2000 at Preston (UK) and from 1978 to 2002 at Rennes (France).
3 Phenological effects
The EXAMINE network has also revealed changes in aphid phenology and the date of the first spring migrations in particular [35]. For example, consider the peach–potato aphid, Myzus persicae (Sulzer, 1776), which, despite its name, feeds on a large number of crop and wild plants. This species also transmits many plant viruses. It is one of the principal pests of crop species and one of the most widely monitored species in agriculture. In Harpenden, UK, the spring migrations of aphids have been recorded for more than 40 years, making it possible to identify the approximate date from which these insects colonise crops each year. These migrations are occurring increasingly early. In the 1960s, they generally began towards the end of May (mean 24 May), whereas they currently begin towards the start of the month (mean 7 May), corresponding to an advance of nearly 3 weeks over the last 40 years (Fig. 3). Over the same period, the mean air temperature in January and February has increased from 3.3 to 4.6 °C. If we apply the current IPCC predictions, then these migrations will occur about a month earlier by the middle of the 21st Century. The effect of winter temperature on the timing of flights of species in the spring seems to be general. About 95% of the 48 species observed by the EXAMINE network are displaying increasingly early spring migration (Table 1). The advance in the timing of migration, however, varies between species and latitudes, from one day per 10 years to more than three days per year, corresponding to one to 10 weeks over the last 40 years. Advances were different between species with different life cycle strategies and between species feeding on herbs and trees, suggesting the possible value of trait-based groupings in explaining responses to environmental changes [35]. At the scale of the European continent, the date of the spring migration of aphids may be accounted for by several factors, including latitude, altitude, annual climatic variation and land use [36].
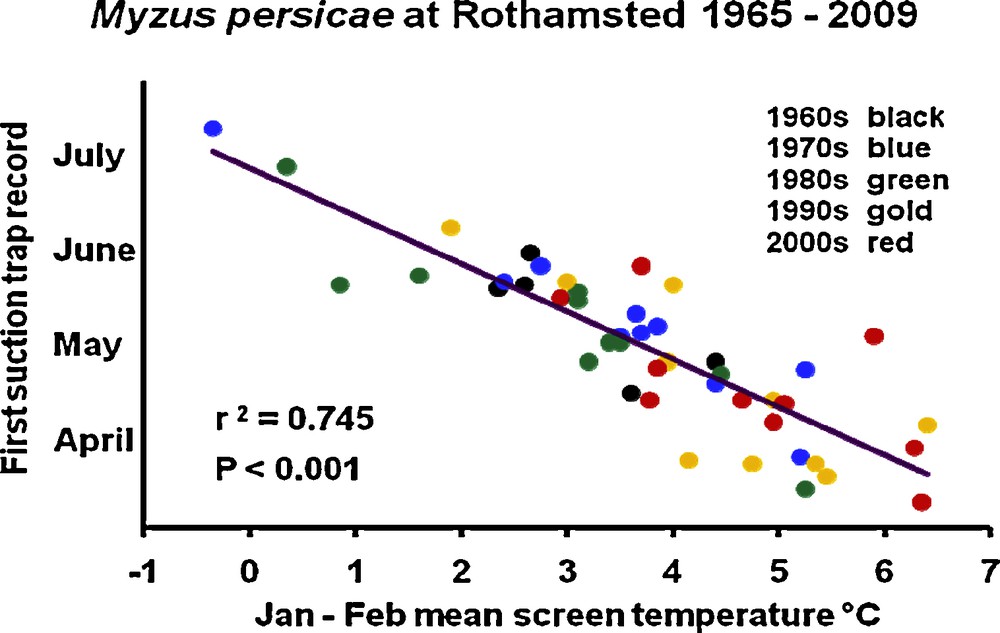
Date of the start of spring migration of the peach–potato aphid (Myzus persicae) at Rothamsted (UK), from 1965 to 2009, as a function of mean daily temperature in January and February.
Mean date of first trap records (for 46 species) in Europe (EXAMINE network) and the range of advances in the timing of this date.
| First trap record | Range of advances by species (number of days/year) | ||
| Minimum | Maximum | ||
| Dundee (Scotland) | 19 June | 0.1 | 1 |
| Rothamsted (England) | 25 May | 0.1 | 1 |
| Rennes (Northern France) | 6 May | 0.3 | 3.8 |
| Montpellier (Southern France) | 28 March | 0.2 | 2.1 |
4 Effects on interactions between aphids, their host plants and their natural enemies
Aphids are at the centre of a system including the plants on which they depend and an array of organisms dependent on aphids for food. The natural enemies of aphids include predators such as ladybirds, and parasitoids, small wasps that lay their eggs in the bodies of aphids. All these organisms are subject to the effects of temperature and may react differently to changes in temperature [37]. There is thus a risk of spatial or temporal desynchronisation with the other members of the community and there will presumably be consequences for the functioning of these communities.
