1 Introduction
The examination of the internal anatomy of alcohol-preserved specimens belonging to anatomic and taxonomic collections using magnetic resonance imaging (MRI) is becoming more and more frequent and we can imagine that it will be one of the routine techniques of anatomical studies in the coming years. Numerous works have shown that this technique allows non-invasive and non-destructive acquisition of high-resolution images in intact opaque animals or organs [1–11] and Digital Fish Library (http://www.digitalfishlibrary.org/index.php)]. MRI delivers high contrast and good resolution images for soft tissues and internal anatomy without affecting or destroying the examined specimens and can be conducted on still-in-the-jar specimens [2]. This absence of alteration is very important for rare [1,4,6–8] and endangered species [9,10] and for historical specimens [10] or those belonging to taxonomic medical collections [11]. Nevertheless, this kind of examination requires special care and several parameters need to be taken into consideration before conducting an MRI sequence on a specimen, such as the size of the specimen and the intensity of the magnetic field [2]: an imager with a 1 Tesla magnetic field is not powerful enough to obtain high resolution images on a specimen smaller than 15 cm [2,5]. The aim of the present work is to insist on the methodology and, especially, the influence of metallic objects in order to propose a protocol for MRI acquisitions on dry or alcohol-preserved specimens belonging to anatomic and taxonomic collections.
2 Materials and methods
2.1 Specimens studied
Dead specimens of the Atlantic mackerel (Scomber scombrus (L. 1758), Acanthomorpha, Teleostei, Scombridae) were studied: a fresh specimen (Standard Length: 33.8 cm) caught by Baillet Pêche (F-44/Carquefou) and an alcohol-preserved specimen (Standard Length: 26.9 cm), preserved in the “Collection Pédagogique” of the University of Nantes (Faculté des Sciences et Techniques, Nantes, France) under the reference: UNSCIBA.Z 000974. This specimen was added to the collections in 1970, and has been in alcohol for 40 years. There is no record indicating that the specimen was fixed in formalin, before storage in alcohol.
2.2 Radiography
Specimens were X-rayed, in lateral recumbency; using a Convix 30 Machine with a Universix 120 command at 46 kV and mA at 6.4 for 17 ms. Images were developed using a Fuji FCR 5000. For one of the radiographs, a staple was placed in the mouth, on the tongue, of the fresh specimen.
2.3 Magnetic resonance imaging
The MRI images were acquired using a 1 Tesla superconducting magnet (Harmony, Siemens) with a 30 cm horizontal bore super-conducting magnet. The matrix was 256 × 256. Specimens were kept at room temperature (20 °C) and placed on the scanning table in lateral recumbency for the fresh specimen and in the jar for the alcohol-preserved one. A standard head coil was used. Sagittal localizer series were performed in order to delineate the slices of the images for each pulse sequence series. The acquisitions were performed using turbo spin echo sequences with a 3 or 4 mm slice thickness. Sagittal T1 weighed images (TR = 650 ms and TE = 13 ms) and T2 weighed images (TR = 5300 ms and TE = 105 ms) were recorded of the whole fish. As the specimens were dead animals, no injection of contrast agent, such as gadodiamide [12], could be performed. An MRI sequence was also conducted on the mackerel with a staple on its tongue.
3 Results
The conducted MRI sequences have shown once again [1,2,4] that this technique is interesting for investigating the internal anatomy of alcohol preserved still-in-the-jar specimens (Figs. 1–3). The differences between Figs. 2 and 3 are explained by the different weightings (T1 versus T2) [2]. This kind of examination complements conventional radiographs (Fig. 4) and provides valuable information on soft anatomy of such preserved animals [2,4]. Nevertheless, with the presence of a metallic object, a zone with no signal appears (Fig. 4); the whole head of the mackerel is no longer visible. It is the result of an artefact of magnetic susceptibility: every metallic object causes large distortions in the magnetic field [13,14]. This perturbs locally the normal examination and the acquisition of images of organs located close to this object. A no-signal-zone is then produced with a distortion of the image at the interface zone. Non-ferromagnetic metals–such as lead, gold or platinum, etc.–do not disturb such examinations [13].
