1 Introduction
During the 7th to 4th millennia before our era, European human populations underwent dramatic transformations in many different ways: social, demographic, economical, nutritional and genetic. All these were linked to the adoption of a new way of life, mostly based on food production, formerly introduced to southeastern Europe together with domestic animals and plants by immigrant farmers coming from the Near-East, where this new life-style emerged during the 10th–9th millennia BC [1–4]. From southeastern Europe, the so-called Neolithic package (including farming practices, domestic plants and animals but also ceramics, timber or dry stone houses, the generalization of storage and a strong demographic increase [5]) was disseminated following two main streams, across the continent (Danubian stream) and along the northern coastline of the Mediterranean Sea (Mediterranean stream). But the role of the last hunter-gatherers of Europe in the dissemination and adoption of this Neolithic way of life should not be underestimated, though it remains difficult to assess. By and large, two contrasting views are often debated regarding this specific aspect: (i) a completely foreign-induced, immigration-based Neolithisation process; and (ii) a mostly local process, relying on the adoption and subsequent dissemination of foreign practices as well as domestic plants and animals by the European hunter-gatherers themselves. Recent results obtained by classic archaeozoology and archaeobotany, but also by the development of more recent techniques (palaeogenetics, stable isotope analyses, geometric morphometrics) and of existing data organization and analysis (development of bioarchaeological databases) have provided new bases to assess the likelihood of these two contrasting hypotheses.
2 The last hunter-gatherers of Europe and their role in the establishment of the Neolithic in Europe: what do the margins tell us?
Weighing the impacts of time scale distortions, spatial heterogeneity and archaeological evidence disproportions between the two periods considered is one of the main challenges the archaeology faces regarding the investigation of Mesolithic/Neolithic interactions. These distortions and disproportions are very likely at the basis of the difficulties we have in Europe in highlighting the process by which the local hunter-gatherers either disappeared at the contact of or blended into the Neolithic society. In this respect, it is probably not an accident that the northern and western margins of Europe yielded most of the convincing evidence of interactions [6]. They are the furthermost regions from the area where the Neolithic cultures entered Europe during the 7th/6th millennia BC (southeastern Europe). It stems from this that they are also the latest regions to have been reached by the Neolithisation wave. This had several consequences on the Neolithisation process in these marginal areas: Mesolithic societies survived later there than elsewhere in Europe and the Neolithic impact was somehow buffered after having travelled for several thousands years and over a very long distance. In concrete terms, this could have resulted in a lesser disparity of the size of the populations involved (if we admit the hypothesis of a foreign population-induced process) or of the objects and practices that circulated between the communities (if we admit a hypothesis of acculturation of local populations) and thus of their archaeological perceptibility. Conversely, in central and eastern Europe, the very end of the Mesolithic is difficult to grasp and the general impression is one of abrupt disappearance preceding the arrival of the Neolithic by a few decades or centuries. This situation is likely an illusion and probably results from the near-immediacy of the disappearance of the Mesolithic society as such, once it had been in contact with the incoming Neolithic. The conclusion of this development is that the late Mesolithic immediately preceding the Neolithic appearance is best perceived on the northern and western fringe of Europe.
