1 Introduction
The potential input of molecular phylogenies to modern taxonomy is considerable [2–5], to the extent that a DNA-based approach to taxonomy is being envisaged [6–8]. It is arguable that molecular phylogenies should provide the basis to taxonomy in the cases where conflicts or uncertainty persist from classifications based on morphology, morpho-anatomy, and other phenotypic characters. Unlike molecular phylogenies, traditional taxonomy based on morphological characters can for instance be misled by phenotypic plasticity, morphological convergence, and arbitrary character weighting [8,9].
In the last 130 years, up to 281 nominal species and 43 nominal genera (Table 1) have been proposed for the Mugilidae [11]. The first thorough taxonomic revision of the Mugilidae was produced by Schultz [38], who mainly used mouth anatomy to define species and genera. Schultz [38] validated only ten previously defined mugilid genera and described three new ones, a revision that was subsequently questioned (review in [43]). The taxonomy and nomenclature of Mugilidae have still not been finalized [44], with between 14 and 20 genera being recognized as valid according to the most recent revisions [11,12,45]. The Integrated Taxonomic Information System (http://www.itis.gov/; information retrieved on 16 August 2011) recognizes 16 valid genera, while Eschmeyer and Fricke [13] list 20 valid genera. Two genera, Liza and Mugil, currently represent 40% of the species richness of the family Mugilidae [13]. While the taxonomy and nomenclature of species in the genus Mugil are mostly stable, those in the genus Liza have undergone changes since [38] (Table 1), reflecting disagreement among authors regarding the taxonomic placement of some of the species currently under this genus.
Nominal genera of the Mugilidae in chronological order of appearance, with their status according to Senou [10], Thomson [11], Ghasemzadeh [12], Eschmeyer and Fricke [13] and this study.
| Genus | Author and date | Ref. | Type species | Genus assigned by author (date) | ||||
| Senou (1988) | Thomson (1997) | Ghasemzadeh (1998) | Eschmeyer and Fricke (2011) | This study | ||||
| Mugil | Linnaeus 1758 | [14] | Mugil cephalus Linnaeus 1758 | Mugil | Mugil | Mugil | Mugil | Mugil |
| Chelon | Artedi 1793 | [15] | Mugil chelo Cuvier 1829 | Chelon | Chelon | Chelon | Chelon | Chelon |
| Cephalus | Lacepède 1799 | [16] | Mugil cephalus Linnaeus 1758 | Mugil | Mugil | Mugil | Mugil | Mugil |
| Agonostomus | Bennett 1832 | [17] | Agonostomus telfairii Bennett 1832 | Agonostomus | Agonostomus | Agonostomus | Agonostomus | Agonostomus |
| Cestraeus | Valenciennes 1836 | [18] | Cestraeus plicatilis Valenciennes 1836 | Cestraeus | Cestraeus | Cestraeus | Cestraeus | Cestraeus |
| Dajaus | Valenciennes 1836 | [18] | Mugil monticola Bancroft 1834 | Agonostomus | Agonostomus | Agonostomus | Agonostomus | Dajaus |
| Nestis | Valenciennes 1836 | [18] | Nestis cyprinoides Valenciennes 1836 | Agonostomus | Agonostomus | Agonostomus | Agonostomus | Agonostomus |
| Arnion | Gistel 1848 | [19] | Mugil cephalus Linnaeus 1758 | Mugil | Mugil | Mugil | Mugil | Mugil |
| Ello | Gistel 1848 | [19] | Mugil cephalus Linnaeus 1758 | Mugil | Mugil | Mugil | Mugil | Mugil |
| Joturus | Poey 1860 | [20] | Joturus pichardi Poey 1860 | Joturus | Joturus | Joturus | Joturus | Joturus |
| Myxus | Günther 1861 | [21] | Myxus elongatus Günther 1861 | Chelon | Myxus | Myxus | Myxus | Myxus |
| Chaenomugil | Gill 1863 | [22] | Mugil proboscideus Günther 1861 | Chaenomugil | Chaenomugil | Chaenomugil | Chaenomugil | Chaenomugil |
| Rhinomugil | Gill 1863 | [22] | Mugil corsula Hamilton 1822 | Rhinomugil | Rhinomugil | Rhinomugil | Rhinomugil | Rhinomugil |
| Gonostomyxus | Macdonald 1869 | [23] | Gonostomyxus loaloa Macdonald 1869 | Cestraeus | Cestraeus | Cestraeus | Cestraeus | Cestraeus |
| Neomyxus | Steindachner 1878 | [24] | Myxus (Neomyxus) sclateri Steindachner 1878 | Neomyxus | Chaenomugil | Neomyxus | Neomyxus | Neomyxus |
| Querimana | Jordan and Gilbert 1883 | [25] | Myxus harengus Günther 1861 | Mugil | Mugil | Mugil | Mugil | Mugil |
| Aeschrichthys | Macleay 1883 | [26] | Aeschrichthys goldiei Macleay 1883 | Cestraeus | Cestraeus | Cestraeus | Cestraeus | Cestraeus |
| Liza | Jordan and Swain 1884 | [27] | Mugil capito Cuvier 1829 | Chelon | Liza | Liza | Liza | Chelon |
| Trachystoma | Ogilby 1888 | [28] | Trachystoma multidens Ogilby 1888 | Chelon | Myxus | Trachystoma | Trachystoma | Trachystoma |
