1 Introduction
The genus Gerbillus Desmarest, 1804 is one of the most diversified groups of rodents inhabiting arid and semiarid areas. It is known for its morphological homogeneity [1–3] but high karyotype heterogeneity with a diploid number ranging from 2n = 34 to 2n = 74 [3–9]. Gerbillus has never been comprehensively revised [10] and its taxonomy is still holding a number of controversies. Among these, the genus Gerbillus is still a matter for discussion for taxonomists ([10] and reference herein). In fact, whether this genus is holding subgenera or good to split off into several genera is yet debated.
Since its early description, three different subgenera were created for the genus Gerbillus [11,12]. These are Gerbillus, Dipodillus and Hendecapleura and this on the basis of some morphological features.
The subgenus Gerbillus is characterized by the presence of well developed auditory bullae of which the posterior parts reach or even exceed the level of the occipital bone, a maximum number of five metatarsal tubercules, one carpal tubercule, and the presence of opposite cusps in the first upper molar and haired hind feet. The latter is bare in the species of the subgenus Hendecapleura which share some of the other characteristics of the subgenus Gerbillus (e.g. the well developed auditory bullae).
On the other hand, the subgenus Dipodillus shows a mediocre development of the auditory bullae, a higher number of metatarsal tubercules (six), a first upper molar with alternate cusps and hairless plantar surfaces. Even though these three taxa were mostly accepted by most of authors, there was no general agreement about the taxonomic rank to assign to them, in particular, regarding the Dipodillus species. In fact, during the last century, this taxon has been regarded as a subgenus [13–15] or as a genus [16–22]. However, Lay [23], studying the most important characters used to separate these subgenus, recognized only one genus “Gerbillus”.
To solve these controversial allocations, the genus Gerbillus was the subject of new molecular studies [24,25]. These investigations allowed an insight in the systematic, the taxonomy and the evolutionary pattern of Gerbillus. Particularly, the mitochondrial DNA analysis [25] confirmed the subdivision into three distinct taxa as previously identified on the basis of morphology [11,12] and revealed that the elevation of Dipodillus to a genus rank will make Gerbillus a paraphyletic genus. On the basis of this analysis, it was concluded that the three taxa Dipodillus, Gerbillus and Hendecapleura must be considered as three distinct subgenera belonging to a unique monophyletic genus [25].
In the present work, we adopted a taxonomic scheme emerging from the molecular investigation and, in order to provide a new insight into the Gerbillus systematics and taxonomy, we used a geometric morphometric approach to investigate the patterns of morphological differentiation among species and subgenera. In fact, the geometric morphometric approach has proved to be a useful technique to investigate morphological similarity due to ecological convergence and to solve taxonomic issues in small mammals, particularly in rodents [26–29]. In order to investigate the morphological differentiation among Gerbillus species and its implication in systematics, we studied skull size and shape differentiation among six species from Tunisia: G. simoni and G. campestris belonging to the subgenus Dipodillus, G. gerbillus, G. tarabuli and G. latastei belonging to the subgenus Gerbillus, and G. nanus belonging to the subgenus Hendecapleura.
2 Material and methods
A total of 148 specimens were analysed, representing six species of the genus Gerbillus from eight localities of Tunisia (Table 1). Samples were unambiguously identified by cytogenetic analysis. Only adult specimens were used in this study. Images of all dorsal and ventral sides of the skulls were digitized using a Nikon D100 camera. Successively, 28 landmarks were collected on the dorsal and 22 on the ventral side (Fig. 1) using the program Tps-Dig2 [30]. The obtained landmark configurations were successfully aligned using the generalized procrustes analysis (GPA) and analysed using the MorphoJ program [31].