3 From harvesting the seashore to cattle rearing in northwestern Europe: a glimpse into the Mesolithic way of life and its Neolithic transformation
A large number of sites – among which many shell middens – dating to the late Mesolithic have been excavated along the Atlantic façade of Europe, from Portugal up to Scotland. They harbored large quantities of organic remains and provided an important amount of documents, which allowed a precise insight into late Mesolithic hunting, fishing and gathering strategies as well as into Mesolithic diet. The analyses of this relatively rich documentation revealed that, in some places at least, sites had probably been occupied all the year round, given the seasonal availability of the resources represented (auks, migratory birds, seashells, fishes, fruits) [7]. The diet of these populations was mostly relying on marine resources, as indicated by stable isotope analyses (mostly δ13C and δ15N) carried out on human skeletons in Portugal [8], Brittany [9], Scotland [10] and Ireland [11]. Archaeozoological studies allowed identifying more precisely the resources exploited: most of them are available on the seashore but studies of specific cases also showed that the territory exploited included all the different natural environments present in the vicinity of the sites, from inland woods to marshes, sheltered sandy shores or cliffs [7,12]. The picture is one of broad basis subsistence economy relying on a well-diversified exploitation of the landscape in relatively small-scale territories. With the arrival of the Neolithic – at dates that vary from ca. 5200 cal. BC for Portugal to 5000 cal. BC in Brittany and 4000 cal. BC in Ireland and Britain – there was an important switch of diet toward terrestrial resources, as revealed by stable isotope analyses [7,8,13] (Fig. 1). As indicated by zooarchaeological data available, this switch is very likely linked to the arrival of domestic animals, which replaced in an important proportion the various marine products that were dominating the diet until then [4,7,13–15]. The appearance of domesticates and husbandry practices also had other effects on the Atlantic seaboard: the use of some marine resources for husbandry purposes. A survey of archaeological, historical, and toponymic sources allowed showing the general use of small islands to keep animals since the Neolithic [13,15]. Seaweed used to feed domesticates, a widespread practice in the recent past of northwestern Europe, was also detected in the Neolithic of the Orkney Islands, north of Scotland [16].
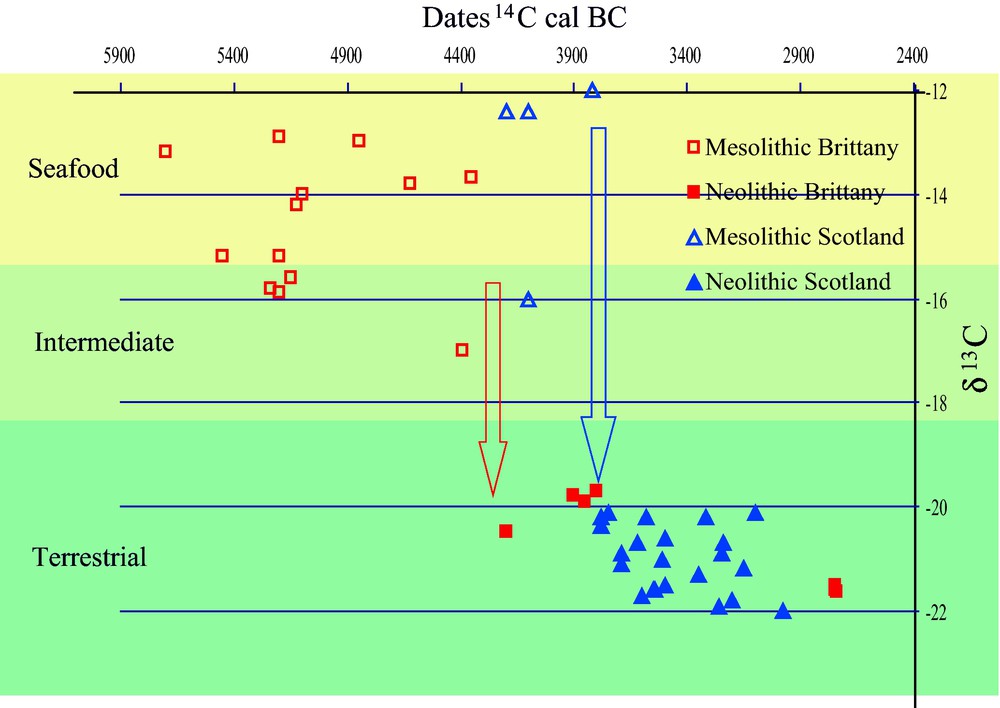
Scatter diagram of stable isotope and 14C dating for a set of Neolithic sites in Brittany and Scotland, as examples of the drastic Early Neolithic diet change at seashore sites on the European Atlantic coast [13].
