1 Introduction
One of the important factors influencing zooplankton communities is predation [1] and Cladocerans, which are key elements in freshwater communities, are important prey-items to planctivorous fish [2], Chaoborus larvae [3–5] and Copepods [6,7].
Recent focus on predator-prey interactions in aquatic systems has revealed that chemicals released by a potential predator into the environment may cause a phenotypic response in some morphological characters [8–12], behavior [13–15] and life history [16–18] of prey.
In Daphnia, the body size, crests, and helmets and spines appear to be effective defenses against predators which are detected by kairomones such as fish [19], Notonectids bugs [20–22], Copepods [23] and Chaoborus larvae [3,4,24]. Such changes are presumed in most cases to be adaptive by either enhancing the probability of survival of prey like the induction of vertical migration in the presence of fish chemicals [25–27], or by shifting prey life-histories to maximize fitness [16,17].
Most work has concentrated on examining the effect of predator-released chemicals on morphology and behavior changes. For example, Stibor [16] showed that chemicals released by vertebrate fish and invertebrate Chaoborus predator caused shifts in the life history of the freshwater Cladoceran Daphnia hyalina. He found that animals treated with water, which previously contained fish, have reproduced early at a smaller size and exhibited a greater reproductive investment, compared to control. In addition, he observed that animals reared in water conditioned by a predator Chaoborus showed a delayed maturity with a larger size. Dodson and Havel [22] showed that Daphnia pulex treated with water, which previously contained the invertebrate predator Notonecta undulata, exhibited a shorter development time with smaller body size. Daphnia magna living in North African temporary ponds may have been subjected to a distinct history of selective pressures compared to populations inhabiting permanent ponds. Selective predation pressures over evolutionary time may promote divergence among prey by conferring an advantage to anti-predator adaptations. Major invertebrate predators in seasonal ponds not only include aquatic insects like odonata, notonectids and aquatic beetles [28,29] but also flatworms [30–32]. The present study was designed to compare the effects of predator-released chemicals from both vertebrate Gambusia holbrooki (Baird and Girard) which is known to invade temporary ponds [33] and invertebrate Notonecta glauca Linnaeus predators on life-histories traits of North African Daphnia magna Straus.
2 Material and methodology
D. magna used in this study has been maintained in the culture collection at the Laboratoire de recherche des zones humides (Université d’Annaba) for a number of years. D. magna was isolated from a seasonal pool, Joanonville, and reared in an aquarium containing dechlorinated tap water and fed commercial yeast every other day and daily a mixture of extracts of Beta vulgaris maritima.
The vertebrate predator G. holbrooki sampled from a seasonal pool Berrihane, was reared in an aquarium containing 20 L of dechlorinated and oxygenated tap water and at a density of 1 fish/L. Animals were fed every day commercial fish food. N. glauca was sampled from another pool, reared in oxygenated water at a density of 1 Notonecta/L and fed each day with zooplankton. Half of the water in these aquariums was changed every other day and feces were removed simultaneously.
Laboratory life table experiments were conducted at room temperature. Experiments were started with neonates released from three matured females originated from one clone. Animals were given water with and without chemical substances from the predators G. holbrooki and N. glauca. Animals were reared individually in three different tubes in 20 ml aged tap water with three different treatments: dechlorinated tap water for the control, dechlorinated tap water which has previously contained fish G. holbrooki (second treatment) and dechlorinated tap water that included adult of N. glauca (third treatment). Treatments will be called control for Daphnia treated with tap water without predators, fish treatment for Daphnia treated with Gambusia and invertebrate treatment for those treated with Notonecta. Animals were checked every 24 hours, refreshed and fed daily commercial yeast and extracts of B. vulgaris maritima. Room temperature, which was measured daily at 12 a.m. was about 17.13 ± 0.95 °C. Size (body length) was measured with an ocular micrometer, to the nearest 0.05 mm from the top of the head to the base of the tail spine. The following life history traits were measured: age at first reproduction; size at first reproduction (SFR); brood size from the first broods and size of neonates also from the first broods. Results were compared using a one-way ANOVA [34].