| Neomugil | Vaillant 1894 | [29] | Neomugil digueti Vaillant 1894 | Agonostomus | Agonostomus | Agonostomus | Agonostomus | Dajaus |
| Oedalechilus | Fowler 1903 | [30] | Mugil labeo Cuvier 1829 | Oedalechilus | Oedalechilus | Oedalechilus | Oedalechilus | Oedalechilus |
| Squalomugil | Ogilby 1908 | [31] | Mugil nasutus de Vis 1883 | Rhinomugil | Rhinomugil | Rhinomugil | Rhinomugil | Squalomugil |
| Xenorhynchichthys | Regan 1908 | [32] | Joturus stipes Jordan and Gilbert 1882 | Joturus | Joturus | Joturus | Joturus | Joturus |
| Ellochelon | Whitley 1930 | [33] | Mugil vaigiensis Quoy and Gaimard 1825 | Ellochelon | Liza | Ellochelon | Ellochelon | Ellochelon |
| Protomugil | Popov 1930 | [34] | Mugil saliens Risso 1810 | Chelon | Liza | Liza | Liza | Chelon |
| Sicamugil | Fowler 1939 | [35] | Mugil hamiltoni Day 1869 | Sicamugil | Sicamugil | Sicamugil | Sicamugil | Sicamugil |
| Gracilimugil | Whitley 1941 | [36] | Mugil ramsayi Macleay 1883 | Chelon | Liza | Gracilimugil | Liza | Gracilimugil |
| Moolgarda | Whitley 1945 | [37] | Moolgarda pura Whitley 1945 | Moolgarda | Valamugil | Valamugil | Moolgarda | – |
| Planiliza | Whitley 1945 | [37] | Moolgarda (Planiliza) ordensis Whitley 1945 | Chelon | Liza | Liza | Liza | Planiliza |
| Aldrichetta | Whitley 1945 | [37] | Mugil forsteri Valenciennes 1836 | Aldrichetta | Aldrichetta | Aldrichetta | Aldrichetta | Aldrichetta |
| Xenomugil | Schultz 1946 | [38] | Mugil thoburni Jordan and Starks 1896 | Mugil | Mugil | Mugil | Xenomugil | Mugil |
| Crenimugil | Schultz 1946 | [38] | Mugil crenilabis Forsskål 1775 | Crenimugil | Crenimugil | Crenimugil | Crenimugil | Crenimugil |
| Heteromugil | Schultz 1946 | [38] | Mugil tricuspidens Smith 1935 | Chelon | Liza | Liza | Liza | Chelon |
| Oxymugil | Whitley 1948 | [39] | Mugil acutus Valenciennes 1836 | Chelon | Liza | Liza | Liza | Planiliza |
| Pteromugil | Smith 1948 | [40] | Mugil diadema Gilchrist and Thompson 1911 | Chelon | Liza | Liza | Liza | Planiliza |
| Strializa | Smith 1948 | [40] | Mugil canaliculatus Smith 1935 | Chelon | Liza | Liza | Liza | Chelon |
| Valamugil | Smith 1948 | [40] | Mugil seheli Forsskål 1775 | Moolgarda | Valamugil | Valamugil | Valamugil | Crenimugil |
| Plicomugil | Schultz 1953 | [41] | Mugil labiosus Valenciennes 1836 | Oedalechilus | Oedalechilus | Oedalechilus | Oedalechilus | Plicomugil |
| Osteomugil | Lüther 1977 | [42] | Mugil cunnesius Valenciennes 1836 | Moolgarda | Valamugil | Valamugil | Valamugil | Osteomugil |
| Minimugil | Senou 1988 | [10] | Mugil cascasia Hamilton 1822 | Minimugil | Sicamugil | Sicamugil | Sicamugil | Minimugil |
| Paracrenimugil | Senou 1988 | [10] | Mugil heterocheilos Bleeker 1855 | Paracrenimugil | Crenimugil | Crenimugil | Crenimugil | ND |
| Pseudoliza | Senou 1988 | [10] | Mugil parmatus Cantator 1849 | Pseudoliza | Liza | Paramugil | Paramugil | Planiliza |
| Paramugil | Ghasemzadeh 1998 | [12] | Mugil parmatus Cantator 1849 | Pseudoliza | Valamugil | Paramugil | Paramugil | Planiliza |
| – | – | – | Mugil falcipinnis Valenciennes 1836 | Chelon | Liza | – | Liza | Neochelon gen. nov. |
| – | – | – | Mugil grandisquamis Valenciennes 1836 | Chelon | Liza | Liza | Liza | Parachelon gen. nov. |
| – | – | – | Mugil capensis Valenciennes 1836 | Chelon | Myxus | Myxus | Myxus | Pseudomyxus gen. nov. |
Molecular phylogenetic investigations of the Mugilidae have been mostly regional in scope, with a majority of studies concerning mugilid species sampled from the Mediterranean region, and a few other studies concerned with species from the Atlantic waters of South America, or from India, or from eastern Asia (reviewed in [1]). More recent studies have attempted to expand taxonomic sampling by including species and genera from various locations worldwide in addition to their initial treatment of Mediterranean Mugilidae [46,47]. The most comprehensive phylogenetic survey of Mugilidae published thus far concerned 55 species representing 19 of the 20 currently recognized genera [1] (Fig. 1). A substantial proportion of the species in particularly speciose genera Chelon (5/7 of currently recognized species), Liza (14/19), Mugil (9/12), Moolgarda (4/5) and Valamugil (3/4) were included. Broad geographic sampling was undertaken for the ubiquitous genera Chelon, Liza, and Mugil. Emphasis was also put on sampling several widely-distributed species of these and other genera, including C. macrolepis, Crenimugil crenilabis, Moolgarda cunnesius, M. seheli, Mugil cephalus, M. curema and Valamugil buchanani.