Evidence from other parts of Europe, as the Iron Gates area for example (lower Danube region), revealed similar diet shifts at the Mesolithic/Neolithic transition. In this region, both zooarchaeological and isotopic data show that Mesolithic diet was relying to a large extent on freshwater fishes, a resource which was nearly abandoned with the transition to the Neolithic [17], during which diets were mostly based on terrestrial – and according to zooarchaeological evidence – domestic resources. More sporadic data collected throughout Europe suggest that the late Mesolithic was relying in most of the territory on a very large diversity of animal resources, including mollusks (marine on the coast, terrestrial snails inland), crustaceans, fishes (marine or freshwater), birds and mammals implying an extensive knowledge of resources and of their location in the landscape. Neolithic diets contrast sharply with this picture: resources exploited appear to have been less diverse, mostly terrestrial and often domestic in a number of regions.
According to archaeozoological and archaeobotanical analyses, the following domestic plants and animals entered the early European Neolithic diet: emmer (Triticum dicoccum) and einkorn (Triticum monococcum) wheats, barley (Hordeum vulgare), pea (Pisum sativum), chick pea (Cicer arietinum), bitter vetch (Vicia ervilia), lentil (Lens culinaris), cattle (Bos taurus), sheep (Ovis aries), goat (Capra hircus) and pig (Sus scrofa domesticus) [18]. Milk consumption, as fermented milk, butter or cheese at least, has been suspected for a long time, as early as the Neolithic [19–21]. It has now been clearly established as present from the start of the Neolithic in certain regions of Europe at least, as age profiles of sheep and goat have evidenced husbandry for milk in the early Neolithic on the Northern Mediterranean shore, from the Near East (ca. 8000 cal BC) to Southern France (ca. 5300 cal BC) [22] and as dairy lipid residues have been found in pottery sherds from the early 6th millennium in the Balkans [23].
A recent reappraisal of the Neolithic animal diet transition at the scale of the Near East and Europe suggested that, beyond the large regional and temporal variability of the scenarios, it mainly resulted in a smoothing of the seasonal variations, in the apparition of new sources of lipids (milk, pig seasonal fat) in spite of the reduction of the diversity of eaten species, and a global increase of food availability [24]. Though it definitely cannot explain alone the origin of Neolithisation, this latter aspect of the diet transition should have interacted with the Neolithic demographic transition in a complex snowball effect, which might have played a driving role in the spread of the Neolithic new way of life to Europe and other continents.
Given the magnitude of the diet shifts observed in Europe with the transition to farming, the following questions become crucial: how was the foreign component important within the European Neolithic population? Was the Neolithisation process mostly relying on a colonization movement from pioneers acquiring and exploiting new territories? What was the origin of the domestic resources that appeared in Europe with the Neolithic?
4 The appearance of domesticates and cultigens in Europe: native or imported?
A key question in this debate is the origin, local or foreign, of domestic plants and animals. It is relatively easy to answer in some specific cases. Sheep and goat, as well as some cereals (emmer wheat and einkorn) and pulses (lentil, pea, chick pea, and bitter vetch) had no wild ancestors in Europe during the Holocene. These species were introduced to South-East Europe at the beginning of the Neolithic and their origin can be traced back to the Near East, or in some cases to western central Asia [25–31]. From these origins, these domesticates and cultigens were then disseminated across Europe, following the Mediterranean and Danubian routes during the 7th to 4th millennia cal. BC (Fig. 2; see [4,24,32]). The question of a possible native status remained open for a number of taxa: barley, the wild form of which (Hordeum spontaneum) existed in the Aegean at the beginning of the Holocene; domestic cattle that stems from the aurochs (Bos primigenius) present in Asia, Europe (with the exception of Ireland) and North Africa at the beginning of the Holocene; domestic pig, the wild form of which (S. scrofa scrofa) was also present in roughly the same area (but including Ireland this time) at the onset of the Holocene. The case of the dog (Canis familiaris) is not envisioned here: its earliest domestication events (from the wolf Canis lupus) are as early as the upper Paleolithic in Europe [2,33] but there might have been later ones in Eurasia and thus the origin of European Neolithic dogs is still debated.
