1 Introduction
Diet constitutes an important source of water in desert environments. Both food selection with a high water content and renal adaptations are mechanisms for water ingestion and conservation where its availability is limited [1,2]. Rodents are considered highly suitable animals for raising in arid and desert regions. They are able to survive on a dry diet and may go into a state of dehydration for a long period during the dry summer season [3]. These small mammals have developed different combinations of ecological, anatomical, behavioural and physiological adaptation strategies that enable them to survive under these conditions of extreme heat and water stress [4,5]. Several reports have documented that the rodent kidney and colon have developed morphological adaptations, which give them the opportunity to deliver highly concentrated urine [2,6–8]. Activity of both organs is controlled by the hormonal system including vasopressin and aldosterone, which are the main hormones involved [9,10]. Various research programmes have shown that the desert rodents have a high rate of vasopressin and aldosterone, that allows them a great water and electrolyte reabsorption through the renal tubule and gastrointestinal reservoir [3,4,11,12]. Aldosterone, a major mineralocorticoidal hormone, stimulates the transcription of genes encoding Na+/K+ ATPase, sodium channels and increases their numbers at the basolateral membranes and the epithelium of the collecting duct, distal tube and the ascending limb of loop of Henle, thus, minimizing urinary Na+ and water output [13–15].
In rodents adapted to water deprivation, the water economy is the result of the complementary action of vasopressin and aldosterone on the kidney. High levels of plasma aldosterone promote significant Na+ reabsorption in the kidney and a gradient corticopapillar rise, which increases water reabsorption in the presence of a high level of vasopressin [16]. Aldosterone is essentially synthesized in the adrenal zona glomerulosa [17–19], whose cells have the ability to adapt their morphology to environmental conditions [20–22].
Chatelain et al. [20] showed that in dehydrated adult female rats suffering water deprivation for three days, the adrenal zona glomerulosa cells present hyperplasia and a rise in aldosterone secretion. Moreover, from eight days of water deprivation, the plasma aldosterone level increased in Merion shawi. This level increased even more after 30 days [16]. In Gerbillus campestris, the level of plasma aldosterone increased under a dry diet [23]. According Sellami et al. [16], in rodents, the increase level of aldosterone in response to water deprivation seems to be a desert adaptive feature.
Gerbillus tarabuli, a nocturne rodent, inhabits arid and semi-arid localities in Algeria, Tunisia, Morocco, Senegal, Mauritania, Niger and Tchad [24–29]. It is well adapted to water scarcity and presents a large capacity to maintain hydromineral balance under water restriction during a long period [30,31]. G. tarabuli can survive exclusively on a dry diet and be independent of exogenous water. It is marked by a lack of sweat glands, excretion of highly concentrated urine and a significant production of metabolic water [32,33].
The objective of this work is to evaluate the aldosterone level and to study the gerbil adrenal zona glomerulosa morphology in the presence of an important water diet content during seven days.
2 Materials and methods
2.1 Animals
Thirty adult male and female gerbils (G. tarabuli), weighing between 32 and 53 g, from the Algerian Arid Zones Station were maintained under a controlled temperature (25 °C) and relative humidity (20–25%) with a 14 h photoperiod. The animals were given barley and dates ad libitum. Water has not been supplied. Gerbils were acclimatized for 48 h prior to the start of the experiment. Animal manipulations were performed according to the recommendations of the Algerian ethical committee and under the supervision of authorized investigators.
2.2 Experimental design
The animals are housed in large cages equipped with a metal lid and filled in 1/4 with sand from the Beni Abbes region. Each cage contained five adult male and female gerbils. Gerbils were divided into two groups. The first group (n = 15) served as controls. The gerbil controls were given barley grain (100 g containing: 10.4% water; 9.6% protein; 50.3% sugar; 50.3% cellulose; total mineral content: 2.7%) and dry dates (100 g containing: 15% water; 2.5% protein; 69% sugar; total mineral content: 1.5 to 1.8 g; 7 g fiber) ad libitum. The second group (n = 15) was given barley, dates and of fresh lettuce leaves (100 g containing: 95% water; 1.7 g protein; 2% sugar; total mineral content: 2 to 2.6 g: 1% fiber) ad libitum for seven day.