3 Results
Data revealed that individuals of D. magna reared under fish treatment of G. holbrooki reproduced early when compared with control groups, whereas in the invertebrate treatment, animals appeared to extend their age at first reproduction (Fig. 1). Statistics showed a significant effect of fish kairomones but no effect of Notonecta kairomones (Table 1).

Age at first reproduction of Daphnia magna reared under control water, fish Gambusia and invertebrate Notonecta kairomones.
One way ANOVA testing the difference in the age at first reproduction of Daphnia magna in relation to chemical cues from Gambusia and Notonecta waters.
| Source of variance | DF | SS | MS | F Ratio | P | |
| G. holbrooki | Factor | 1 | 18.04 | 18.04 | 12.65 | < 0.01** |
| Error | 51 | 72.75 | 1.43 | |||
| Total | 52 | 90.79 | ||||
| N. glauca | Factor | 1 | 1.58 | 1.58 | 1 | 0.325 |
| Error | 31 | 48.94 | 1.58 | |||
| Total | 32 | 50.52 |
The presence of chemical cues of both Gambusia and Notonecta reduced the SFR of D. magna when compared with control treatment (Fig. 2). From ANOVA table, it can be concluded that fish and invertebrate treatments had a significant effect on SFR of D. magna (Table 2). Brood size in animals treated with fish was larger than control treatment (Fig. 2). In contrast, animals treated with invertebrate show smaller broods than control (Fig. 3) but differences of the two treatments were not significant comparatively to control treatments (Table 3).
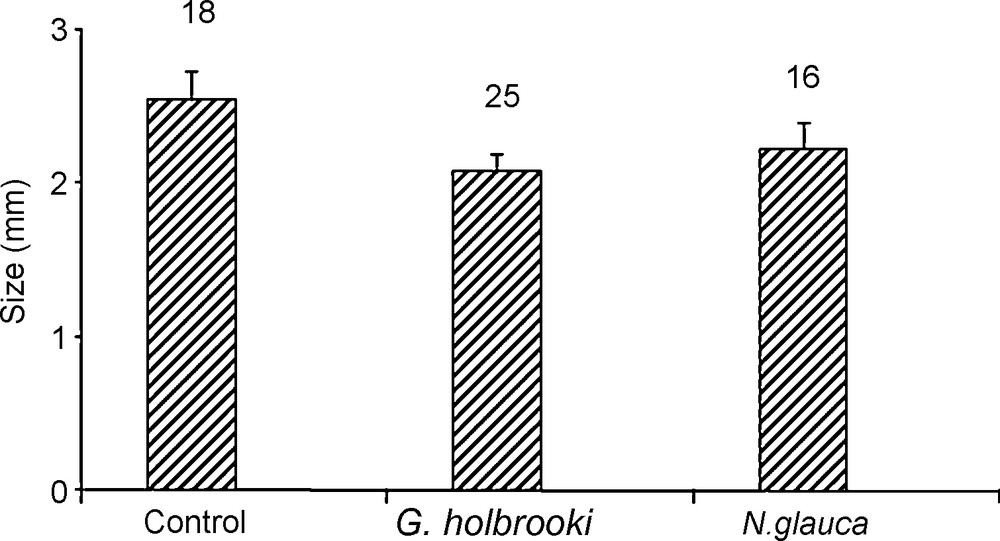
Size at first reproduction of Daphnia magna reared in control water, fish Gambusia and invertebrate Notonecta kairomones.
One way ANOVA testing for the influence of chemical cues from Gambusia (Gambusia water versus control water) and Notonecta (Notonecta water versus control water) on the size at first reproduction of Daphnia magna.
| Source of variance | DF | SS | MS | F Ratio | P | |
| G. holbrooki | Factor | 1 | 2.3271 | 2.3271 | 95.60 | < 0.001*** |
| Error | 41 | 0.9980 | 0.0243 | |||
| Total | 42 | 3.3251 | ||||
| N. glauca | Factor | 1 | 0.8909 | 0.8909 | 25.94 | < 0.001*** |
| Error | 32 | 1.0988 | 0.0343 | |||
| Total | 33 | 1.9897 |

Brood size of Daphnia magna reared in control water, fish Gambusia and invertebrate Notonecta kairomones.
