1 Introduction
Ant-plant associations range from simple opportunistic relationships and relationships that are mutually beneficial, to complex, multiple interactions [1,2]. Indeed, ants, which in some cases can be pollinators [3] and can play a major role in seed dispersal [4], are frequently involved in protecting plant leaves and reproductive organs from herbivores and other enemies [5,6]. However, ants may also impose reproductive costs on their host plants when they prey on pollinators or when they damage the plants’ reproductive parts [7–11]. Also, the sap-sucking Hemiptera they exploit may have a detrimental impact on the host plants’ reproductive biology [12]. Thus, the identity of the mutualistic ant species is an important factor positively or negatively influencing plant fitness in obligate ant-plant interactions.
Plant-ants are obligatorily associated with myrmecophytes that provide them with a nesting place (i.e., hollow structures called domatia) and frequently extrafloral nectar (EFN) and/or food bodies (FBs). In return, plant-ants protect their host myrmecophytes from a broad range of herbivores plus competitors and pathogens [5] and/or provide them with nutrients (myrmecotrophy) [13]. There seems to be a continuum in the means by which plant-ants protect their host plants from defoliating insects, ranging from predation to aggressiveness related to an exacerbated territoriality and the absence of predatory behavior when the myrmecophyte provides the ants with protein-rich FBs [14–18]. An intermediary step is when the workers retrieve as food only a part of the insects that they kill while discarding the rest [16,19].
Most myrmecophytic Cecropia (Cecropiaceae) are associated with plant-ants from the genus Azteca (subfamily Dolichoderinae [20–22]). The ants, which protect their host plant from different kinds of enemies (mostly defoliators), are in turn provided with shelter (hollow branches) and food through the glycogen-rich Müllerian bodies produced by the trichilia situated at the base of each leaf petiole, and with lipid- and amino acid-rich pearl bodies produced on the abaxial leaf surfaces [20]. Stable isotope ratios from Costa Rican C. peltata and C. obtusifolia sheltering Azteca spp. colonies showed that, in addition to relying on the food provided by their host Cecropia, Azteca ants likely forage for exogenic food sources [23,24].
In this study, using the myrmecophyte C. obtusa and its two main associate plant-ants, A. ovaticeps and A. alfari, (both are predators that capture insects landing on their host-plant foliage [16]) as focal taxa, we aimed to determine if exogenic food plays an important role for both partners. We hypothesized that prey acquisition favors (1) the development of the colonies, particularly the production of winged sexuals that require proteinaceous food to form alary muscles and (2) the plant's acquisition of nutrients.
2 Materials and methods
2.1 Study site and species characteristics
This study was conducted around the field station at Petit Saut, Sinnamary, French Guiana (05°03′30.0′′N; 52°58′34.6′′W; elevation a.s.l 100 m). The climate is tropical moist with ca. 3500 mm of yearly precipitation distributed over 280 days. The major dry season occurs between July and November, and another shorter, more irregular dry period occurs in March. The maximum monthly temperature averages around 33.5 °C, and the monthly minimum around 20.3 °C.
2.2 Field sampling and experiments
The first field study was conducted in May 2009 in order to quantify the natural abundance of δ15N in C. obtusa leaves according to the presence or absence of ants (as in [23]). We firstly searched for trees not associated with ants (control lot) by conducting several series of observations, including at night, to be sure that they did not shelter ant colonies. We then selected the two closest C. obtusa individuals of a similar size sheltering an A. alfari and an A. ovaticeps colony, respectively. This sampling method was chosen so as to homogenize the local environment (i.e., similar amounts of soil nitrogen) for the three lots. A total of 69 trees, 23 for each of the three lots, from 2.5 to 4 m in height were studied. A ca. 5 cm2 piece of the youngest, most well-developed C. obtusa leaf was harvested from each of the three lots.