2.3 Body and absolute organ weights
The animals were weighed in the morning of the first day and in the end of experiment using a balance (FKBA-KERN). The adrenal glands were weighed individually with precision balance (Sartorius Talent-Germany).
2.4 Blood sample determination
After light anesthesia with chloroform, the gerbils were scarified by rapid decapitation and their blood, collected in heparin, was analyzed for hematocrit, plasma sodium and potassium (NaK radiometer analyser, Copenhagen, Denmark) and for aldosterone concentrations by standard radioimmunoassay [34] (Diagnostic Products, Los Angeles, CA), the sensitivity of which was approximately 31 pmol/l. The within- and between-assay coefficients of variation were 3% and 8%, respectively.
2.5 Estimation of plasma volume
The estimation of the relative change in plasma volume was estimated by the Van Beaumont et al. [35] formula:
2.6 Urine collection
Urine was collected daily in plastic bottles fitted beneath the metabolic cages. To each bottle, liquid paraffin was added with the object of stopping evaporation. The volume of urine voided by each animal was measured in a measuring cylinder to the nearest 15 ml.
2.7 Histology and ultrastucture
Small pieces of the adrenal gland of each animal from the control and treatment groups were fixed in 10% formal saline solution [36]. After routine processing, paraffin sections of each tissue were cut at 5 μm thicknesses and stained with hematoxylin and eosin for microscopic examination. For fine structural studies, a samples were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH = 7.4) for 2 h at 4 °C, post fixed for 1 h in 1% osmium tetroxide, in 0.1 M phosphate buffer, dehydrated through a graded series of ethanol and embedded in araldite-epon [37]. Selected fragments of the araldite-epon blocks were sectioned with an ultra microtome, and stained with uranyl acetate and lead citrate. Sections were observed by Jeol transmission electron microscope.
2.8 Statistical analysis
The results are presented as mean ± SD. An analysis of the variance and the Student t-test were performed for the comparison of the gerbil control group and the gerbils under the water-rich diet. The differences are considered significant when P < 0.05. We will represent three classes of results:
- • –P < 0.05;
- • –P < 0.01;
- • –P < 0.001.
3 Results
3.1 Food consumption
Under the rich-water diet, the gerbil orients his dietary choice towards the lettuce.
3.2 Body and adrenal weight
All the animals under the water-rich diet lost weight by the end of the experiment, while the control animals gained weight. The adrenal gland did not illustrate any change in their absolute weight compared to control (Table 1).
Body and adrenal gland weight in Gerbillus tarabuli under water-rich diet.
| Treatment group | Body weight (g) | Absolute adrenal gland weight (g) | ||
| Initial weight | Final weight | Left adrenal | Right adrenal | |
| Control (n = 15) | 42.12 | 43.6 | 0.015 ± 0.003 | 0.012 ± 0.03 |
| Water-rich diet (n = 15) | 43.22 | 40.71 | 0.015 ± 0.005a | 0.014 ± 0.003a |
a Difference is not significant (P < 0.05).
3.3 Blood variables
The water rich-diet highly increased the hematocrit, total protein and aldosterone concentrations and decreased plasma volume (–52.8%). Plasma electrolytes (Na+, K+) had non-significant changes in concentration (Table 2).
