1 Introduction
Ant–plant interactions are very common and this relationship can vary from a facultative non-specific ‘diffuse’ relationship to an obligatory specific and symbiotic association. In diffuse relationships, ants forage for prey on the foliage of plants that attract them with food rewards such as extra-floral nectar (EFN) and/or food bodies (FBs), and in turn are protected against defoliating insects [1,2]. In myrmecophytes, or plants housing ants in hollow structures called domatia, the relationship is strict and necessary to the survival of both partners. In exchange for housing and sometimes EFN and/or FBs, the ants protect their host myrmecophytes from herbivores, competing plants, encroaching vines and fungal pathogens, or supply them with nutrients [2–4].
As EFN is mainly composed of sugary substances, FBs play an essential role in myrmecophyte–ant symbioses as they often contain relatively large amounts of proteins in addition to lipids and/or carbohydrates [1–3,5–7]. Also described on several non-myrmecophytic plants, the importance of FBs was noted in myrmecophytic Acacia, Cecropia, Macaranga and Piper [5–10].
O'Dowd [5], who reported that the elements stored in the FBs' inner cells occupy most of the cytoplasm, classified FBs according to their development and anatomy. They can derive from (1) epidermal tissue and can be single (Piper) or multi-celled, or from (2) sub-epidermal tissue (Cecropia, Macaranga), and they possibly bear an epidermal cell layer (Cecropia).
We examine here the structure of the FBs produced by the Amazonian myrmecophyte Cordia nodosa Lamark (Boraginaceae) [3], and how best to categorize them using O'Dowd's classification scheme [5].
2 Materials and methods
Samplings were conducted in the French Guiana primary rainforest around the field station Les Nouragues (4°5′N, 52°41′W), where the density of C. nodosa individuals varies from 3 to 20 per hectare, with Allomerus octoarticulatus Mayr being the most frequent associated ant species [11,12]. We looked for FBs on 50 C. nodosa individuals 1.5–2.4 m in height (the largest individuals can exceptionally reach 9–10 m) bearing fruit and noted the behaviour of patrolling ants.
After zones of FB production were located, we collected samples of C. nodosa foliage and inflorescences in order to conduct histological studies. Samples for light microscopy were fixed with FAA (formalin, acetic acid, and alcohol), embedded in paraffin and tissue sections were tinted with toluidine blue contrasted with sodium molybdate. The samples for transmission electron microscopy (TME) were fixed with glutaraldehyde and osmic acid, and embedded with Epon. Contrasts were made using uranyl acetate and lead citrate [9].
3 Results
3.1 Food body distribution and production
We noted the presence of FBs in very different places on the plant: ovoid FBs (up to 0.2 mm in length; Fig. 1.1) were found near the veins on the abaxial face of the leaves, and spheroid FBs were found along young stems, the floral axis of the inflorescences and on immature fruits. Their production, although continuous, was very irregular and thus difficult to quantify. Both Allomerus and Azteca workers gathered them whole as is known for all other FBs (i.e. the presence of the extrafloral nectar-like secretion does not engender a particular behaviour).
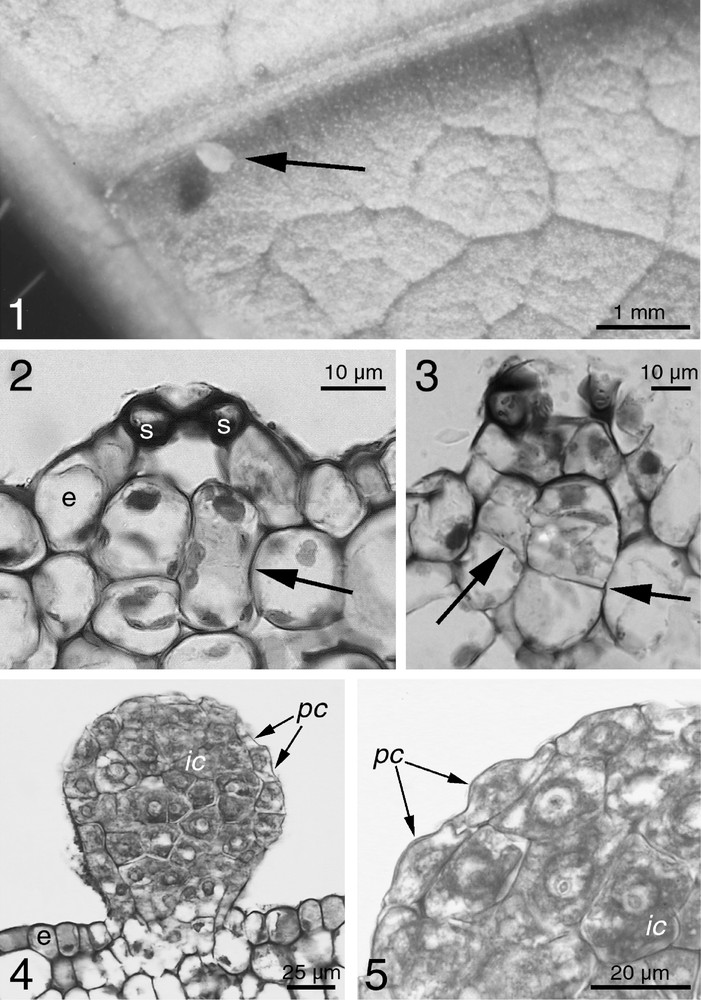
(1) A food body (arrow) on a Cordia nodosa abaxial leaf face. The form is more ovoid than those developing on the stems or the fruits. (2) Light microscopy of the first stage of the emergence of an FB on the stem of C. nodosa. Sub-epidermal cells elongate, beginning to divide (arrow) under a stomata (s: guard cells of the stomata; e: epidermal cell layer). (3) Synthesis of the cell wall between two sub-epidermal cells at the end of division (arrows). The FB begins to emerge. (4) Fully emerged FB on C. nodosa from a young stem. Inner cells (ic) can be distinguished from peripheral cells (pc) flatter and in continuity with the epidermis (e). (5) Close-up of the peripheral cells (pc) and the inner cells (ic) of an FB of C. nodosa. Note the polyhedral form of the inner cells and the flat form of the peripheral cells.