A second field study was conducted between June 2009 and January 2010 in a pioneer growth situated at Keren Roch, ca. 400 m from the field station at Petit Saut. At the beginning of this study, four lots of 32 C. obtusa (n = 132) were selected; two lots were composed of trees sheltering an A. ovaticeps colony, the two others sheltered an A. alfari colony. For each Azteca species, we separated the ants into a control lot and an experimental lot. We paired C. obtusa trees of similar sizes (from 2 to 3 m in height) from the control and experimental lots.
The colonies from the two experimental lots were provisioned with surplus “prey” twice a week during 6 months. At the beginning of the experiment, the “prey” consisted of insects captured using a light trap and then frozen until being furnished to the Azteca ants. These “prey” were replaced by canned tuna in water later in the experiment. After that, we provisioned the colonies of the two experimental lots with food artificially enriched with 15N during 1 month. The enriched food consisted of 500 mL of a solution containing 10 g of ammonium nitrate (NH415NO3, 10 atom% 15N, Isotec) and 10 g of ammonium nitrate (15NH4NO3, 10 atom% 15N, Isotec) mixed with honey to make an aqueous honey solution 50% w/v. This artificially-enriched food was kept in a refrigerator at 4 °C during the entire experimental period. Every 2 days, we used a micropipette to place 1 mL of this 15N-enriched food into small plastic cups that we then covered to exclude food-robbing insects. These cups were attached to the trunk of the Cecropia trees at ca. 1.5 m in height. There was no contact between the food contained in the cups and the trunks of the trees. Before supplying each colony with fresh 15N-enriched food, we cleaned the cups, removing any remaining food.
A ca. 5 cm2 piece of the youngest, most well-developed leaf from C. obtusa belonging to each of the four lots was harvested before and after the 4-week-long 15N enrichment experiment. By the end of the experiment, due to the extremely severe dry season, some trees had become unhealthy enough not to be taken into consideration in the δ15N analyses. As a result, we were able to analyze the δ15N for 26 trees from both the control and experiment lots associated with A. ovaticeps, and 28 and 29 trees from the control and experiment lots, respectively, associated with A. alfari.
2.3 Isotopic analysis
All of the leaf samples were cleaned, then vacuum-dried and ground into a homogeneous powder using a mixer mill. Around 1 g of plant samples were analyzed for their δ15N content. Stable isotope analyses were conducted at the Scottish Crop Research Institute (SCRI, Invergowrie, Dundee, DD2 5DA, UK) using a Thermo-Finnigan Deltaplus Advantage gas isotope-ratio mass spectrometer interfaced with a Costech Analytical ECS4010 elemental analyzer. The natural abundances of 15N were calculated as follows:
2.4 Evaluating the size of the colonies
At the end of the second field study, in January 2010, all of the Cecropia trees healthy enough to be analyzed were cut at ca. 20 cm height from the ground (the internodes situated below this height are seldom hollow and do not shelter Azteca ants), permitting the trees to regenerate themselves with the next rainy season. Each tree was cut into three or four pieces and quickly placed into large plastic bags containing 0.5 l of 96% ethanol. We placed a label identifying the tree inside each bag, closed the bag, and then tagged the outside of the bag with the same code. From September 2010 to April 2011, we quantified the size of the ant colony for each of the four lots by opening and carefully washing pieces of the tree trunks with 70° ethanol to collect all of the ant colony members. Workers, queens, winged sexuals, male and female worker nymphs, large last-instar female larvae and other larvae were counted separately for each C. obtusa tree.
Due to the amount of time necessary for us to complete this task (i.e., 8 months), we were unable to properly conserve all of the colonies so that we were only able to conduct censuses on 20 trees associated with A. ovaticeps for both the control and experiment lots and for 21 trees associated with A. alfari.
2.5 Statistical analyses
We compared the number of ant individuals from different categories (total number of sexuals; worker larvae, nymphs and adult individuals) between the control and experimental lots using Student's t-test. The same comparison was made for the ratio between sexuals and workers. Because we hypothesized that the experimental lots would have larger means than the control lots, we used one-tailed P values.