List of the specimens included in the analysis; collections are preserved in the laboratory of Animal Ecology “research unit: Ecology and Population Biodiversity”; Faculté des Sciences de Tunis, Tunisia.
| Species | Locality | n | Coordinates |
| G. campestris | Bouhedma | 9 | 34°48’N – 09°65’ E |
| chenini | 4 | 32°54’N – 10°17’E | |
| Djebil | 8 | 33°01’N – 09°03’E | |
| Kondar | 10 | 35°55’N – 10°22’E | |
| G. latastei | Sidi toui | 11 | 32°44’N – 11°17’E |
| Bouhedma | 15 | 34°48’N – 09°65’E | |
| Faouar | 8 | 33°16’N – 08°29’E | |
| G. tarabuli | Dghoumes | 12 | 34°04’N – 08°56’E |
| Faouar | 10 | 33°16’N – 08°29’E | |
| G. simoni | Kerkennah | 24 | 34°42’N – 11°11’E |
| G. gerbillus | Faouar | 20 | 33°16’N – 08°29’E |
| G. nanus | Sidi toui | 14 | 32°41’N – 11°44’E |
| Dghoumes | 4 | 34°04’N – 08°56’E | |
| Bouhedma | 4 | 34°48’N – 09°65’E |
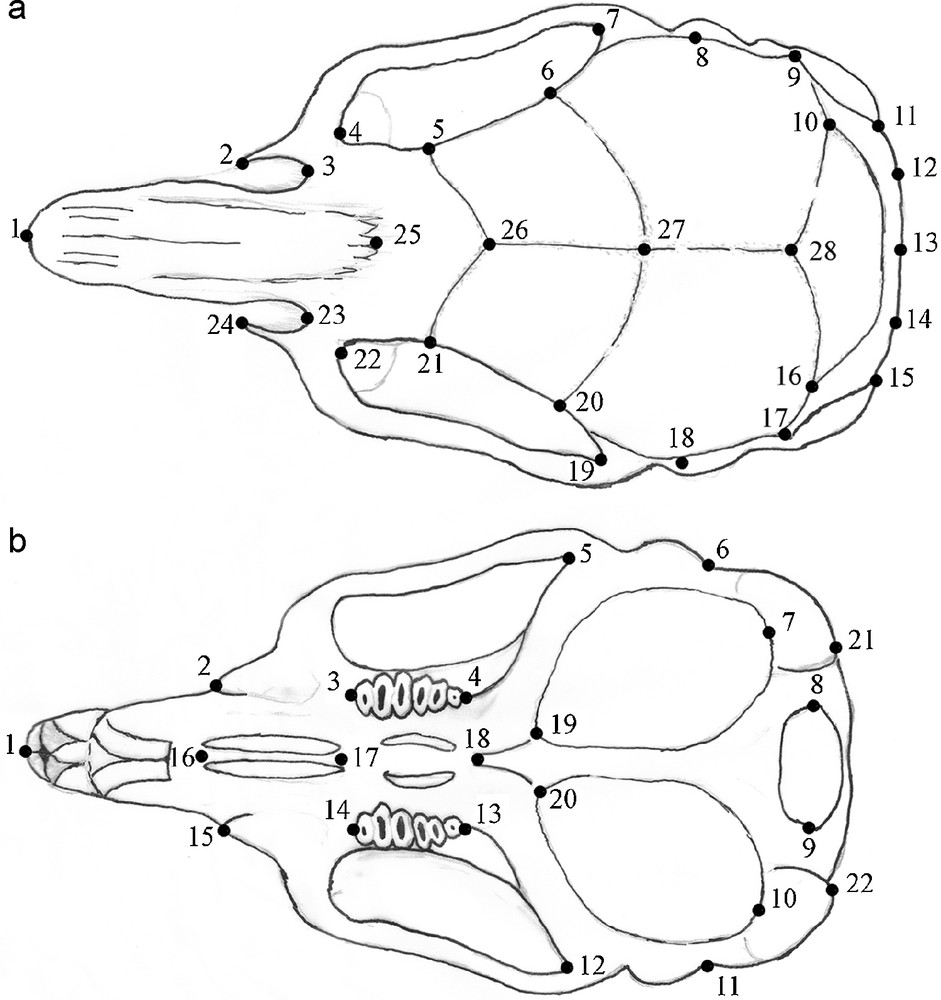
Collected landmarks. Dorsal side (a): tip of the nasal (1), front of the zygomatic plate (2, 24), inferior base of the zygomatic plate (3, 23), posterior edge of the postorbital bar (4, 22), infraorbital constriction (5, 21), frontal-parietal structure (6, 20), back of the zygomatic notch (7, 19), squamosal structure (8, 9, 17, 18), junction of parietal and squamosal and occipital (10, 16), posterior limit of parietal structure (11,15), limit foramina jugular on the posterior edge of auditory bulla (12, 14), occipital tip (13), parietal-temporal suture (28), diagonal intersection of frontal bone (26), frontal-parietal suture (27), nasal-frontal structure (25). Ventral side (b): tip of the nasal (1), inferior margin of infraorbital foramen (2, 15), anterior extremity of molar row (3, 14), posterior extremity of molar row (4, 13), back of zygomatic notch (5, 12), tympanic bulla at the posterior border of the external auditory meatus (6, 11), posterior extremity of tympanic bulla (7, 10), posterior limit of accessory bulla (21, 22), anterior extremity of foramen (16), posterior extremity of foramen (17), junction between tympanic bulla and pterygoid process (19, 20), anterior limit of mesopterygoid fossa (18), posterior intersection between foramen magnum and occipital condyle (8, 9).