Hematocrit, total protein, aldosterone, electrolytes concentration and urine volume in Gerbillus tarabuli under water-rich diet.
| Blood parameters | Urine volume (ml/24h) |
|||||
| Hematocrit (%) |
Total Proteins (g/l) |
Aldosterone (pg/ml) |
Electrolytes (mEquiv/L) |
|||
| K+ | Na+ | |||||
| Control (n = 15) | 29.66 ± 3.07 | 51.66 ± 4.16 | 164.78 ± 46.3 | 96.1 ± 1.01 | 152.3 ± 9.68 | 0.66 ± 0.92 |
| Water-rich diet (n = 15) | 47.18 ± 2.45*** | 64.25 ± 4.02*** | 2531.74 ± 100.2*** | 8.12 ± 4.48 | 150.49 ± 8.74 | 11.3 ± 4.34*** |
3.4 Urine volume
The water rich-diet increased urine volume with a highly significant variation (P < 0.01) between the animals on the water addition diet and the control group (Table 2).
3.5 Histological and ultrastructural findings
3.5.1 Control
The adrenal zona glomerulosa cells of the control gerbils are more densely packed, one against the others, with reduced intercellular spaces. They are arranged in clusters or arches surrounded by a network of fenestrated capillaries (Fig. 1). The nuclei varied in size and showed a nucleolus. Numerous lipid droplets with limiting membranes, which were rounded in shape and varying greatly in size, were present in the cytoplasm. Some of these appeared to be confluent. Many spherical mitochondria filled the cytoplasm (Fig. 2). They are located very close to the lipid droplets (Fig. 3).
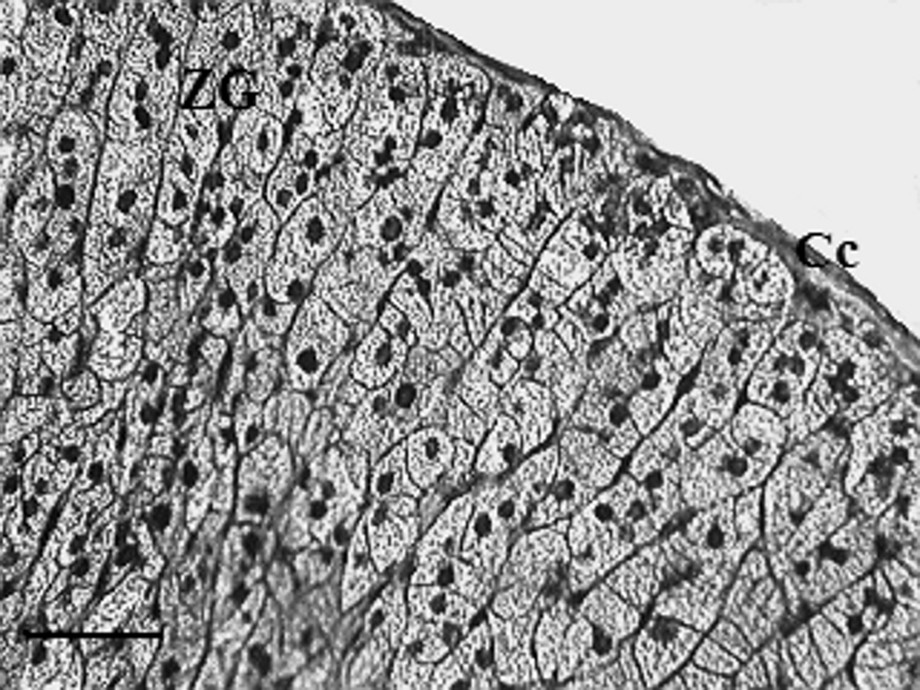
Adrenal zona glomerulosa of control gerbil (scale bar = 100 μm). The zona glomerulosa was constituted of the tight cells arranged in clusters surrounded by a network of fenestrated capillaries. The nucleus (N) was rounded in shape. Cc: capsular; ZG: zona glomerulosa.