For the δ15N values from the first field study, we used a repeated measures ANOVA (both similarity of variance and normality tests passed) followed by a Newman-Keuls’ post hoc test. For the second field study (15N-enriched food), we used the Kruskal-Wallis test followed by a Dunn's post hoc test (GraphPad Prism 5.02, Inc. software).
3 Results and discussion
3.1 Impact of over-provisioning the colonies on their growth
We noted that both A. ovaticeps and A. alfari colonies relied on the exogenic food we furnished in addition to the Müllerian and pearl bodies supplied by the host plant. Each time we provisioned the colonies, we indeed saw the workers that discovered the prey recruiting numerous nestmates. These numerous individuals then retrieved the pieces of prey or 15N-enriched food.
Accordingly, for Azteca ovaticeps, the numbers of sexuals as well as the quantity of worker brood and adult individuals were significantly higher in the experimental colonies than in the control colonies (Table 1). This was not the case for the A. alfari colonies (Table 1) possibly due to differences in the speed of colony growth for trees of similar sizes and to the influence of seasonality. Indeed, the production of winged sexuals, distributed throughout the entire year, increases slightly during the rainy season, reaching its maximum level in March for A. ovaticeps and in June for A. alfari (AD, pers. obs.).
Mean number (± SE) of each type of individual at the completion of the experiment. Statistical comparisons: Student's t-test and one-tailed P values.
| Azteca ovaticeps | ||||
| Control lot | Experimental lot | Statistical comparisons | ||
| (n = 22) | (n = 20) | |||
| Total sexual brood | 5.59 ± 2.43 | 12.15 ± 6.05 | ||
| Gynes and queens | 1.54 ± 0.37 | 3.5 ± 1.75 | ||
| Males | 0.91 ± 0.55 | 8.3 ± 5.26 | ||
| Total sexuals | 8.04 ± 2.78 | 23.95 ± 9.28 | t = 1.71; df = 40; P = 0.047 | P < 0.05 |
| Worker larvae | 439.0 ± 85.8 | 744.8 ± 97.7 | t = 2.36; df = 40; P = 0.012 | P < 0.05 |
| Worker nymphs | 443.5 ± 56.5 | 876.2 ± 135.7 | t = 3.04; df = 40; P = 0.002 | P < 0.05 |
| Workers | 2375.0 ± 248.6 | 3414.0 ± 466.4 | t = 2.02; df = 40; P = 0.025 | P < 0.05 |
| Total | 3266.0 ± 356.7 | 5059 ± 634.6 | ||
| Ratio: sexuals/workers | 0.003 ± 0.001 | 0.006 ± 0.002 | t = 1.18; df = 40; P = 0.12 | NS |
| Azteca alfari | ||||
| Control lot (n = 21) | Experimental lot (n = 19) | Statistical comparisons | ||
| Total sexual brood | 9.62 ± 3.64 | 10.16 ± 4.13 | ||
| Gynes and queens | 3.24 ± 1.08 | 1.95 ± 0.75 | ||
| Males | 0.24 ± 0.17 | 0.89 ± 0.74 | ||
| Total sexuals | 13.14 ± 4.64 | 13.00 ± 5.01 | NS | |
| Worker larvae | 347.0 ± 74.73 | 312.5 ± 36.8 | NS | |
| Worker nymphs | 225.2 ± 37.47 | 364.8 ± 65.2 | t = 1.902; df = 38; P = 0.032 | P < 0.05 |
| Workers | 1569.0 ± 193.6 | 1462.0 ± 370.8 | NS | |
| Total | 2155.0 ± 266.0 | 2152.0 ± 442.5 | NS | |
| Ratio: sexuals/workers | 0.005 ± 0.0014 | 0.0046 ± 0.001 | NS |
In these monogynous species (only one queen was noted per tree for both A. ovaticeps and A. alfari), our hypothesis that the production of sexuals can be “proportionately” increased by over-provisioning the colonies was not confirmed, even for A. ovaticeps (non-significant differences in the ratios between sexuals and workers; Table 1). This is likely due to the relatively small size of the selected host trees and, consequently, the Azteca colonies. Indeed, many of the colonies were not mature enough to produce sexuals during the experimental period (in total, colonies producing sexuals: 59.5% [n = 42] and 55% [n = 40] for A. ovaticeps and A. alfari, respectively). We chose not to work on larger trees due to the major organizational constraints selecting such trees would have imposed including: (1) conducting the experiment over a much wider area; (2) the need to place larger quantities of wood into plastic bags and then transport them to the laboratory; and (3) finding the appropriate means of conserving the colonies over a very long period of time before completing their censuses.