Size was computed as the centroid size (CS, the square root of the sum of the square of the distance between landmark and the centroid [32]). Size differences among species were tested by Anova and visualized using a boxplot. The significance of the CS pairwise differences among species was tested through the Tukey HSD test.
Shape differences among species were investigated by a principal component analysis (PCA) and by a canonical variate analysis (CVA). A multivariate analyses of the variance (Manova), computed on principal component scores matrix, was used to test the significance of the observed shape differences. The percentages of correct classifications were calculated using the leave-one-out cross-validation procedure. A regression between shape variables and centroid size was computed in order to investigate the influence of size on shape.
The presence of a significant sexual dimorphism for size and shape was tested by Anova and Manova computed separately for each species. Finally, unweighted pair group method with average (UPGMA) were computed from the interspecific Procrustes distances computed among reference configurations of the dorsal and ventral views. The UPGMA were computed using Mega 4.0 program [33].
All the statistical analyses were performed using the software R version 2.8.1 [34].
3 Results
3.1 Size
A significant size variation (Fsex = 70.856, P < 0.001) is found among species (Fig. 2). A significant sexual dimorphism is absent in all the species with the exception of G. simoni. However, the Anova suggests that sexual dimorphism found in this species did not influence the observed species differences (Fsex*species = 0.977, P = 0.43).
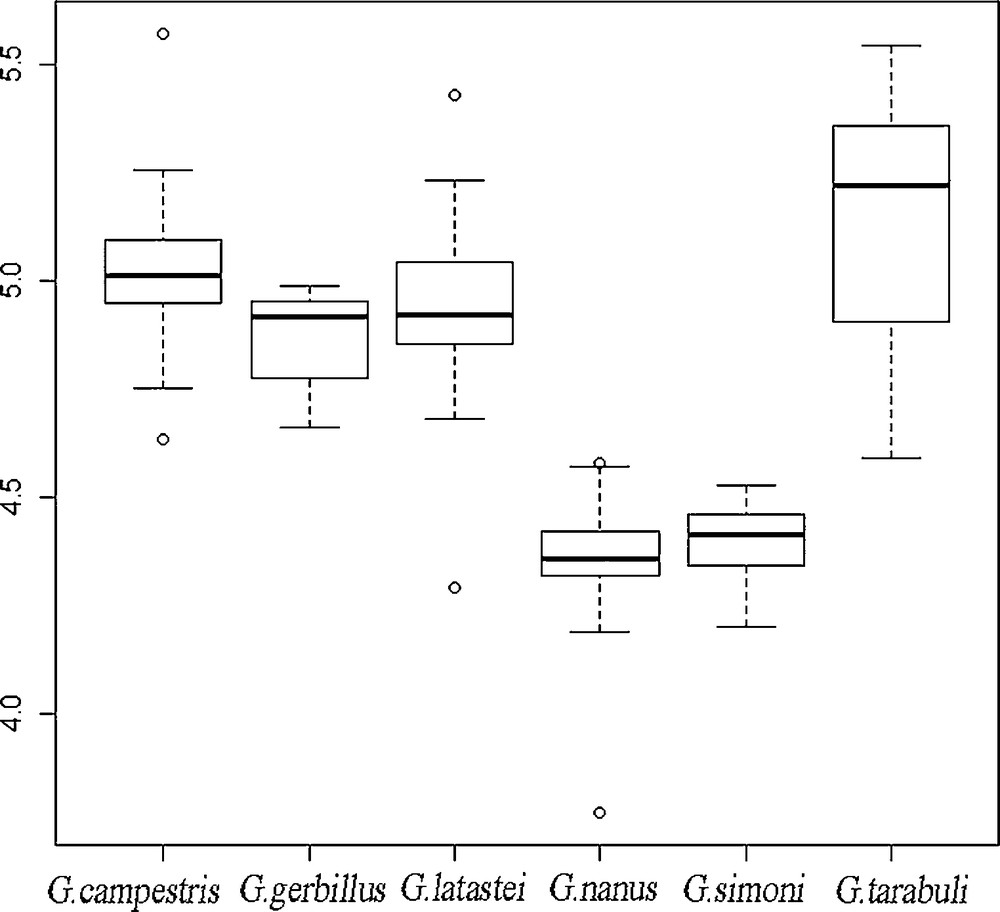
Box plot showing the average of centroid size (based on dorsal configurations) of each species. The inner line represents the median. Box margins are at 25th and 75th percentiles, bars extend to 5th and 95th percentiles, circles represent outliers.