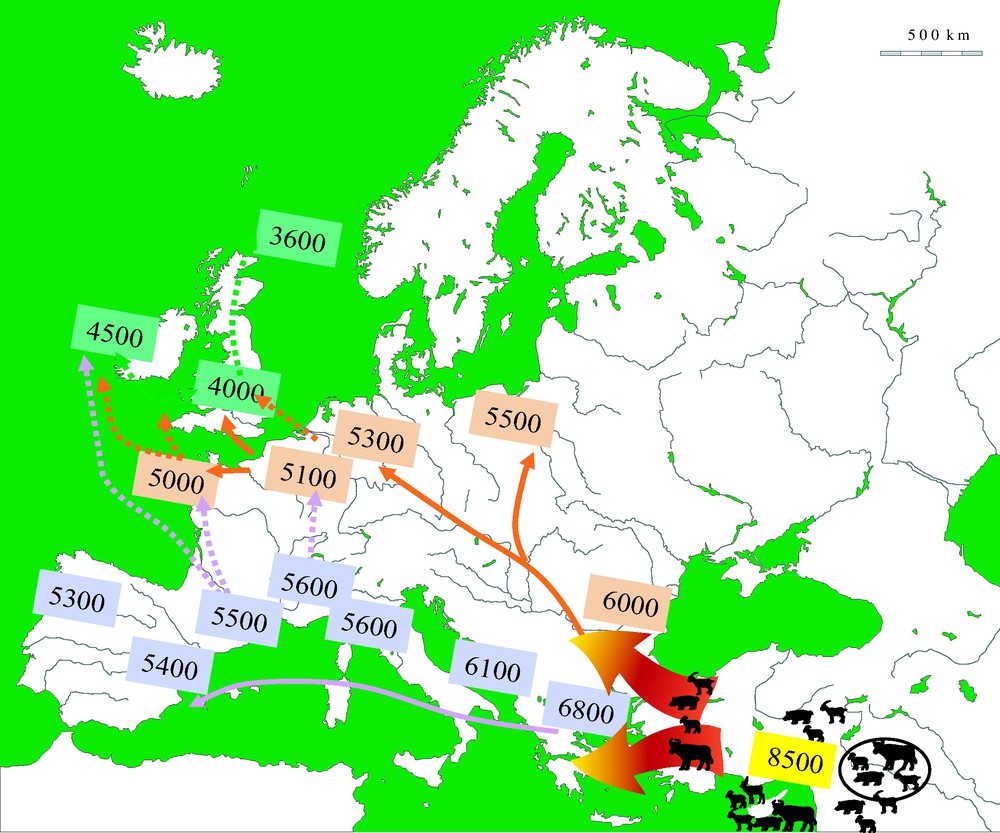
Chronology and main routes of dissemination of domesticates in Europe during the Neolithic [4].
The origin of Neolithic European cattle – imported from the Near-East or domesticated from the European aurochs – has been debated for ages on morphological bases (essentially size and a few cranial criteria [34,35]) but none of the elements taken into account allowed to rule out one hypothesis or the other. However, the analyses of prehistoric bovine mitochondrial ancient DNA (aDNA; control region) from Europe and south-west Asia ([36–39]; [32] for an overview) has confirmed that European domestic cattle belonged to a different haplogroup than European aurochs for maternal lineages, the former being very likely of Near-Eastern origin. A local domestication of aurochs in the late Mesolithic (Ertebølle, 5th millennium cal. BC) of Northern Germany has been suggested by Nobis [40] on the basis of the small size of bovines found at Rosenhof, Schleswig-Holstein. A combination of aDNA and morphometric analyses has recently revealed that these small bovines were females, and very likely undomesticated (aurochs), despite their small size [41]. Thus, even if marginal introgressions of European auroch genes in the cattle domestic lineages cannot be completely ruled out [42], there are strong parallels between the process of appearance of domestic cattle in Europe and the pattern of diffusion of domestic sheep and goat.