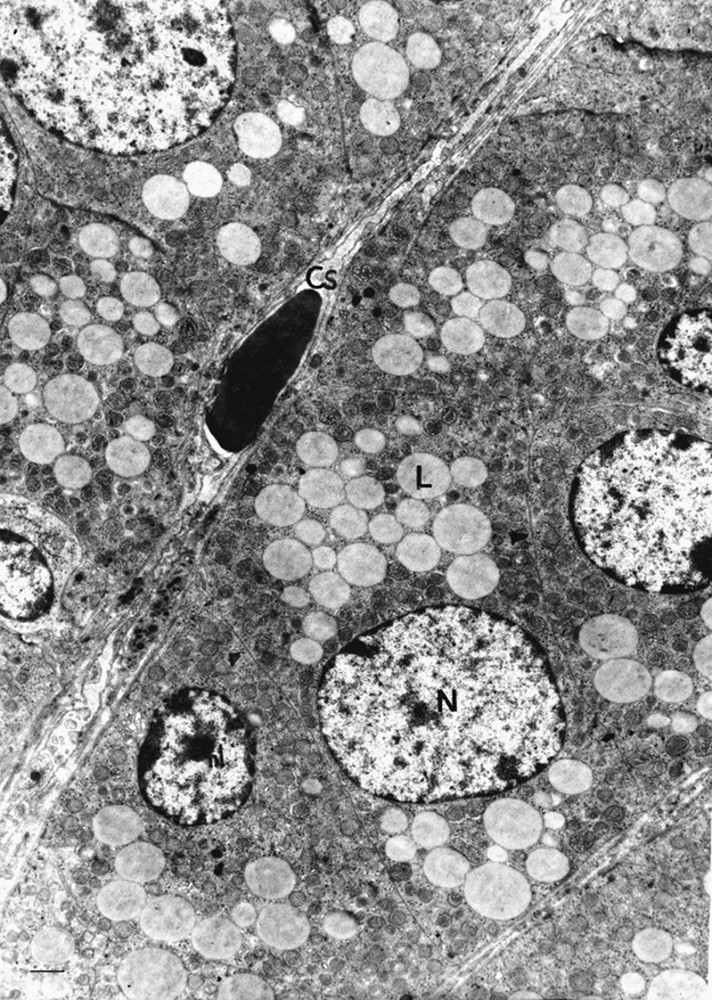
Adrenal zona glomerulosa cells of a control gerbil (scale bar = 10 μm). The cytoplasm contained a great number of lipid droplets (L) and spherical mitochondria (m). Some droplets appear to be confluent (*). The nuclei (N) were varied in size and contained a distinct nucleolus (nu). Chr, Heterochromatin; Ne: nucleus of endothelial cell; Cs: sinusoid capillaries.
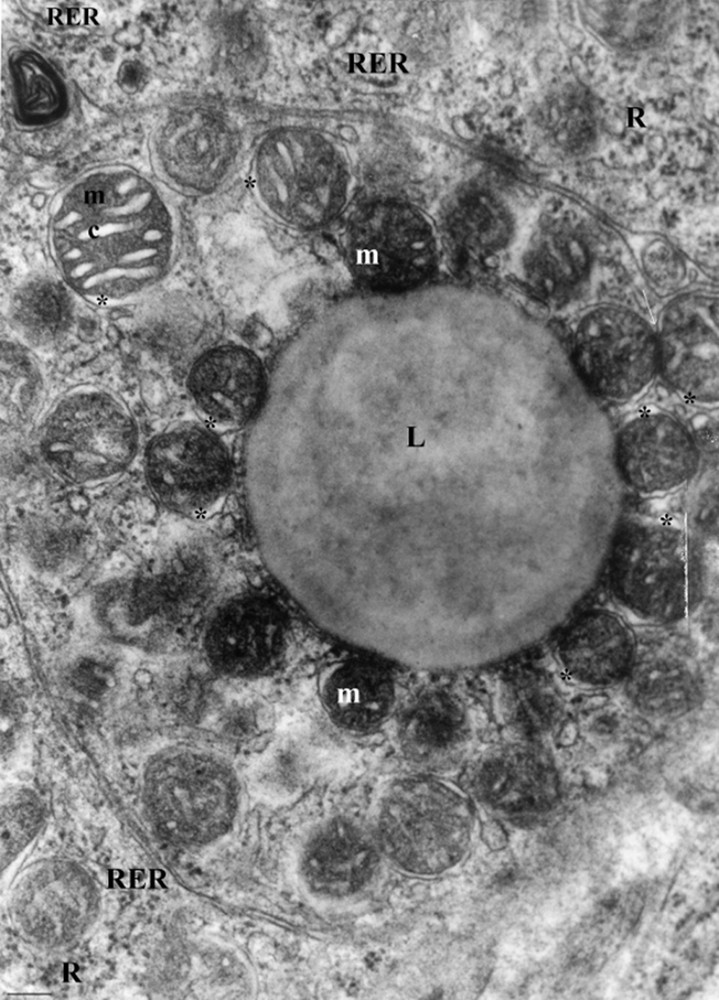
Adrenal zona glomerulosa cells of a control gerbil (scale bar = 100 μm). Numerous spherical mitochondria (m) are located very close to the lipid droplet (L). Cytoplasm is rich with free ribosome (R). *Dilated intermembrane space; RER: rough endoplasmic reticulum elements.
3.5.2 Under rich-water diet
Compared with the control group, the gerbil adrenal cortex and zona glomerulosa maintain their general structure organization under the rich-water diet (Fig. 