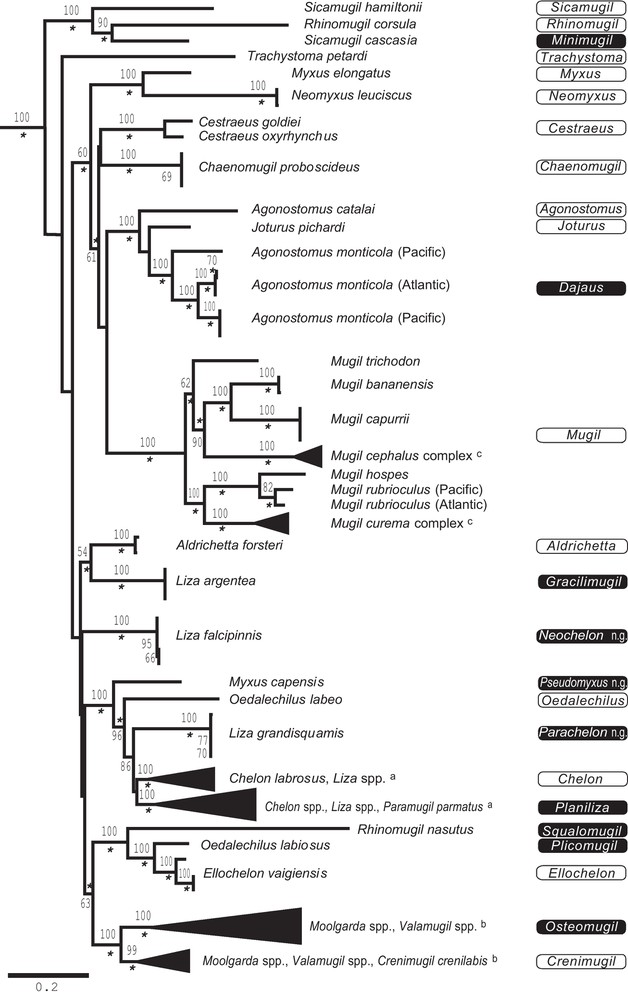
Revised genus names in Mugilidae, superimposed on the phylogenetic tree of Mugilidae (55 species from 19 genera) inferred using partitioned maximum-likelihood analysis of 3885 aligned nucleotides from three mitochondrial gene loci (modified from [1]). Taxon names at extremity of branches according to the current nomenclature [13]; when species identification was uncertain, an unknown species or “sp.” was assigned to the recognized genus for the taxon. Proposed new genus designations are shown on the right-hand side of the figure; black background: resurrected genera or newly proposed genera (‘n.g.’); open: genera maintained in their current name. Numbers on the branches are ML bootstrap values, with those below 50% not shown. Asterisks indicate nodes with a posteriori probability from partitioned Bayesian analysis ≥ 0.95 [1]; scale bar: 0.1 inferred nucleotide substitution/site under (GTR + G + I) model. a Details in Fig. 2A; b details in Fig. 2b; c details in Fig. 2C.
Durand et al.’s [1] phylogeny allowed one to test previous phylogenetic hypotheses based on morphology and morphoanatomy, themselves in contradiction with one another (Fig. 1A–E of [1]). It was found that several genera in the Mugilidae actually were polyphyletic or paraphyletic and that the molecular phylogeny matches no one of the previous, morphology-based classifications. Here, we propose a revised classification based on these results.
2 Methods
We thus examined the phylogenetic placement of each of the 19 mugilid genera in the mitochondrial phylogeny of Mugilidae produced by [1] (Fig. 1). In addition, we determined the phylogenetic placement of the genus Xenomugil (represented by its single species X. thoburni).