3.2 Food body development and morphology
FB development occurs in a substomatal location (Fig. 1.2). The first stage consists of periclinal divisions in the subepidermic cell layer (Figs. 1.2 and 1.3). While growing, the first cells of the FB fill the substomatal gap and continued cell divisions cause an upward extension of the epidermis, which results in surface bumps (Fig. 1.3). Anticlinal divisions occur when the dividing cell layers emerge. Different division rates between the top and the base of the FB produces the basal neck and the 50-cell-plus FB takes a sub-spherical form (Figs. 1.1 and 1.4). Inner and peripheral cells do not mature in the same way. Inner cells enlarge, take a polyhedral form and have an active nucleus. The cells become flatter and more crenate (Figs. 1.4 and 1.5) towards the peripheral layer that is continuous and can be considered as an epidermis. The guard cells, never observed on the apical zone of the FBs, probably necrotise during FB development. Neither vascular tissue nor transfer cells have been found between the FBs and the underlying vascular tissues.
3.3 Food body ultrastructure
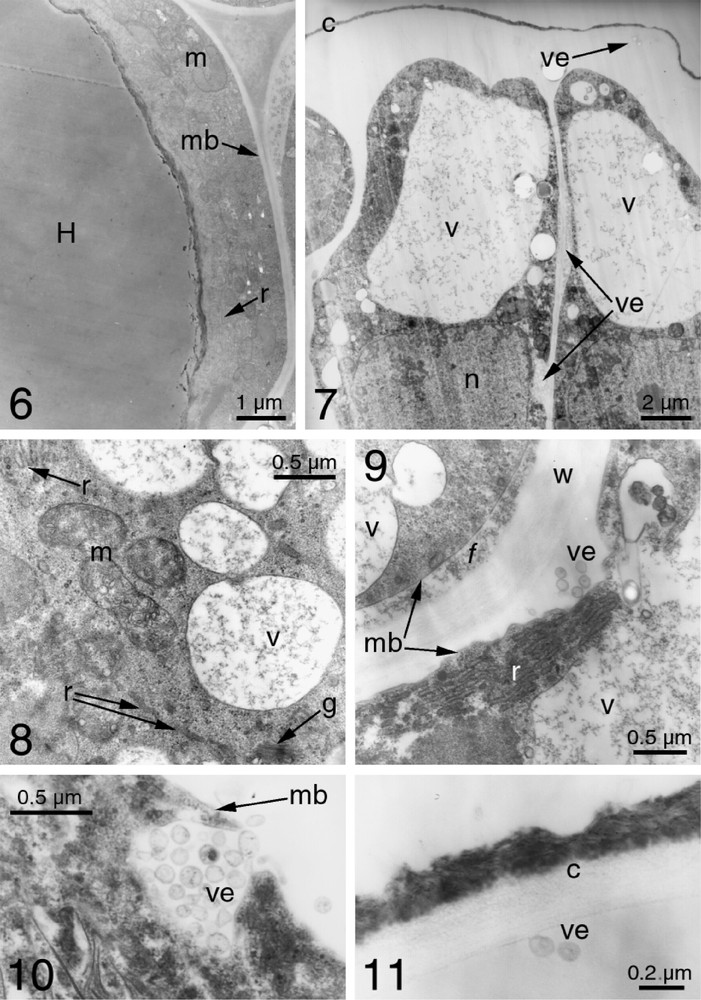
The TEM observation of C. nodosa FBs confirms the differences seen by light microscopy between inner and peripheral cells. The inner cells are characterized by vacuoles occupying most of the cell volume and showing an important mass in the cytoplasm, whose content appears homogeneous (Fig. 1.6). Cytoplasmic activity is reflected in the numerous mitochondria and a lobed nucleus.