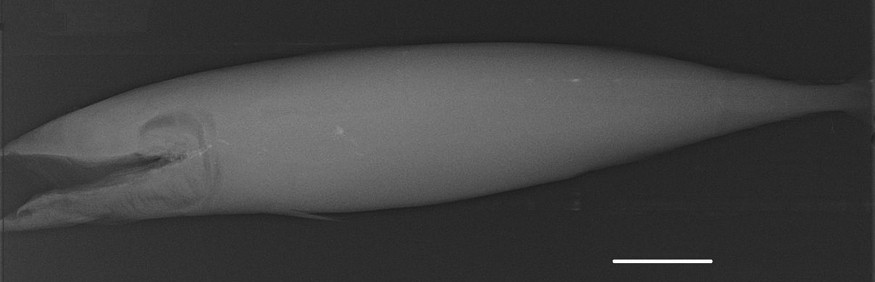
Radiography of an alcohol-preserved Atlantic mackerel (UNSCIBA.Z 000974). Scale bar indicates 5 cm.
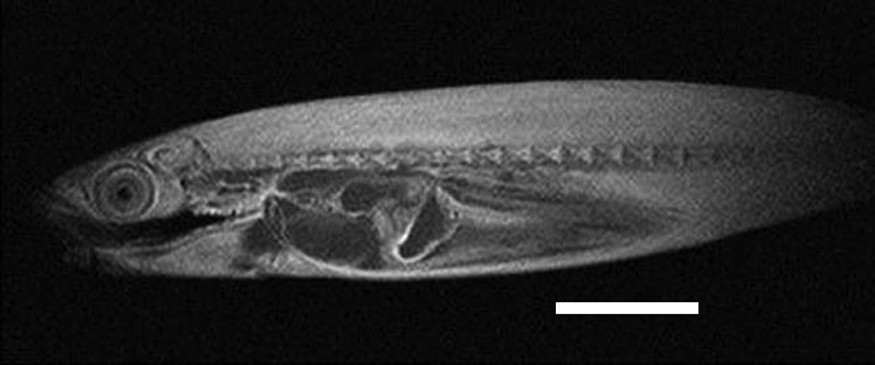
Virtual parasagittal section obtained by MRI (T1 weighted) of an alcohol-preserved Atlantic mackerel (UNSCIBA.Z 000974). Scale bar indicates 5 cm.
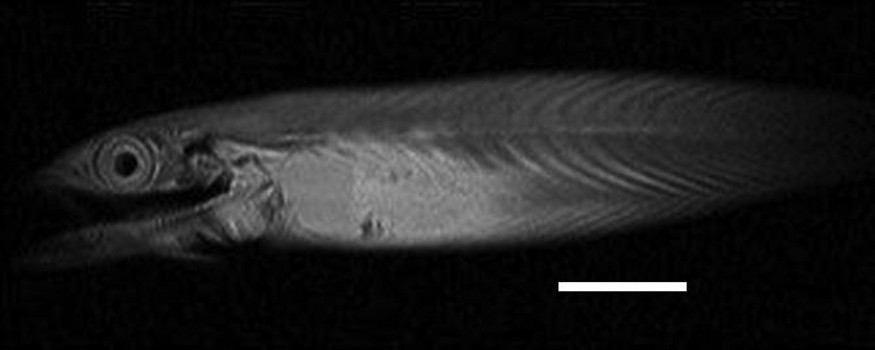
Virtual parasagittal section obtained by MRI (T2 weighted) on of an alcohol-preserved Atlantic mackerel (UNSCIBA.Z 000974). Scale bar indicates 5 cm.