The Tukey HSD test suggests that G. nanus and G. simoni are significantly smaller than all the other species (P < 0.001). G. tarabuli is the largest one (P < 0.001), while G. latastei, G. gerbillus and G. campestris exhibit an intermediate size value. Among these, G. gerbillus shows a significant difference with G. campestris (P = 0.004) while G. latastei exhibits an intermediate size without significant differences.
3.2 Shape
The Manova did not reveal the presence of sexual dimorphism both on dorsal and ventral configurations in all the species. The regression between shape and size shows that a significant component of the shape can be explained by the size both in ventral (P < 0.0001) and dorsal (P < 0.0001) configurations. However, the variation of the shape related to the size in ventral and dorsal views remains low (2.94% and 7.3%, respectively).
The Manova performed on dorsal configuration suggests the presence of significant shape differences between species (Wilks’ λ = 0.001, P < 0.0001). The scatter plot of the first two principal component axis (Fig. 3a) shows a good discrimination of G. campestris, G. simoni and G. nanus. These species are located on the two extreme points of the variation described by PC1 with G. campestris and G. simoni characterized by positive values of PC1 while G. nanus is characterized by negative values. By contrast, G. latastei, G. tarabuli and G. gerbillus show an intermediate position and are located in the same portion of the morphospace. The second PC axis allows only a partial discrimination between G. campestris and G. simoni. The CVA performed on the dorsal shape variables (Fig. 4a) shows a good discrimination of G. campestris, G. simoni and G. nanus. Differently from PCA, G. gerbillus is also discriminated from other species but it still shows a partial overlap with G. tarabuli and G. latastei. By contrast, the two latter species are not discriminated by CVA analysis. These results are confirmed by the cross-validation (Table 2) which shows a high percentage of correct classification for all the species (> 95%) with the only exception of G. tarabuli and G. latastei who respectively show a 36.37% and 26.48% of misclassified specimens. Wireframes obtained for the dorsal view (Fig. 4a) show that G. campestris and G. simoni (positive values of CV1) are characterized by a restriction of the interparietal and of the occipital bones. On the other hand, the remaining species (negative values of CV1) show an opposite trend, with a tendency towards a lateral extension of the parietal bone that is particularly evident in G. nanus. Wireframes associated to the CV2 (Fig. 4) suggest that G. gerbillus and G. simoni share a thinner rostrum while G. campestris, G. tarabuli, G. latastei and G. nanus have a larger and shorter rostrum.

Principal component analyses (PCA) of dorsal (a) and ventral (b) configurations. Symbols represent different species: G. campestris (star), G. simoni (white circle), G. nanus (white triangle), G. gerbillus (cross), G. tarabuli (white square), G. latastei (black circle).