A partial phylogeny of Mugilidae based on all available nucleotide sequences of a 300-bp fragment of the cytochrome b (cytb) gene had initially shown X. thoburni haplotypes to be embedded within the Mugil curema lineage (Appendix A). Consequently, here we run a new phylogenetic analysis (following procedures described in [1]) of the genera Mugil and Xenomugil, using a new matrix of sequences that comprised representatives from all Mugil spp. lineages of [1], the new sequences of two X. thoburni individuals, and the additional sequences of two M. cephalus individuals from the Galapagos Islands. Both X. thoburni and additional M. cephalus from the Galapagos Islands were collected at Bahia Divine, Isla Santa Cruz on 15 June 2011 by T. Ballesteros. Their partial nucleotide sequences at the 16S rRNA (16S), cytochrome-oxidase I (COI) and cytb loci [GenBank (http://www.ncbi.nlm.nih.gov) accession numbers JX559523 to JX559535] were obtained using the same experimental protocols as [1].
For the present revision, a genus name was conserved if the topology of the tree supported the monophyly of the genus. When a genus currently considered valid was paraphyletic or polyphyletic, we split it into the minimum necessary number of genera according to the topology of the tree. The current genus name was conserved for the type species (Table 1) and, when applicable, its sister species in the same genus. For the other monophyletic groups under the same genus, former genus names were resurrected whenever applicable. For this, we considered the history of genus nomenclature in Mugilidae and the validity of the 43 genera proposed thus far (Table 1). The principle of priority [51] was followed when a previously proposed genus name was available. When no genus name was available for a given lineage, we proposed a new genus name.
Thus, our concern was to minimize disruption to the existing nomenclature. This accords with the principles of the PhyloCode [52].
3 Results and discussion
A summary of Durand et al.’s [1] phylogenetic results (Fig. 1) and their taxonomic implications at the genus level are examined in the following. We added information on the distribution of each genus. Genera are listed in alphabetical order.
3.1 Agonostomus
Agonostomus was paraphyletic with respect to Joturus; A. monticola was phylogenetically closer to J. pichardi than both were from A. catalai (Fig. 1). At locus 16S, the nucleotide divergence between A. monticola (GenBank JQ060644–JQ060652) and A. catalai (GenBank JQ060643) was 13.3–13.5% (Kimura 2-parameter; MEGA 5 [49]) while the estimated divergence between A. telfairii (GenBank DQ532834) and A. catalai was 0.2%. Since A. telfairii, which is the type-species of the genus, is genetically closer to A. catalai than A. monticola, it is the latter that should be placed under a different genus name, namely Dajaus which is the earliest genus name available for A. monticola (Table 1). The genus Agonostomus under its present, revised definition exclusively occurs in the South-West Indian Ocean [11].
3.2 Aldrichetta
Aldrichetta was found it to be the sister subclade of Liza argentea; no taxonomic change is needed for Aldrichetta, which is monotypic [13]. The distribution of this genus is restricted to the temperate coastal waters of Australia and New Zealand [11].
3.3 Cestraeus
The genus Cestraeus, represented by two species (C. goldiei and C. oxyrhinchus) in [1] was found to be monophyletic and a brother genus to Chaenomugil, Mugil, and (Agonostomus + Joturus); no taxonomic change is needed regarding Cestraeus because of its monophyly. The genus Cestraeus is present in the Indo-Malay-Papua archipelago, in New Caledonia and in Fiji [11].
3.4 Chaenomugil
This genus was found to be a brother genus to Cestraeus, Mugil and (Agonostomus + Joturus). No taxonomic change is needed for Chaenomugil, which is monotypic [13]. C. proboscideus, the only species in the genus, occurs in the eastern Pacific, from Baja California to Peru [11].
3.5 Chelon
Chelon labrosus grouped with L. aurata, L. ramada, L. saliens, L. richardsonii, L. bandialensis, L. dumerili, Liza sp. (S. Africa) and L. tricuspidens to form a subclade (Fig. 2A), which turned out to exclusively comprise species distributed in Atlantic and Mediterranean waters or species apparently endemic to eastern southern Africa (L. tricuspidens, L. richardsonii and an apparently undescribed Liza sp. [1]). The other Chelon species sampled, all from the Indo-Pacific, formed a distinct subclade together with Indo-Pacific Liza spp. and Paramugil parmatus. All species in the ‘Atlantic’ subclade (Fig. 5A of [1]), which includes Chelon labrosus (the type species of the genus Chelon) should be placed under Chelon by the principle of priority (Table 1). The other Liza and Chelon species sampled should be placed under different genera (see below).