4). In zona glomerulosa cells, the lipid droplet number decreases in the cytoplasm, with the appearance of coated pits in the cell membrane. The mitochondria, with irregular shape and size, are encircled by the elements of the developed smooth endoplasmic reticulum (Fig. 5). Their crests are tubular and dilated (Fig. 6). The Golgi apparatus shows a hypertrophic appearance (Fig. 7).

Adrenal zona glomerulosa of gerbil under water-rich diet (scale bar = 50 μm). The zona glomerulosa maintain its general structure organization. Cc: capsular; ZG: zona glomerulosa.
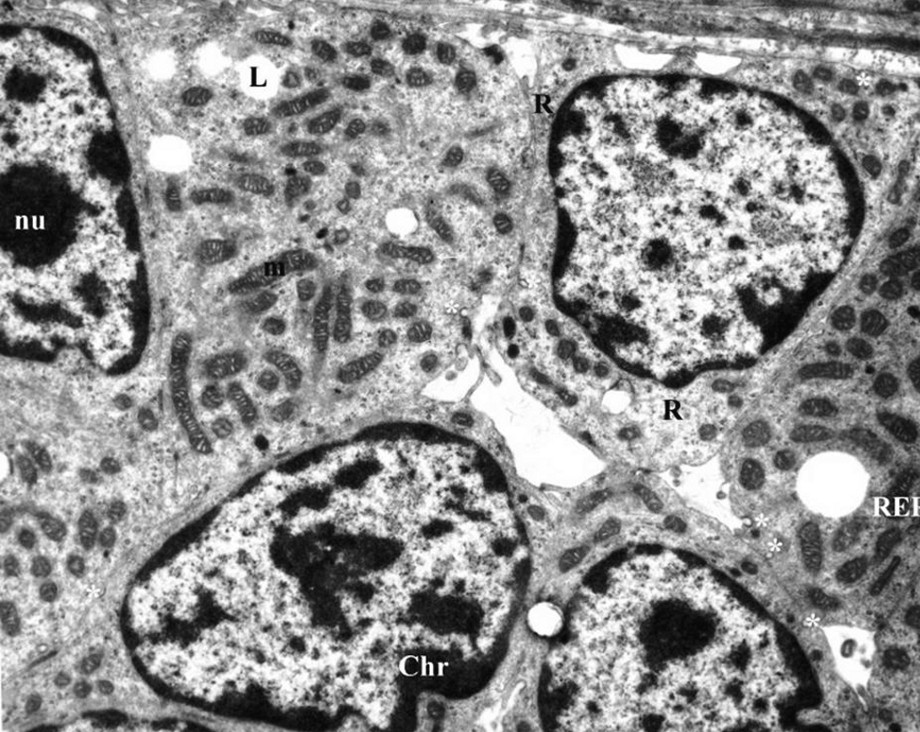
Parts of glomerulosa cells of gerbil under water-rich diet (scale bar = 10 μm). Notice presence of numerous coated pits (*), decreased in number Lipid droplets (L), and numerous mitochondria (m) were varied in shape and size. nu: nucleolus; Chr: chromatine; R: ribosome; RER: rough endoplasmic reticulum.