One way ANOVA testing the effect of the presence or absence of Gambusia and Notonecta kairomones on the brood size of Daphnia magna.
| Source of variance | DF | SS | MS | F Ratio | P | |
| G. holbrooki | Factor | 1 | 0.86 | 0.86 | 0.18 | 0.673 |
| Error | 34 | 161.89 | 4.76 | |||
| Total | 35 | 162.75 | ||||
| N. glauca | Factor | 1 | 10.80 | 10.80 | 2.31 | 0.139 |
| Error | 28 | 130.67 | 4.67 | |||
| Total | 29 | 141.47 |
Size of neonates of D. magna treated previously with fish or invertebrate was significantly smaller than control treatments (Fig. 4, Table 4).
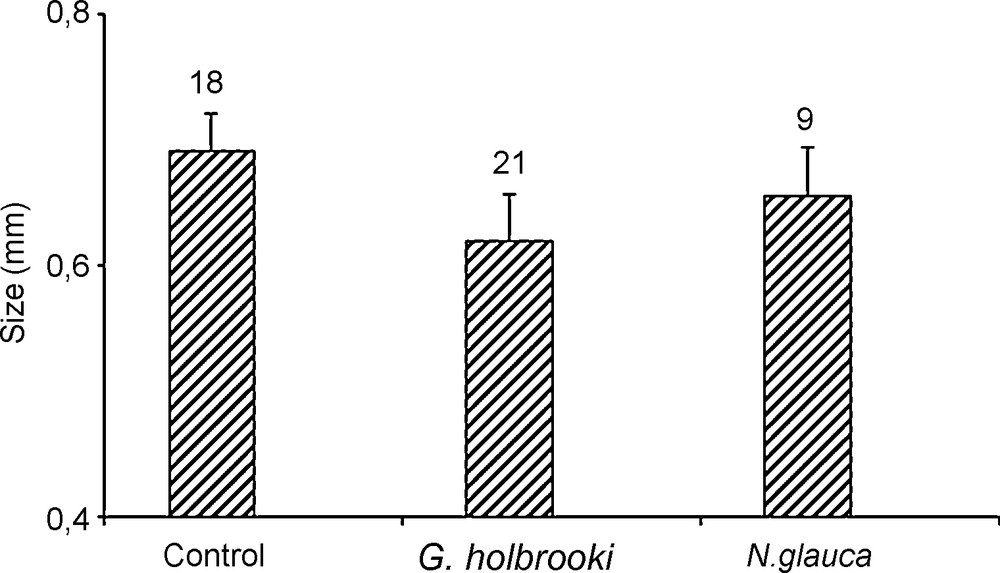
Size of neonates of Daphnia magna reared in control water, fish Gambusia and invertebrate Notonecta kairomones.
One way ANOVA testing the effect of the presence or absence of Gambusia and Notonecta kairomones on the size of neonates of Daphnia magna.