The early history of the domestic pig in Europe is rather different. Molecular analysis (mtDNA, control region) of a large set of ancient and modern suid samples from Europe and the southwest Asia showed the presence of very distant haplogroups between 6000 and 4000 cal. BC in Europe [43]. Some of these haplogroups, identified as corresponding to domestic animals on metrical bases, do not stem from the European wild boar, and they are presumably of Near-eastern origin. This part of the history of domestic pigs in Europe is very similar to that observed for sheep and goat, and for cattle as recently highlighted (see above and [32]). However, these exogenous pig haplogroups seem to have been completely wiped out from Europe during the 4th millennium cal. BC and replaced with domestic animals stemming from the European wild boar. This suggests that a European domestication event took place before, or just after, the 4th millennium cal. BC. A Mesolithic legacy cannot be definitely rejected, as a number of late hunter-gatherer societies focused their hunting activities on suid exploitation [44,45], which could have eventually led to “proto-domestications”. But post-Linearbandkeramik1 economic transformations that took place between 4800 and 4000 cal. BC in several regions of central and western Europe could be related to a pig domestication event. In the west of central Europe in particular, cultures such as Rössen and Michelsberg and affiliated groups focused their animal exploitation on pig husbandry [4,14,46]. It seems very likely that the development of pig husbandry and the European domestication of pig are linked. Ongoing research on this subject is now focusing on identifying where in Europe this latter phenomenon emerged.
As for barley (Hordeum vulgare), recent archaeobotanical and molecular studies have clearly established the origin of the domestic form in the Near and Middle East [47–49]. Archaeobotanical data have also evidenced the discontinuity in Aegean stratigraphies between wild Mesolithic barley and the Neolithic domestic form [50], the latter being intrinsically linked to a set of crops (emmer, einkorn, pea, chick pea, bitter vetch…), the Near-eastern origin of which is indisputable.
5 Acculturated European hunter-gatherers or foreign farming pioneers?
Menozzi, Piazza and Cavalli-Sforza's works on human protein polymorphisms and current geographic distribution of human genes (ABO, HLA…) in the 1970’ to early 1990’ have opened the way to a large reflexion on human population turnover in Europe in connexion to the Neolithisation process. In a paper published in 1978 [51], these scientists showed that the modern Near-eastern and European distribution of a set of several tens of genes was reflecting ancient events such as a near-eastern population inflow in Europe, which very likely coincided with the 10,000 year-old Neolithic expansion. Following these seminal works (see also [52–57]), the invention and the generalization of ancient DNA amplification allowed to directly work on ancient human genes distribution. These new data led to rule out the two extreme and opposite scenarios of Neolithisation process: the completely foreign-based process hypothesis and the completely native-based process hypothesis. But the diversity of interpretations given to the sets of data produced [58–62], which goes from an hypothesis of very minor genetic inflow of foreign population to an hypothesis of major input, reveals both the complexity of the phenomenon underlying them and their partial character. The latter stems from the fact that most of the aDNA sequences produced are mitochondrial. Work carried out on nuclear aDNA has been very restricted so far, as it is much more difficult to handle than mt aDNA. This results in the fact that we only have an idea of the matrilineal side of the Neolithisation process, the patrilineal side remaining completely unknown from us. There is no reason to believe that the two sides – female and male – of the Neolithisation history were congruent; there are indeed good reasons to believe that this was not the case. As pointed out by Hurles et al. [63], modern European colonizations often offer contrasting patterns regarding their female and male components: the Oceania cases examined by these authors revealed a major European admixture with indigenous Polynesians when analyzing Y chromosome diversity in modern populations of Polynesia, while mt DNA analyses revealed their strictly Oceanian maternal origin. This of course results from the specificity of the modern European colonization processes, mostly based on the immigration of European men, settling in colonized territories and marrying native women. We cannot prove of course that this scenario can apply to the Neolithisation process of Europe, but this is certainly a hypothesis to consider. If it were the right scenario, this would mean that the real inflow of foreign genes in Europe was much more important than indicated by mt aDNA data. It is hoped that ongoing research on nuclear aDNA provides us with new decisive data on this issue in the near future.
6 Evolution and adaptation of domestic plants, animals and humans to new conditions in Europe in the course of the Neolithic
As well as the earliest transportations of the domesticates out their natural area of distribution within the Near East [33], the importation of domesticates, cultigens and the immigration of humans – to some extent at least – to Europe led to necessary adaptations to local environments and/or to new social practices (e.g. diet changes). In some cases, a genetic determinism of this adaptation can be demonstrated; in others the determinism of the adaptation seems more complex.