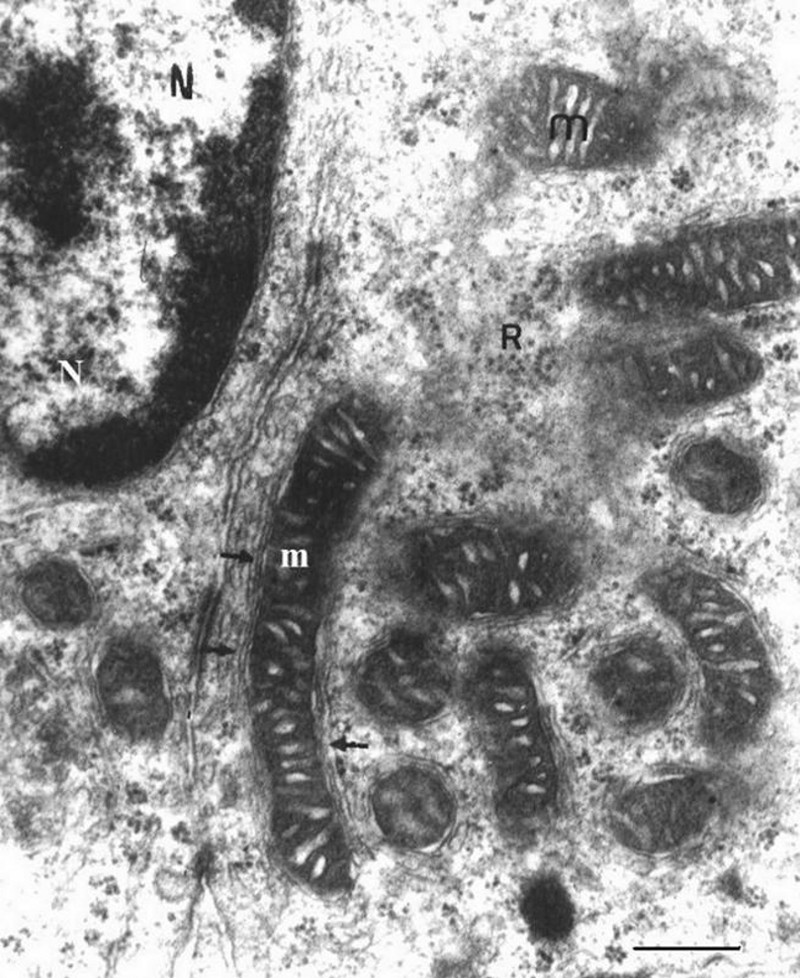
Parts of zona glomerulosa cells of gerbil under water-rich diet (scale bar = 1 μm). Notice the intimate contact of the mitochondria (m) smooth endoplasmic reticulom (arrow) and dilated mitochondria cristae. The cytoplasm was rich in free ribosome (R). N: Nucleus.

Parts of glomerulosa cells of hydrated gerbil (scale bar = 1 μm). Notice development of the Golgi apparatus (Go) with numerous coated vesicles (Vc) and divided lysosomes (arrow). L: lipid droplets; m: mitochondria; C: cristae; REL: smooth endoplasmic reticulum; RER: rough surfaced endoplasmic.