3.2 Impact of over-provisioning the colonies on the host myrmecophytes
In natural conditions, δ15N values from Costa Rican C. peltata sheltering Azteca sp. colonies resulted in a significantly lower δ15N value for trees sheltering Azteca sp. or even for the ants themselves than for trees without ants [23]. The interpretation of this result is difficult as in this case the exogenic food gathered by Azteca sp. would have a particularly low δ15N value [26]. Yet, it remains possible that the compared trees originated from different areas with different soil composition and different 15N concentrations resulting in a bias. Indeed, in a study on detritivorous animals, differences of up to 7‰ δ15N were found in the litter between different sites; this value then shifted to the entire animal community [27].
In our first field study, also conducted in natural conditions, we showed that the δ15N values from C. obtusa trees sheltering colonies of A. ovaticeps or A. alfari, both predatory [16], were significantly higher than those from the trees without ants (controls) (Fig. 1).
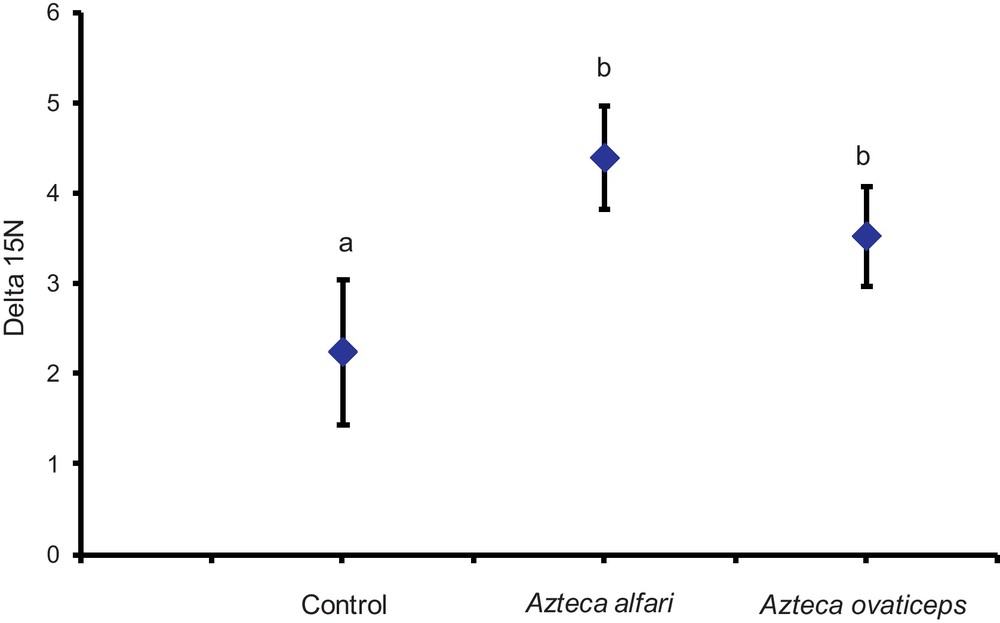
Comparison of the δ15N values (‰) between Cecropia obtusa sheltering a colony of Azteca alfari, A. ovaticeps or unoccupied by ants (control lot). Statistical comparisons (means ± SE); repeated measures ANOVA: F3, 69 = 6.827; P = 0.0026 (the pairing is significantly effective; P < 0.0001); Newman-Keuls post hoc comparison: different letters indicate significant differences at P < 0.05).