One of the most important challenges faced by Neolithic farmers has probably been to adapt the biological cycles of plants and animals and the conditions of their development to European environments. Diets and birth seasons in domestic mammals, and growing conditions and flowering times in cultigens would have been profoundly affected by their transfer to new environments with climatic characteristics, circadian rhythms, vegetation types, and soil qualities very different from the ones that prevailed in the areas where they were first domesticated then already acclimated in the Near East. In particular, difficulties may have been acute when the domestic species were transferred from the South to the North of Europe, which represents a much more rapid climate gradient than the longitudinal one (with the exception of the Atlantic fringe). Today, traditionally bred unimproved ruminants have slightly different birth seasons along a south/north gradient in Europe. In cattle, this lag is essentially due to differences in the seasonal availability of vegetation, which modulates the timing of the reproduction cycle. In sheep, it is due to differences in the seasonal day length at different latitudes of Europe, which strongly drives the reproduction cycle. Such differences have been highlighted in the Neolithic sheep of Western Europe, during the 4th millennium cal. BC, between animals from the Paris Basin and the Orkney Isles [64] using a methodology based on the isotopic ratios of oxygen (δ18O) to trace seasonal cycles within tooth crowns of different individuals. The results suggest that reproduction cycle adjustments had taken place before the 4th millennium cal. BC or just after. A similar methodology – but this time using δ13C to trace diet source changes – has revealed that some of the Neolithic sheep from Orkney had also been fed on marine resources – very likely seaweed – during winter [16]. This constitutes a strong adaptation to the marine environment, and predates by several millennia the oldest known writings on this practice [65]. This major diet shift probably required a number of metabolic adjustments that can be underlain by genetic or epigenetic adaptations, but this still needs to be demonstrated.
Cereal growing probably underwent similar adaptations to local conditions. There are currently in relation to the photoperiod two variants of barley in Europe: a photoperiod responsive and a photoperiod nonresponsive. Their distribution shows a clear pattern along a south northern gradient. The nonresponsive variant is dominant in the north and the responsive variant in the south. It is known that this variation is genetically determined and strongly correlated to a single nucleotide polymorphism (SNP) [66]. Both forms exist in the wild, and allow the flowering time to be driven by or divorced from day length. Recently, it has been suggested on molecular grounds, that the clearly differentiated distribution of these variants in Europe resulted from a differential selection within the plant populations conveyed along the two main routes of Neolithic dissemination after they had both reached Europe [49].
Not only domestic animals and plants, but also microorganisms (namely pathogens) and humans, may have undergone physiological adaptations to new life conditions. As mentioned earlier, dairying was amongst the items introduced by early farmers to southeastern Europe at the start of the Neolithic on this continent and spread to most of Europe following the Neolithic expansion. Adult humans can digest processed milk such as cheese without difficulty, as nearly all lactose molecules have been hydrolyzed down. It is not the case for fresh milk, the digestion of which requires the production of one enzyme – the lactase – that hydrolyses the lactose into two smaller molecules: glucose and galactose that are absorbed and metabolized separately. Lactase production is a characteristic of all mammals during childhood; it disappears with weaning, except in some human populations where it persists at different degrees during adulthood. This characteristic, which results in adult lactose tolerance, is genetically determined and one may wonder whether it was or not present in the Neolithic populations who introduced and conveyed dairying practices in Europe. In the latter case, only cheese and other processed milk preparations involving lactose degradation would have been consumed in significant quantities.