The peripheral cells can have globular vacuoles forming a single volume (Fig. 1.7), or a system of numerous vesicles with a reticulate 3D form that could derive from the edges of the endoplasmic reticulum's cisternae (Fig. 1.8). Their content is always light and fleecy. There is a high level of synthesis because the cytoplasm of the peripheral cells have numerous mitochondria, dictyosomes, and a well-developed endoplasmic reticulum (Figs. 1.8 and 1.9). Secretory activity is illustrated through small vesicles (less than 0.1 μm) that form outside the cells, whose content originates in the vacuoles (Figs. 1.7, 1.8 and 1.10). They are expelled in groups and can evolve in two ways. Some of them migrate through the apoplasm to the surface of the FB (Fig. 1.11). Others seem to burst between the membrane and the cell wall and pour their fleecy content into the apoplasm. The released substances can passively spread to the surface of the FBs, whose cuticle has two distinct layers (Fig. 1.11). The inner layer, the cuticle itself, is light and homogeneous, while the dark, heterogeneous and irregular external layer probably represents the dried accumulation of the secreted substance.
4 Discussion
FBs are produced on all the young parts of C. nodosa individuals, including the inflorescences, as described for Ampelopsis [5] and some Macaranga species [8]. This differs therefore from that of myrmecophytes, whose FBs are produced in specific zones or organs, such as the trichilia, situated at the base of the leaf petioles of Cecropia, with FB production limited to the afternoon [9,10], the stipules or sheathing leaf bases of Piper [13], the leaflets of Acacia [6], or both the leaves and stipules in other Macaranga species [8]. Because FB production is unpredictable, limited neither to a particular part of the plant nor time of day, the plant induces its associated ants to continually patrol its foliage, inflorescences and even immature fruits.
Unlike for Cecropia peltata or C. obtusa, the original guard cells do not remain on the top of C. nodosa FBs [9,10] nor do the stomata remain in most FBs [5]. Most of the cytoplasm of the inner cells of C. nodosa FBs is occupied by stored elements similar to those of Acacia or Macaranga that contain lipids [2,6,8], but further analysis will determine which types of metabolites are stored in these inner cells. All the stages in the secretory activity of the peripheral cells are similar to typical nectar synthesis, progression and secretion in the peripheral cells of extrafloral nectaries (EFNs): a pre-nectar is temporarily accumulated in the vacuoles; then, during nectar secretion, the endoplasmic reticulum is very abundant, vesicles form then fuse with the plasmalemma and discharge the nectar outside the secretory cells by exocytosis [14], where it accumulates and becomes available for ants. The similarity between C. nodosa FBs (i.e., numerous vesicles; single-volume vacuoles absent) and EFN vesicles is great as vacuoles are not prominent in nectar-secreting cells [15]. As a result, peripheral cells likely secrete sugary substances, but the secretion of lipids cannot be excluded as this occurs frequently in secretory trichomes. Due to the cellular structure of FBs some proteins are also present, but they cannot satisfy the needs of the ant colonies. In this case the plant fuels ant foraging activity through its energy-rich FBs, and receives biotic protection [16], mostly through the predatory behaviour of associated ants. A. octoarticulatus, the ant most frequently associated with C. nodosa, is a remarkable group hunting species [12,17]; however, contrary to what was first thought, A. octoarticulatus colonies do not provide their host C. nodosa with nutrients [4].
By their anatomy and development, C. nodosa FBs belong to the second type defined by O'Dowd [5]: of sub-epidermal origin, they are multicelled, have a continuous external cell layer, a sub-spherical form and a pedicel. Nevertheless, C. nodosa FBs differ from all other FBs. Their structure, noted for the first time to our knowledge, permits two types of activity: the intracellular storage of metabolites in inner cells, a typical FB role, and a nectary-like secretion by peripheral cells. EFNs are considered as more archaic structures than FBs, so that in the genus Macaranga a ‘graded myrmecophily’ was characterized by the relative presence of EFNs and FBs among the species [8,18]. The origins of EFNs and FBs are similar. For example, patches of glandular trichomes in Leguminosae could be primitive homologues of more organized EFNs [19,20], while FBs originate in the trichomes [5,10].
In conclusion, C. nodosa belongs therefore to the restricted group of myrmecophytes that, in addition to housing ants, furnishes them with food in the form of FBs. Their dual production, likely sugary substances secreted by peripheral cells and lipids stored by inner cells, could indicate that these FBs have conserved an archaic character from secretory trichomes while acquiring inner storage cells, or that in general FBs and EFNs are not only two complementary structures, but perhaps also related. This production induces workers of associate ant species to forage on all parts of the host plant and fuels them with energetic food, while they hunt for the prey to satisfy their nitrogen requirements. Consequently, they protect their host plant, particularly the young, most vulnerable parts where FBs are produced.
Acknowledgements
We are grateful to Andrea Dejean for proofreading the manuscript. This work was supported by grants from the ‘Conseil régional de Guyane’ and by the French ‘Ministère de l’Écologie et du Développement durable' (SOFT program, research agreement GIF ECOFOR No. 98, and the ‘Tropical Ecosystems’ program No. 02-E2002).