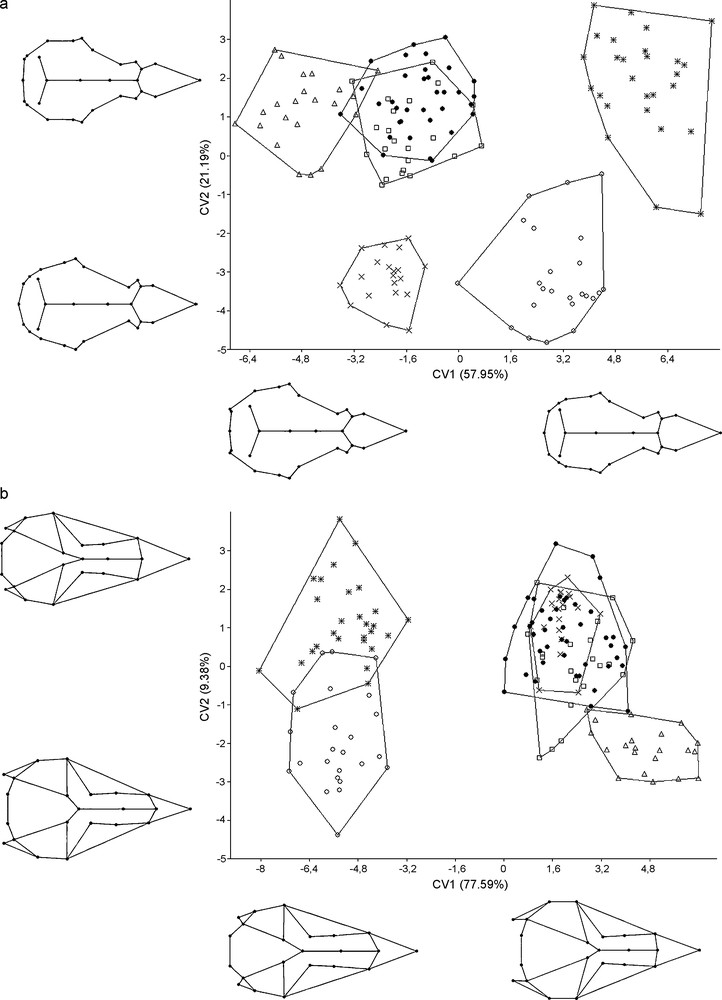
Canonical variate analyses (CVA) of dorsal (a) and ventral (b) configurations. Symbols represent different species: G. campestris (star), G. simoni (white circle), G. nanus (white triangle), G. gerbillus (cross), G. tarabuli (white square), G. latastei (black circle). Shape differences along the CV1 and CV2.
Classification results of the discriminant analyses performed on the dorsal configuration according to the leave-one-out cross-validation procedure. In the diagonal there are the percentages of correct classification for each species.
| G. campestris | G. gerbillus | G. latastei | G. nanus | G. simoni | G. tarabuli | |
| G. campestris | 26 (96.29%) | 0 | 0 | 0 | 1 | 0 |
| G. gerbillus | 0 | 20 (95.23%) | 0 | 0 | 0 | 1 |
| G. latastei | 0 | 1 | 25 (73.52%) | 2 | 0 | 6 |
| G. nanus | 0 | 0 | 1 | 21 (95.45%) | 0 | 0 |
| G. simoni | 1 | 0 | 0 | 0 | 23 (95.83%) | 0 |
| G. tarabuli | 0 | 1 | 7 | 0 | 0 | 14 (63.63%) |
Similarly to the dorsal configuration, the ventral one shows a significant shape difference between the species (Manova: Wilks’ λ=0.0027, F = 12.1, P < 0.0001). However, both PCA and CVA analyses suggest that these shape differences observed within the same subgenus are of a lower magnitude regarding the ventral configuration (Figs. 3 and 4). This is particularly evident for the species of the subgenus Gerbillus. Conversely, a higher phenetic divergence is observed between the subgenus Dipodillus and the subgenera Gerbillus and Hendecapleura. In fact, the PCA (Fig. 3b) performed on the ventral configurations shows that G. campestris and G. simoni are well discriminated from the other species while G. nanus partially overlaps with G. latastei, G. tarabuli and G. gerbillus. The three latter species are completely overlapping. The CVA (Fig. 4b), explaining the 86.97% of the total shape variation, differently from the CVA obtained for the dorsal configuration, does not allow any discrimination between the three taxa. G. nanus is well discriminated by CVA but it is located close to the species belonging to the subgenus Gerbillus. The other two species belonging to the subgenus Dipodillus, i.e. G. simoni and G. campestris, are largely differentiated from the other species but show a higher degree of overlapping if compared to the results obtained from the CVA performed on the dorsal shape variables (Fig. 4a). Cross-validation (Table 3) shows a high percentage of correct classification for G. campestris. The other species show a higher number of misclassified specimens regarding the dorsal configuration with G. tarabuli which is nearly reaching 50% of misclassified individuals (Table 3). The wireframes obtained for the ventral view (Fig. 3b) suggest that the major deformations are associated to the shape of the tympanic bullae and the maxillary bone. The first CV axis describes clearly an opposite tendency in shape characterizing respectively the subgenera Gerbillus and Hendecapleura versus the subgenus Dipodillus. In G. campestris and G. simoni, the auditory bullae are of mediocre development while G. nanus shows a particularly hypertrophic tympanic bullae and a very well developed accessory bulla. The second CV axis describes shape difference in the position of the junction between tympanic bullae and pterygoid process (landmarks 19 and 20), more backward in both G. simoni and G. campestris comparing to the other species (Fig. 4). These two species, according to the wireframes described by CV2, show also a larger palate due to the enlargement of the posterior extremity of the molar row.