| Source of variance | DF | SS | MS | F ratio | P | |
| G. holbrooki | Factor | 1 | 0.05111 | 0.05111 | 43.34 | < 0.001*** |
| Error | 37 | 0.04363 | 0.00118 | |||
| Total | 38 | 0.09474 | ||||
| N. glauca | Factor | 1 | 0.00782 | 0.00782 | 6.87 | 0.015* |
| Error | 25 | 0.02847 | 0.00114 | |||
| Total | 26 | 0.03629 |
4 Discussion
D. magna exhibited phenotypic plasticity for life history traits in the presence of chemicals released by vertebrate and invertebrate predators. It decreased its age at first reproduction when reared in water conditioned with fish, whereas no similar pattern was detected with the invertebrate predator. The presence of chemical cues of both Gambusia holbrooki and Notonecta glauca had the effect of reducing the SFR and the size of neonates but had no effect on the brood size. With fish predators as Gambusia, Daphnia reproduced early at a smaller size before exceeding a size when it became vulnerable to predators and produced smaller neonates in order to avoid predation for its neonates. With Notonecta, Daphnia did not reduce its age at first reproduction but reached maturity at smaller size and also produced smaller neonates. These changes of behavior or life history traits induced by kairomones are thought to be adaptive [35,36]. Similar effects on the age at first reproduction and size were noted by Stibor [16] in response of Daphnia hyalina to fish kairomones and a delayed age with larger size for those treated by Chaoborus larvae water. The earlier age at maturity with reduced size and smaller neonates of Daphnia magna in response to fish kairomones were also noted by Weider and Pijanowska [19] but with Notonecta, they had noted a delayed maturity with larger size. Results most similar to ours on the effect of fish Perca fluviatilis kairomones on the hybrid Daphnia galeata x hyalina [37] and the effect of Gambusia holbrooki-treated water on Daphnia chevreuxi [17] had been reported. In each case, Daphnia reduced its age at maturity, its size at first reproduction and the size of its neonates.
Investigations of Dodson and Havel [22] have found that Daphnia pulex treated with Notonecta undulata kairomones reduced its size and the size of its neonates, reduced its age at maturity but displayed no effect on its brood size. Daphnia pulex treated with fish Lepomis macrochirus and Notonecta undulata kairomones, reduced its size and the size of its neonates [10]. The two species Daphnia retrocurva and Daphnia galeata mendotae reduced their size in the presence of fish Lepomis. However, with Notonecta undulata and Chaoborus americanus kairomones, they develop a high helmet as a response to a tactile predator [9]. Under size-selective predation, as exemplified by zooplankton and fish, earlier maturation at a reduced size by a zooplankter has an adaptive advantage because it will enhance its chances of successfully reproducing before attaining a size where it became more vulnerable to fish predation [38]. In contrast, a zooplanker's vulnerability to invertebrate predation will be reduced by attaining a larger size or elongated heads and spines especially Chaoborus, a tactile predator which selects smaller sizes than fish [10]. However, we observed a smaller size at first reproduction for animals when reared in water treated with Notonecta. In the case of predation by Notonecta, it is not clear whether increased or decreased body size results in a decreased vulnerability. According to Dodson [13], Grant and Bayly [20], Scott and Murdoch [21], Giller and McNeil [39], Cooper [40] and Reynolds and Geddes [41], Notonecta is a size-selective predator taking the larger size classes of zooplankton. Thus, any decrease in body size, especially of the adults may be advantageous in the presence of Notonecta [10,22]. Therefore, life-history adaptations to the presence of G. holbrooki and N. glauca usually consist of a reduced size at first reproduction and size of neonates. Thus, the probability that an individual will be eaten before reaching maturity and reproducing will decrease. Although flatworms are known to interact with fish [31] and to exhibit a wide range of foraging methods (toxins, mucus pads, active searching) [32], little is known about how Daphnia respond to such selection. Future studies should investigate the influence of flatworm predation on the behavior and life history traits of local Daphnia populations.
5 Conclusion
Vertebrate and invertebrate predator kairomones caused shifts in the morphology and life history of the freshwater Cladocera Daphnia magna. Daphnids cultured in the fish-conditioned water reproduced early at a smaller size and produced smaller neonates, compared to controls. In contrast, Daphnids reared in water treated with Notonecta glauca had no effect on the age at first reproduction but females were also smaller and produced smaller neonates. Thus, North African Daphnia magna, a resident of temporary ponds seems to react in a similar way to populations of permanent habitats but more studies are needed to unravel processes by which local cladocera can coexist with their predators.
Acknowledgements
This research was carried out with the support of the Algerian Ministère de l’Enseignement Supérieur et de la Recherche Scientifique (MESRS) and DSFP, King Saud university (KSA).