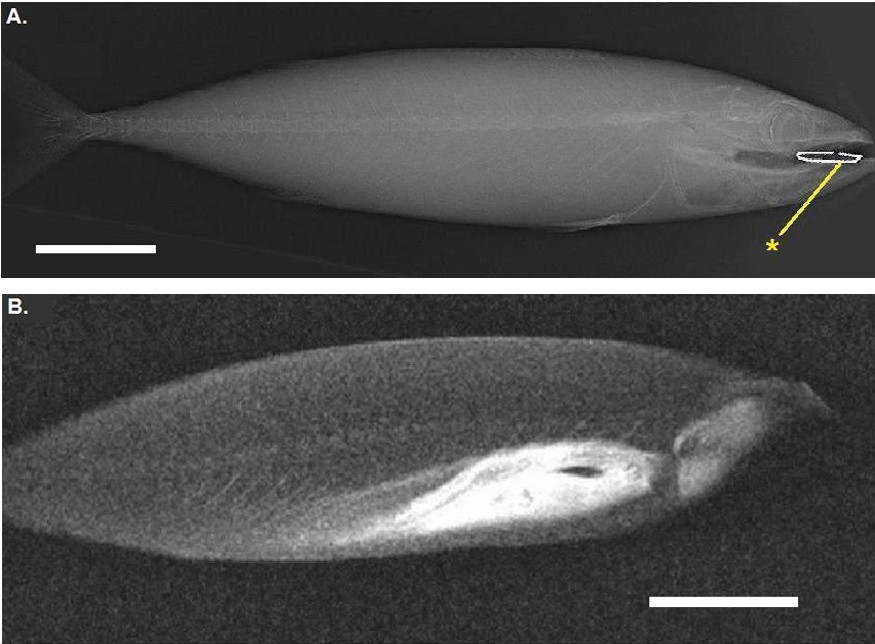
A. Radiography of a fresh Atlantic mackerel with a staple (*) in its mouth. B. Virtual parasagittal section obtained by MRI (T1 weighted) on the same animal. Scale bar indicates 5 cm.
4 Discussion
The present study points out a well-known problem for medical examinations [13,14]; by interferences between magnetic fields, metallic objects disturb the acquisition of high quality images by MRI. Moreover, for many, often old, collection specimens, the exact way of preservation of is often unknown; some metallic objects (as needles, nails or hooks) may be present in specimens or may be on the jar and the labels. They can be attracted by the magnetic field, and then damage, even destroy the specimen, the jar and the MRI equipment. Surprisingly, this point is very rarely quoted or evocated in the literature dedicated to such examinations of collections specimens.
Consequently, regarding both the quality of observation and the preservation of material (specimens and MRI equipment), we would like to suggest a simple protocol for MRI examinations on specimens belonging to anatomic and taxonomic collections. A simple radiograph of the specimens should be carried out before conducting an MRI sequence. Moreover, museums and institutions loaning specimens for such examinations should recommend or impose it both to preserve specimens and specify responsibilities in case of damage. Radiographs will help to choose or prepare a convenient specimen, by avoiding the ones containing hidden or forgotten metallic objects or eliminating these elements. As MRI equipment is becoming more and more powerful, with magnetic fields of several Tesla in a few years, detecting the presence of metallic objects will be quite decisive for high-quality examinations and to preserve materials. Unlike in medicine, the specimens present in the collections are not patients; they cannot answer questions about whether they had been in contact with metallic objects! Thinking of performing radiographs before MRI sequences is only a way to preserve materials, specimens, jars and data.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We are indebted to M. Fusellier, S. Madec and C. Raphael (Unité d’Imagerie Médicale, ENVN-Oniris, Nantes, France) for their technical help in MRI examinations, J. Baudet and A. Lequet (Faculté des Sciences et Techniques, Université de Nantes, Nantes, France) for access to specimens, C. Ozouf-Costaz, A Dettaï and G. Lecointre (Department Systématique et Évolution, MNHN-CNRS-UMR 7138, Paris, France) and C. Cauchie for their advice and encouragement. A first draft of the manuscript has been greatly improved by Ian Nicholson (ENVN-Oniris, Nantes, France).