Classification results of the discriminant analysis performed on ventral configuration according to the leave-one-out cross-validation procedure. In the diagonal there are the percentages of correct classification for each species.
| G. campestris | G. gerbillus | G. latastei | G. nanus | G. simoni | G. tarabuli | |
| G. campestris | 26 (96.29%) | 0 | 0 | 0 | 1 | 0 |
| G. gerbillus | 0 | 18 (85.71%) | 0 | 1 | 0 | 2 |
| G. latastei | 1 | 2 | 24 (68.57%) | 3 | 1 | 4 |
| G. nanus | 0 | 0 | 1 | 18 (85.71%) | 0 | 2 |
| G. simoni | 4 | 0 | 0 | 0 | 20 (83.33%) | 0 |
| G. tarabuli | 0 | 2 | 8 | 0 | 0 | 12 (54.5%) |
3.3 UPGMA
The UPGMA trees based on Procrustes distances (Fig. 5) show the same topology both for dorsal and ventral views highlighting the occurrence of two clusters reflecting the subgeneric assignment of the species. One cluster includes G. campestris and G. simoni while the other includes the three species belonging to the subgenus Gerbillus, i.e. G. gerbillus, G. tarabuli and G. latastei. G. nanus, the sole species of the subgenus Hendecapleura, shows a phenotypic similarity with the subgenus Gerbillus. According to the PCA and CVA, the UPGMAs suggest that the subgenus Dipodillus has a higher phenetic distance from the other subgenera, particularly in the ventral configuration. On the other side, the Procrustes distances between species belonging to the same subgenus are lower in the ventral configuration with respect to the dorsal one.

UPGMA phenograms based on Procrustes distances for dorsal (a) and ventral (b) configurations of the skull.
4 Discussion
Although skull features were one of the main arguments used to assess the systematics and the taxonomy of the genus Gerbillus, most previous studies regarded only a limited data set of measures mainly related to the dental and bullae morphology [9,20,35–37]. This is the first study which investigated the systematic relationships within the genus Gerbillus by a statistical quantification of the skull shape differences and suggests the study that the magnitude of differentiation among species and subgenera is different in ventral or dorsal configurations of the skull. The latter aspect should indeed be taken into account when morphology is used to assign a taxonomic rank to the different taxa.
Recent molecular data [25] proved that the subgenera Dipodillus and Gerbillus are actually sister taxa which comes across the view of two subgenera or even two different genera. However, according to our results and in particular those obtained for the ventral configuration (Figs. 3, 4 and 5), G. campestris and G. simoni, the two species belonging to the subgenus Dipodillus, appear as the most differentiated. Moreover, we found a close phenotypic similarity between the subgenus Gerbillus and G. nanus (subgenus Hendecapleura). These results contrast with the molecular systematics of Gerbillus [25] and agree with the previous morphological investigations that supported the separation of Dipodillus from the rest of the genus Gerbillus.