4.1 Spatial synchronisation
Warmer conditions enable species limited by low temperatures to extend their range polewards [38]. Over the course of the next century, the IPCC predicts a mean increase in temperature of 2 °C, corresponding to the poleward movement of climatic conditions by 600 km, at a rate of 6 km per year. Aphids are highly mobile insects and are therefore clearly capable of following this movement, but what about their host plants? With the exception of a few plant species with a high colonisation capacity, most plants are unable to colonise new territories at this speed. This is likely to limit the expansion of specialist aphids living on only one type of plant. Other more generalist species, by contrast, may colonise new plant species facilitating the expansion of their range. The effects of global warming on plants may be unpredictable, because some species display changes in the content of certain secondary compounds, implicated in defence against phytophagous insects, with increasing temperature [39].
Changes in spatial synchronisation may also occur for parasitoids, the geographic expansion of which depends on their own optimum temperature and the presence or absence of the aphids on which they feed [40]. If, for physiological reasons, a parasitoid cannot follow its prey into their new distribution area, then the aphids may flourish due to the absence of one of their natural enemies. Such changes would probably result in local changes to natural equilibria.
4.2 Temporal synchronisation
The presence at the same site of the three protagonists of the system – the plant, the aphid and its natural enemies – is not sufficient for the system to be in equilibrium. The various components of the system must also be synchronised in time. Global warming changes plant phenology [41]. The development of a time lag between the phenology of the plant and that of the insect may have negative consequences for the long-term survival of the insect [38]. For aphids laying their eggs on trees, for example, egg-hatching coincides with bud burst. This synchronisation ensures that the young colonies of insects have access to particularly nutritious plant organs. Differences in the reactions of the plant and the insect to changes in temperature could lead to the desynchronisation of these events. Aphids hatching too early or too late have very high mortality rates and very low multiplication rates. However, if these changes are not too abrupt, the mechanisms responsible for the synchronisation of aphids and their host plants may continue to operate, enabling the aphids to adapt [42]. The same is true for parasitoids. If a parasitoid begins to develop at a temperature slightly lower than that for the aphid and at a faster rate than the aphid with increasing temperature, then early and warm springs will result in its emergence too early and its death due to a lack of aphids on which to feed. If this phenomenon repeats itself over several years, it may lead to the extinction of the parasitoid [40].
4.3 Too many aphids for the predators, and other possible effects
The effects of increasing temperatures on the aphid/parasitoid relationship may not involve a loss of synchronisation. For example, at temperatures below 11 °C, the rate of reproduction of the pea aphid A. pisum exceeds the rate at which aphids are consumed by the ladybird Coccinella septempunctata (Linnaeus, 1758), whereas the reverse is true at temperatures above 11 °C [43]. Thus, after a particularly mild winter favouring an early start to insect activity, pea aphids flourish at the start of the season without being limited by predation by ladybirds. As temperatures begin to increase and to exceed the threshold for efficient predation by ladybirds, aphid populations become subject to increasingly strong predation pressure, leading to an eventual decrease in numbers [38]. These findings are consistent with models of the Coccinella septempunctata–Sitobion avenae (Fabricius, 1775) interaction predicting higher levels of predation by ladybirds in warmer summers [44]. The effects of climate change on predation efficiency may depend on the species predating on aphids [45]. Exposure to 4 h of heat three times per week (heat shock) increases the predation pressure exerted by Coccinella septempunctata on A. pisum but decreases that by Harmonia axyridis (Pallas, 1773). It is therefore not possible to draw general conclusions about the interactions between aphids and their natural enemies. For example, an increase in temperature has no effect on the capacity of A. pisum to resist attacks from two species of parasitoids and the ladybird Hippodamia convergens (Guérin-Méneville, 1842). By contrast, such an increase in temperature renders this aphid less susceptible to the attacks of a pathogenic fungus [46].
There are many more examples of probable effects of climate change on the relationships and equilibria between aphids and the various organisms with which they interact and, particularly, on the transmission of plant viruses by aphids (review [47]). A final example concerning aphid pests of crops illustrates the complexity of the mechanisms that need to be considered when trying to understand the overall effects of climate change. Barley yellow dwarf virus causes a highly damaging disease of cereals and is transmitted by several aphid species. There are several viral strains transmitted with different efficiencies by different aphid species. One of the strains particularly common on maize is transmitted by maize aphid Rhopalosiphum maidis (Fitch, 1856). In Great Britain, global warming and agricultural policy have favoured an increase in the area under maize. Higher temperatures may also allow other aphid species to transmit this viral strain to other cereals, such as wheat [48], for which the economic consequences of infection are much more severe than for maize. Thus, warmer climatic conditions, through their effects on the host plant, on different aphid species and on the virus, may, for the first time, render this maize-specific strain a danger to wheat crops in Great Britain [38].
5 Conclusion
We describe here some of the most significant effects of climate change, and of global warming in particular, on aphids, as well as the effects of changes affecting individuals at the population and community levels. Aphids, with their high rates of multiplication and sensitivity to the environment, are good indicators that climate change has an impact on organisms. Their reactions to these changes are rapid and are of particular ecological importance, because of the central role of these insects in natural and agricultural ecosystems. However, given the many interactions to be taken into account when evaluating the impact of global changes, it is difficult to generalise from the limited available results. The modelling of biological processes may help to untangle these interactions and to develop possible scenarios. Long-term field observations, like those of the EXAMINE network, should also be useful for demonstrating the consequences and for validating models, because they cover all these interactions.