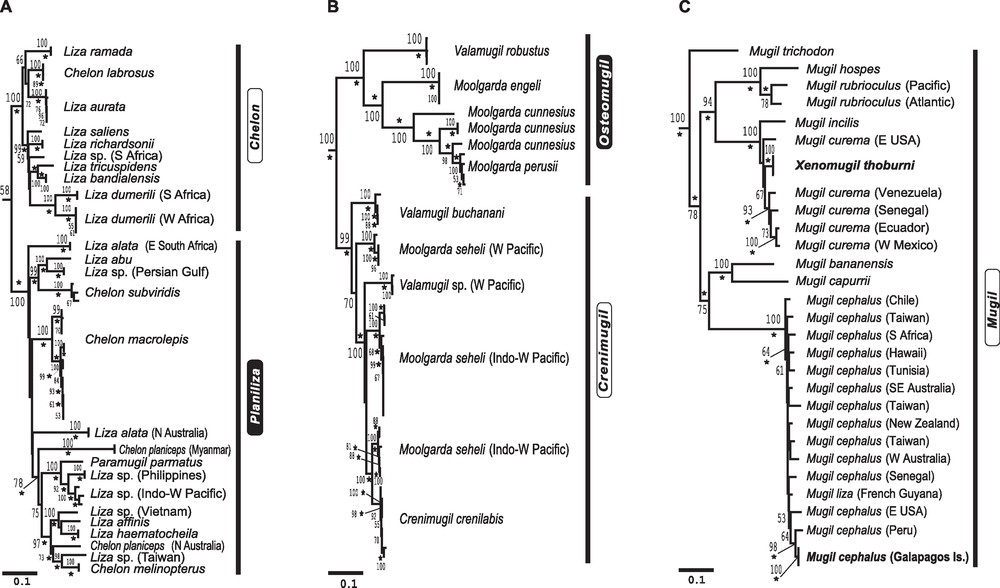
Details of the tree presented in Fig. 1. Taxon names at extremity of branches are given according to the current nomenclature [13]; when species identification was uncertain, an unknown species or “sp.” was assigned to the recognized genus for the taxon. Proposed new genus designations are shown on the right-hand side of each figure; black background: resurrected genera; open: genera maintained in their current name. Numbers on the branches are ML bootstrap values, with those below 50% not shown. Asterisks indicate nodes with a posteriori probability from partitioned Bayesian analysis ≥ 0.95 [1]; scale bars: 0.1 inferred nucleotide substitution/site. A. Revised genus names proposed for species in the current genera Chelon, Liza and Paramugil. B. Revised genus names proposed for the current genera Crenimugil, Moolgarda and Valamugil. C. Phylogenetic tree depicting relationships of Mugil spp. and Xenomugil thoburni. Relationships were inferred using partitioned maximum-likelihood (RAxML [53]) analysis of 2,385 aligned nucleotides from partial 16S, COI and cytb genes (ML score - 13535.1). Myxus elongatus and Agonostomus catalai were selected as outgroup taxa following the mugilid phylogeny of [1] (Fig. 1). Branch lengths are proportional to number of substitutions under the (GTR + G) model. Bold: taxa not included in [1].
3.6 Crenimugil
Crenimugil crenilabis (the type species of the genus Crenimugil; Table 1) grouped with Moolgarda seheli and Valamugil buchanani to form a distinct cluster within the (Crenimugil, Moolgarda, Valamugil) subclade (Fig. 2B). This well-supported lineage should be named Crenimugil because of the priority of the latter to Valamugil (Table 1), and because Moolgarda is both a nomen nudum and a nomen dubium [11]. The close evolutionary affinity of C. crenilabis with M. seheli and V. buchanani has previously been highlighted on the basis of shared morpho-anatomical characters ([10]; Fig. 3). We were unable to obtain a sample of C. heterocheilos, designated by Senou [10] as the type species of his genus Paracrenimugil. Based on the accuracy of the rest of Senou's [10] cladistic tree (Fig. 3), resurrecting the genus Paracrenimugil for C. heterocheilos is an eventuality that deserves consideration. The genus Crenimugil has a wide Indo-West Pacific distribution.

Parsimony tree of Moolgarda spp., Valamugil buchanani and Crenimugil spp. based on a cladistic analysis of 46 morphological characters (redrawn from [10]).
3.7 Ellochelon
The genus Ellochelon was found to be the sister lineage of Oedalechilus labiosus. No taxonomic change is needed for Ellochelon, which is monotypic [13]. E. vaigiensis has a wide Indo-West Pacific distribution, from Natal to Tahiti [11].
3.8 Joturus
The genus Joturus was the sister lineage of Agonostomus monticola. No taxonomic change is needed for Joturus, which is monotypic [13]. This genus is present on both the Pacific and the Atlantic coasts of the American continent, from Mexico to Panama, and in the Caribbean Sea [11].
3.9 Liza
The type species of the genus Liza is Mugil capito (currently L. ramada; [13]). The phylogenetic results of [1] imply that L. ramada be placed under genus Chelon, which in turn implies that Liza is a junior synonym of Chelon. Hence, the name Liza is now unavailable.