4 Discussion
The resistance of rodents to life in an arid environment implies an ability to reduce water intake and urine elimination [3]. Under a water-rich diet, gerbils showed a loss of body weight and a decrease of about 52.8% of plasma volume. This is probably due to the change of feeding behaviour in the animal. Gerbils consumed the lettuce and avoid barley grains and dates. Similar results recorded in dehydrated rats [20,32,38] and Merion shawi [16] confirmed that water deprivation is a potent stimulus, which modifies the water and electrolyte balance in species not adapted to living in an arid environment. On the other hand, the gerbil is a rodent well adapted to water scarcity [30]. Its plasma volume decrease under the water-rich diet, supposedly caused by the loss of body water, may also be due to a significant increase in gerbil urine output.
The control gerbils gained body weight. These results were expected since a marked increase in feed intake was observed in this group. Sellami et al. [16] have shown that dehydrated rodents increase their consumption of rich-carbohydrate grains to ensure a maximum supply of water metabolism. This behaviour compensates the lack of free water provision [39,40]. The reported increase in hematocrit and total protein under a rich diet is clearly associated with reduced plasma volume and is in favour of hemoconcentration [41]. Total protein concentrations are often used to assess the level of hydration of the animal, with high levels indicating water body loss and volume plasma reduce [42,43]. The plasma aldosterone level is so important under a water-rich diet compared with the control group. These results seem to indicate that the change in plasma volume generated a water stress in the gerbil. In response to this state, aldosterone is released to increase the reabsorption of sodium (in exchange for potassium) in distal segments of the nephron, in the colon, in the salivary and in sweat glands [44,45]. As a result, the concentration of plasma electrolytes (Na+, K+) was not significantly changed.
It is known that the metabolism of water and electrolytes are closely related [16] and are controlled by vasopressin and aldosterone [10]. The vasopressin plays a critical role in regulation of water balance [46,47]. It increased the water permeability of the collecting duct of the nephron, and it also changes the transport of electrolytes, particularly sodium and potassium by increasing recycling of these ions in the renal medulla [9,48]. On the other hand, vasopressin stimulates aldosterone biosynthesis by adrenal zona glomerulosa cells via V1 receptors [49].
It is likely that under the water-rich diet period, the vasopressin is involved during perturbation in plasma volume and urine output and in aldosterone secretion. This hypothesis remains to be confirmed with biochemical assay of vasopressin.
Microscopic examination of adrenal zona glomerulosa cells showed a marked stimulation under the water-rich diet. Cytoplasm lipid compartment regression is probably caused by consumption of cholesterol in the synthesis and secretion of aldosterone. The lipid droplets are the site of cholesterol ester storage [50,51]. Their mobilization and hydrolysis by the cholesterol ester hydrolase enzyme stimulates the biosynthesis of steroid hormones such as aldosterone [52]. The decrease of lipid droplet number is associated with the appearance of coated pits, of many coated vesicles, lysosomes and a developed Golgi. These morphological characteristics seem to constitute the main track of low-density lipoprotein capture through endocytosis via low-density lipoprotein-receptor [53,54].
In zona glomerulosa cells, numerous mitochondria contained abundant dilated tubular crestae and established an intimate contact with the smooth reticulum. This structure gives evidence of enzyme activities [55] and constitutes a hallmark of potent steroidogenesis [56]. The presence of elongated mitochondria and division mitochondria images multiplied reaction surfaces and seemed to reflect proliferation and a high biosynthetic activity.
From the results, it is probably that the excess feed water induced water stress in G. tarabuli, presented by the change of physiological parameters. In response to this perturbation, the adrenal zona glomerulosa is stimulated. Their cells increased aldosterone biosynthesis and secretion in order to maintain a reduced water metabolism and electrolytes concentration adjustment. It seems that the adrenal zona glomerulosa participates actively in the development of anatomical and physiological adaptation strategies in G. tarabuli.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.