The differences observed between morphological and molecular data could be explained by a different rate of phenotypic evolution among subgenera. According to molecular data [25], G. nanus, which is phenotipically closely related to the subgenus Gerbillus, is the most basal species of the genus. This suggests that the subgenus Gerbillus, which is from a molecolar point of view a sister taxon of Dipodillus, retained ancestral morphological features while Dipodillus has got a different phenotypic evolution and shows an apomorphic shape comparing to both the subgenera Gerbillus and Hendecapleura. It is worth mentioning that, even if the dorsal and the ventral configurations analyses have led to similar results, we observed a different degree of differentiation among subgenera when ventral or dorsal configurations are considered. In fact, our analyses suggest that the ventral side of the skull is more different in the subgenus Dipodillus compared to the subgenera Gerbillus and Hendecapleura (Figs. 3b and 4b) while a lower degree of differences in the ventral configuration were observed among species within the same subgenera. On the other hand, when the dorsal configuration is considered, we observed a higher degree of differentiation between species belonging to the same subgenus while, especially in the PCA (Fig. 3a), the subgenus Dipodillus appears less differentiated from the subgenus Gerbillus. These different degrees of interspecific differentiation observed when ventral and dorsal configurations are considered could suggest the action of different selective pressures or functional constrains in the morphological evolution of the skull of Gerbillus species. Notably, the phenotytic diversification observed between Dipodillus and Gerbillus subgenera appears mostly related to the ventral side of the skull and the nature of these modifications should be considered when a higher taxonomic rank for Dipodillus is claimed.
Shapes analyses clearly demonstrated that species belonging to the subgenus Dipodillus show modifications in the shape of the rostrum, in the zygomatic plate and especially in the tympanic bullae and in the accessory bullae. In fact, the tympanic bullae in G. campestris and G. simoni show a mediocre development compared to other species and the posterior extremity of the accessory bullae is reduced. Moreover, a narrow zygomatic length which accentuates the angle between the anterior edge of posterior part of zygomatic arc and the dorsal root of squamosal, was observed in G. campestris and G. simoni. Some of these modifications were suggested to have an adaptive value related to auditory and feeding behaviour [28,37–39]. It has also been suggested that different degree of bullae hypertrophy in gerbils is inversely proportional to the population density [38,40] and that different degrees of hypertrophy could influence the efficiency of con-specific mate recognition [38] and the recognition of predators [40], especially in open habitat. The specimens involved in this study were all trapped in southern Tunisia, which is characterized by an arid bioclimate. In general, all species studied here are known to feed on seeds, insect larva and plants stems growing in the desert all over the year but with different proportionality [38,41,42]. Since all these species occur in similar habitats, the morphological features cannot be easily interpreted in the light of different pattern of trophic adaptations. However, with the exception of G. latastei, all of the subgenera Gerbillus and Hendecapleura have a wide distribution range while G. campestris and G. simoni (subgenus Dipodillus) are restricted to North Africa. Evolution of increased specialization in habitat or resources use occurred frequently in animals and it might be regarded as a trend where species with morphological adaptation to specialized habitat evolved from more generalist ancestors [43]. Thus, in the genus Gerbillus, this fact apparently favours a model of phenotypic evolution likely related to a higher habitat (and possibly trophic) specialization of Dipodillus species versus the Gerbillus and Hendecapleura species. In this view, differential selective pressures in the genus Gerbillus led to a peculiar (apomorphic) phenotype of Dipodillus species that can explain the observed incongruence between morphological and molecular data. Thus, phenetic difference could not be representative of the correct systematic relationships among the three subgenera.
In conclusion, the present geometric morphometrics study based on skull shape allowed to discriminate three morphological groups which are congruent with the three subgenera suggested by molecular analyses and by early morphological classification. Moreover, the skull structure, investigated by geometric morphometric study, may play an important role in taxonomy and could be of enough significance to be a valid identification criterion. Furthermore, we suggest that the integration of different techniques might provide a powerful tool to investigate phenotypic evolution and taxonomic issues in the genus Gerbillus and might help to solve systematic issues due to contrasting results from different sources.
Acknowledgments
This work was supported by a grant from the search unit “Biodiversity and Biology of Population”. We would like to thank Professor Said Nouira, the director of the search unit, for his help and support. We are grateful to the team of “Anatomia Comparata Università la Sapienza, Roma”. We are also grateful to the people that helped to collect specimens, especially Lazar Hamdi the ranger of Bouhedma National Park.