Liza argentea and L. falcipinnis were each distinct from the other Liza species, all of which clustered within a single clade (Fig. 1). The latter comprised Myxus capensis, Oedalechilus labeo, and three subclades: one that corresponds to L. grandisquamis, a second one that includes Chelon labrosus and all Liza spp. of the Atlantic and the Mediterranean (see above), and a third sub-clade that includes Chelon spp. and Liza spp. from the Indo-West Pacific only (namely, C. macrolepis, C. melinopterus, C. planiceps, C. subviridis, L. abu, L. affinis, L. alata, L. haematocheila, and Paramugil parmatus) (Fig. 2A). Liza argentea and L. falcipinnis each merit an individual genus name. Liza argentea was previously assigned to the genus Gracilimugil [36] and we here propose that this genus be resurrected for this species; L. falcipinnis should be assigned a new genus name since there does not seem to be any genus name available for that species (Table 1), and L. grandisquamis should similarly be assigned a new genus name [51]. The ‘Indo-West Pacific’ (Chelon spp. + Liza spp. + P. parmatus) subclade contains L. alata, a senior synonym of L. ordensis which is the type species of the subgenus Planiliza [37] (Table 1), hence it should be assigned genus name Planiliza by the principle of priority [51]. The genus Gracilimugil occurs in southwestern Australia.
3.10 Moolgarda
This genus was polyphyletic (Figs. 1 and 2B). The name Moolgarda predates both Crenimugil and Valamugil (Table 1) but the position of M. pura, the type species of the genus [37], is uncertain and the type specimen has been lost [11]. Therefore, Moolgarda should be considered a nomen dubium and no use can be made of this genus name in the present revision. The Moolgarda species that belong to the Crenimugil crenilabis subclade should be placed under Crenimugil (see above). The other species, including M. cunnesius, M. engeli, M. perusii and Valamugil robustus clustered into a distinct subclade (Fig. 2B), hence deserve a different genus name. For this, we propose to use the name Osteomugil following Lüther [42], who described this genus from M. cunnesius (the type species), and who also suggested that it might include M. engeli.
3.11 Mugil
All 9 Mugil species examined by [1] (M. bananensis, M. capurrii, M. cephalus, M. curema, M. hospes, M. incilis, M. liza, M. rubrioculus and M. trichodon) clustered into a single, well-supported clade (Fig. 4A of [1]). Mugil was found to be paraphyletic with Xenomugil (Fig. 2C). The name Mugil remains valid by the principle of priority [51] (Table 1). This genus has a temperate-tropical circumglobal distribution [11].
3.12 Myxus
Myxus was polyphyletic, with M. elongatus (its type-species) pairing with Neomyxus leuciscus, and M. capensis being part of the distinct clade external to O. labeo and the two (Liza spp. + Chelon spp.) subclades. The name Myxus should be maintained for M. elongatus (the type-species of the genus), while M. capensis deserves genus rank. As there is currently no genus name available for M. capensis (Table 1), a new genus name has to be proposed. The genus Myxus under its present, revised definition is restricted to the temperate waters of Australia.
3.13 Neomyxus
Neomyxus is the sister lineage of M. elongatus. No taxonomic change is needed for Neomyxus, which is monotypic [13]. The only representative of this genus, N. leuciscus, occurs around islands of the Central Pacific, from Southern Japanese and Hawaiian islands to Samoa [11].
3.14 Oedalechilus
The mitochondrial phylogeny (Fig. 1) revealed a polyphyletic Oedalechilus: O. labeo, its type species, clustered with Myxus capensis, Chelon spp., Liza spp. and P. parmatus to form a distinct subclade, while O. labiosus paired with E. vaigiensis within another subclade that also included R. nasutus. The genus name Oedalechilus should be maintained for O. labeo (its type-species; Table 1), O. labiosus should be reassigned to the genus Plicomugil following Schultz [41] and Harrison and Howes [54]. Therefore, under its present, revised definition, the genus Oedalechilus is monotypic. It occurs in the Western Mediterranean Sea and in the Azores archipelago [11]. The genus Plicomugil is distributed in the Indo-West Pacific, from the Red Sea to the Philippines [11].
3.15 Paramugil
Ghasemzadeh [12] defined the genus Paramugil for P. parmatus, which was embedded within the Indo-Pacific sub-clade of (Liza spp. + Chelon spp.) (Fig. 2A), for which we argued that the genus name Planiliza be resurrected (see above). Hence, Paramugil should now be regarded as a junior synonym of Planiliza.
3.16 Rhinomugil
Rhinomugil was polyphyletic, with R. corsula being the sister lineage of Sicamugil cascasia, while R. nasutus paired with the lineage that includes Ellochelon and O. labiosus (Fig. 1). The name Rhinomugil should be maintained for R. corsula, its type species (Table 1) but R. nasutus should be assigned a different genus name, namely Squalomugil [31] (Table 1). R. corsula is a freshwater species from India; R. nasutus occurs in the estuarine waters and mangroves of New Guinea and tropical Australia [11].