The second field study permitted us to experimentally show that Nitrogen passed from the food furnished to both Azteca species to their host trees. Indeed, feeding the A. ovaticeps and A. alfari colonies with 15N-enriched food resulted in a significant increase in the δ15N of their host Cecropia trees; this was not the case for the control lot (Fig. 2).
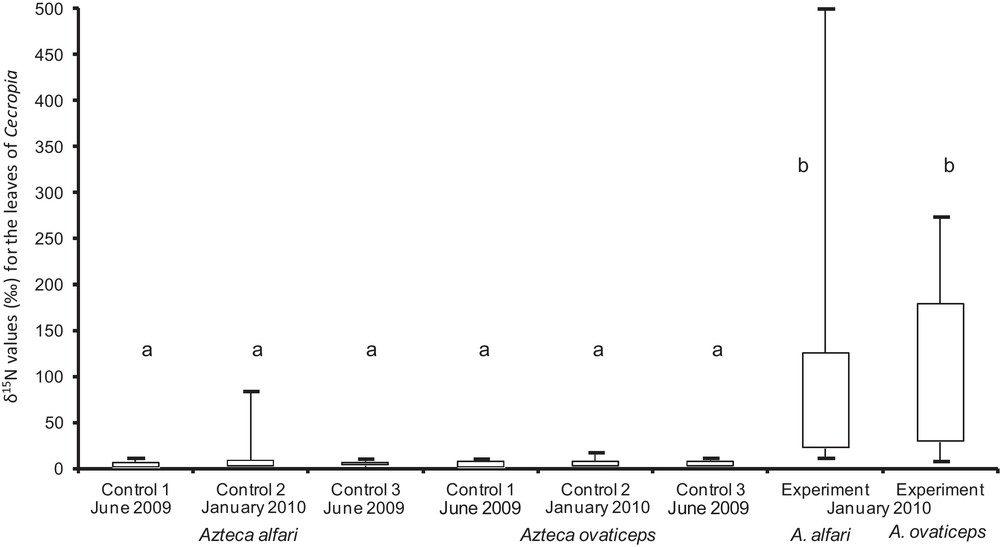
Comparison of the δ15N values (‰) for young Cecropia obtusa leaves before and after the experiment that consisted of providing the guest ants with surplus prey and then 15N-enriched food (for both for A. alfari and A. ovaticeps). ‘Controls 1 and 2’ correspond to the control lot (before and after the experiment). ‘Control 3’ corresponds to the experimental lot before we began providing the ants with food in June 2009. ‘Experiment’ corresponds to the experimental lot at the end of the experiment in January 2010. Statistical comparisons. Kruskal-Wallis test: H6, 107 = 120; P < 0.0001; Dunn's multiple comparison test: different letters indicate significant differences at P < 0.001.
As a result, in the relationship between Cecropia and Azteca, the host plant benefits not only from the well known protection the ants provide it from defoliators and other enemies [16,20,28], but also from nutrients (this study). We therefore demonstrate the existence of a new case of myrmecotrophy this time involving C. obtusa and two Azteca species. Myrmecotrophy is a phenomenon that was first noted for epiphytes. Indeed, certain epiphytes (not to be confused with parasitic plants) compensate the difficulty they have in obtaining nutrients due to the fact that they grow in trees through their association with ants [13,29–35]. Myrmecotrophy was then noted in phanerophytes for which it is recognized as an adaptation to the nutrient-poor, lateritic soils of tropical rainforests [36–39]. In the present study, as shown for both myrmecophytic epiphytes and phanerophytes [33–39], it is likely that 15N passed from the enriched food to the host Cecropia trees via the ants that deposited their clearly visible wastes in certain internodes (that also serve as domatia in myrmecophytic Cecropia).
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We are grateful to Andrea Dejean for proofreading the manuscript, to two referees for their helpful comments and to the staff of the Hydreco Laboratory at the Petit Saut field station for accommodations and technical assistance. Financial support for this study was provided by the Programme Amazonie II of the French CNRS (project 2ID).