In modern Europe, lactase persistence results from the presence of a T allele of C/T polymorphism at −13,910 bp from the lactase gene [67,68] although other SNPs located at other positions (G/A −22,018; [67]; G/A −13,914, [69]) also seem to be associated to lactase production regulation. It is very likely that lactase persistent variants of these SNPs have spread in the European population quite recently: as quoted above, lactase persistence in adulthood is limited to humans among mammals and it is logically assumed that the mutations responsible for it were positively selected in relation to ruminant husbandry. The modern high frequency of lactase persistence variants in Europe led to several hypotheses related to the early history of dairying development on this continent. One is that the mutation would have been positively selected because it procured an advantage within a population already practicing dairying; the other is that dairying appeared within populations where the lactase persistence variant was already frequent. Both scenarios are possible, though the involved population genetic processes are not completely clear. As mentioned above, nuclear aDNA is difficult to handle. However, in the case of SNPs, the difficulty is reduced as only short DNA fragments are analyzed. For this reason, nuclear aDNA analyses have focused on these elements and only two sets of data have so far been obtained for Mesolithic and Neolithic populations in Europe for SNPs associated with lactase persistence [68,70]. They both show the absence of lactase-persistence variant −13,910 T within the analyzed populations except in one heterozygote (C/T) case from Gotland [67]. The SNP–22,018 −A/G has also been typed in continental populations, which only revealed the presence of the G allele and not the A, associated with lactase persistence [68]. Although these data are limited in number (about 20 specimens in total only have been successfully analyzed), they cover a large territory and essentially concern Mesolithic and the earliest periods of the Neolithic. The fact that the occurrence of SNP variants associated with lactase persistence is near to zero suggests that the ability to digest fresh milk was not among the most common physiological characters of early European farmers at the start of the Neolithic. This particularity probably spread later on across this continent. A north-south decreasing gradient has been observed in the modern distribution of lactase persistence alleles in Europe and this gave rise to various conjectures regarding their spatiotemporal origin. The fact that frequencies are higher in the North suggested that it first occurred at high frequencies in that region. On the basis of the decay of long-range haplotypes in the lactase gene region, estimations of the time when lactase persistence began to significantly increase its frequency among the population have been proposed. This moment has been initially estimated to ca. 10,000 to 5000 years from now [71,72]. This range encompasses the Neolithic, but the hypothesis of an early Neolithic phenomenon is somehow contradicted by aDNA analyses published by Burger et al. [68] and Malmström et al. [70]. More recently, approximate Bayesian computation of flexible demic simulation model using data on the modern frequency of 213,910*T allele and farming arrival dates across Europe suggested that the lactase persistence allele first underwent selection among dairying farmers around 7500 years ago in a region between the central Balkans and central Europe: first represented at a low frequency in the population, the 213,910*T allele would have quickly expanded, possibly in association with the diffusion of the Neolithic Linearbandkeramik culture over Central Europe [73]. Another conclusion of this simulation is that natural selection favoring a lactase persistence allele was not higher in northern latitudes through an increased requirement for dietary vitamin D as previously argued. However, this model is only the most parsimonious one among several other models that may have produced the modern pattern. More aDNA work on later Neolithic and Chalcolithic periods will be necessary to reach a conclusion on this subject.
7 Concluding remarks
At the turn of the second millennia before our era, when Bronze Age civilizations began to rise in Europe, the near totality of the European continent was occupied by farmers, from very dry and hot environments of southern Europe to the wet and cold territories of Scandinavia, from mountain valleys to lakeshores, coastlines and islands. In 5000 years, the Neolithisation process, through a series of importations, adaptations and local recomposition of the Near-Eastern package, had profoundly transformed Europe, its human, animal and vegetal populations, its landscapes and its natural settings in general. Starting from that time, the evolution of the so-called natural environments more and more escaped climatic and ecological forcing to be influenced by human activities, which are depending on human biology, but also on cultural and socioanthropological factors [32,74,75]. In many respects, it was a first step toward globalization, opening the way to later agropastoral colonizations, such as the settlement of Iceland, Greenland and Newfoundland during the Middle Ages and later on, the colonization of Americas by Europeans during the Renaissance period. From this point of view, a better understanding of the natural and cultural processes at work in the Neolithic expansion appears as crucial to take up the challenges of sustainable development that our societies will inescapably face in the near future.
Conflict of interest statement
The authors have declared that no conflict interest exists.
1 Or LBK, this term refers to an early Neolithic culture of central Europe.