3.17 Sicamugil
Sicamugil was paraphyletic, with S. hamiltonii being sister group to (R. corsula + S. cascasia) (Fig. 1). Sicamugil should be maintained for S. hamiltonii, the type species of the genus (Table 1), and S. cascasia should be re-assigned to Senou's [10] Minimugil (Table 1). S. hamiltoni occurs in Myanmar; S. cascasia is distributed in the Ganges River and its tributaries [11].
3.18 Trachystoma
Genus Trachystoma formed a distinct clade on its own. The mitochondrial phylogeny confirmed the peculiar systematic status of the monotypic genus Trachystoma (Fig. 1). T. petardi, the only species in the genus, inhabits the rivers of eastern Australia, from Queensland to New South Wales [11].
3.19 Valamugil
Most Valamugil species, along with Moolgarda species, were split into two strongly supported lineages, one of which was paraphyletic with Crenimugil crenilabis. V. robustus belonged to another subclade, which also comprised Moolgarda spp. and Valamugil spp. Valamugil should now be considered as a junior synonym of Crenimugil since V. seheli (currently Moolgarda seheli), the type species of Valamugil, clusters with C. crenilabis (the type species of Crenimugil) into a single, well supported subclade. The subclade that includes M. cunnesius, M. engeli and M. perusii should now be assigned to Lüther's [42] Osteomugil. It is remarkable that Senou's [10] cladistic treatment, based on morphological characters, yielded the same result regarding the Crenimugil/Moolgarda/Valamugil group (Fig. 3) as the present molecular phylogeny (Fig. 2C). Senou [10] however fell short of proposing that M. seheli and V. buchanani be placed under Crenimugil, and that M. cunnesius, M. engeli and M. perusii be placed under Osteomugil. Valamugil robustus was the most externally branching species relative to the other species in our Osteomugil subclade. Therefore, although here we placed V. robustus, M. cunnesius, M. engeli and M. perusii under a single genus, it may be argued that V. robustus be assigned a different genus name because of the large nucleotide distance that separates it from the other species in the sub-clade. The unique position of the first dorsal fin in V. robustus relative to all the other Valamugil spp. and Moolgarda spp. [11] would provide morphological support for this distinction. Nevertheless, we adopted a conservative approach and we leave this taxonomic problem to future research. The genus Osteomugil under its present, revised definition has a wide Indo-Pacific distribution, from South Africa to French Polynesia [11].
3.20 Xenomugil
The Xenomugil thoburni partial haplotypes were found to be embedded within the Mugil curema haplogroup (Fig. 2C). Therefore, there is no phylogenetic rationale to recognizing the genus Xenomugil as valid. The placement of X. thoburni haplotypes within the Mugil spp. subclade implies that Xenomugil is a synonym of Mugil. Further research is needed to clarify the systematics of the M. curema species complex and, in particular, whether X. thoburni is a distinct, biological species.
4 Conclusion
All phylogenetic hypotheses based on morphology and morpho-anatomy proposed within the last few decades for Mugilidae (review in [1]) were in contradiction with one another and the molecular phylogenetic results [1] supported no one. Here, we proposed a new classification that is consistent with the molecular phylogeny of [1], resurrecting genus names previously fallen into oblivion and eventually pointing out the need for new genus names in cases where no name is available [51]. The revised classification of the Mugilidae family proposed here recognizes 25 genera, including 15 genera currently considered as valid (Agonostomus, Aldrichetta, Cestraeus, Chaenomugil, Chelon, Crenimugil, Ellochelon, Joturus, Mugil, Myxus, Neomyxus, Oedalechilus, Rhinomugil, Sicamugil and Trachystoma) and 6 resurrected genera (Gracilimugil, Minimugil, Osteomugil, Planiliza, Plicomugil and Squalomugil). The mitochondrial phylogeny also singled out three isolated lineages (currently L. falcipinnis, Myxus capensis, and L. grandisquamis) for which no genus name is yet available. We here propose the following new genus names: Neochelon gen. nov. (type species: Mugil falcipinnis Valenciennes 1836); Parachelon gen. nov. (type species: M. grandisquamis Valenciennes 1836); and Pseudomyxus gen. nov. (type species: M. capensis Valenciennes 1836). Further genetic evidence is required to confirm or infirm the validity of genus Paracrenimugil proposed by Senou [10] for C. heterocheilos, as no genetic data are yet available for this species. Genus names Liza, Moolgarda, Paramugil, Valamugil and Xenomugil were shown to be invalid and they should now be abandoned.
More research is needed to address taxonomic issues at the infra-generic level. For instance, Agonostomus monticola and several species with large distribution ranges (including Moolgarda seheli, Mugil cephalus and M. curema) consisted of separate lineages whose geographic distribution suggests they are cryptic species (Figs. 1 and 2). Nuclear-DNA markers are powerful to detect reproductive isolation among cryptic species in sympatry. Nuclear genotyping has already led to identifying three biological species within M. cephalus from Taiwan [55] and two biological species within M. curema from the central western Atlantic [1]. Given the general helplessness of morphology and morpho-anatomy to reconstruct a consistent phylogeny of Mugilidae, a central role should now be assigned to molecular phylogenetics and population genetics in the taxonomy of species in this family.
5 Taxonomic description of three new mugilid genera
5.1 Neochelon, new genus
The new genus name Neochelon is here proposed for Mugil falcipinnis Valenciennes 1836 [18], here designated as its type species. The nucleotide sequences examined were those of the cytb gene (GenBank accession No. JQ060212), the COI gene (GenBank JQ060469), and the 16S gene (GenBank JQ060716) of voucher specimen no. MNHN 2009-0730 (from Toubacouta, Saloum estuary, Senegal), and the homologous sequences of specimens collected in St Louis, Senegal (GenBank JQ060213, JQ060470, and JQ060717; JQ060214, JQ060471, and JQ060718) and at the fish market of Lome, Togo (GenBank JQ060215, JQ060472, and JQ060719) (Table 1 of [1]). The new genus is unique by the placement of its mitochondrial haplotypes on the phylogenetic tree of Mugilidae (Fig. 1), alone forming one of the seven major clades that stem from the common ancestor to all current mugilid species. The name of the genus is derived from Chelon, preceded by greek prefix “neo-” meaning “new”. Distribution: West Africa, from Saint-Louis in northern Senegal to Congo [11].
5.2 Parachelon, new genus
The new genus name Parachelon is here proposed for Mugil grandisquamis Valenciennes 1836 [18], here designated as its type species. The nucleotide sequences examined were those of the cytb gene (GenBank accession nos. JQ060218 and JQ060219), the COI gene (GenBank JQ060475 and JQ060476), and the 16S gene (GenBank JQ060722 and JQ060723) of voucher specimens nos. MNHN 2009-731 and SAIAB-83182 (both from Saloum estuary, Senegal), and the homologous sequences of additional specimens collected in the Saloum estuary, Senegal (GenBank JQ060216, JQ060473, and JQ060720) and at the fish market in Bissau, Guinea Bissau (GenBank JQ060217, JQ060474, and JQ060721). This new genus is unique by the placement of its mitochondrial haplotypes on the phylogenetic tree of Mugilidae (Fig. 1), where it forms a subclade sister to Chelon, Oedalechilus, and Planiliza within the clade that also comprises Pseudomyxus gen. nov. The name of the genus is derived from Chelon, preceded by greek prefix “para-” meaning “beside”. Distribution: West Africa, from Senegal to Nigeria [11].
5.3 Pseudomyxus, new genus
The new genus name Pseudomyxus is here proposed for Mugil capensis Valenciennes 1836 [18], here designated as its type species. The nucleotide sequences examined were those of the cytb gene (GenBank JQ060366), the COI gene (GenBank JQ060615) and the 16S gene (GenBank JQ060867) of a specimen collected in the East Kleinemonde estuary, South Africa (sampling details in Table 1 of [1]). The new genus is unique by the placement of its mitochondrial haplotypes on the phylogenetic tree of Mugilidae (Fig. 1): Pseudomyxus gen. nov., together with Chelon, Oedalechilus, Parachelon gen. nov. and Planiliza, forms one of the seven major clades of the Mugilidae family. Pseudomyxus gen. nov. represents the most external lineage within this clade (Fig. 1). The name of the genus is derived from Myxus, preceded by greek prefix “pseudo-” meaning “false”. Distribution: South Africa [11].
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We are grateful to T. Ballesteros, S. Banks, F. Bungartz and N. Tirado from the Charles Darwin Foundation for collecting Mugil cephalus and Xenomugil thoburni tissue samples. We thank J.-F. Trape and S. Trape (IRD, Dakar) for stimulating discussions.
Appendix A
Molecular phylogenetic analysis of Mugilidae partial sequences (300 bp) of the cytb gene, inferred by using the Maximum Likelihood method from Tamura and Nei's [48] model as implemented in MEGA 5 [49]. A. Entire tree with the highest log-likelihood [ln(L) = −5162.2]. Sequences for outgroups Arcos sp. (Gobiesocidae) and Salarias fasciatus (Blenniidae) [50] are from GenBank (http://www.ncbi.nlm.nih.gov/; accession nos. AP004452 and AP004451, respectively). B. Detail of the Mugil subtree; sequences of Xenomugil thoburni and two M. cephalus from the Galapagos Islands were kindly provided by S. Livi (pers. comm.). The percentage of pseudotrees generated by bootstrap resampling (500 runs), in which the associated individuals clustered together is shown next to the branches (bootstrap scores below 70% not shown). Branch length is proportional to the number of substitutions per site. Specimen numbers refer to Table 1 of [1].


