1 Introduction
Multiple species groups can be defined from different perspectives, which led to the introduction of a complex terminology in community ecology [1]. Community is used to simply indicate groups of organisms living in the same place at the same time. A community is therefore not restricted by phylogeny or resource use. Restriction by phylogeny or resources, or both, is described by alternative terms. Guilds are groups of species (without regard to taxonomy position) that exploit the same class of environmental resources in a similar way. A phylogenetically bounded group of species that use a similar set of resources within a community forms an “ensemble” [2–5].
Studies in insect multispecies groups have been largely addressed to investigate communities, and very rarely guilds, because the lack of adequate single species ecological information makes it difficult to group species into guilds [6,7 – as examples regarding beetles]. Even more difficult is to study ensembles, because this implies to study a group of species that: (i) belong to the same taxon (e.g. to a given family); (ii) are synchronically present in the same place; and (iii) are comparable in their ecology. Thus, the study of ensembles has received less attention in comparison with community and guild studies [3,4], and – as far as we know – no study has been attempted to investigate if structural parameters (i.e. relative abundances of species and diversity) of a given ensemble can be tuned by small grain spatial variation in environmental characteristics.
The tenebrionid beetles (Coleoptera Tenebrionidae) inhabiting Mediterranean sand dunes offer such an intriguing opportunity. Several aspects make these animals excellent model organisms for ensemble studies. First, tenebrionids belong to a monophyletic taxon, so they form a phylogenetically bounded group. Second, they are abundant and fairly easy to sample on coastal dunes, which makes it possible to obtain sufficiently large samples of individuals in short time even in small sampling plots. A synchronous sampling from small areas is important to ensure that the sampled species belong to the same spatial and temporal community. Also, it is important that sampling of different biotopes is conducted synchronically to avoid that differences among biotopes are in fact a result of temporal differences in insect activity (e.g., day versus night or spring versus summer). Finally, tenebrionids living on coastal dunes are detritivorous and opportunistic animals, and competition in these insects is considered not an important driver of community organization [see 8 for an extensive discussion]. In general, dune ecosystems include an outstanding ecological diversity in terms of environmental heterogeneity and species composition [9,10]. Furthermore, the dynamic nature of sandy coastal habitats and the strong zonation patterns exhibited by the vegetation make dune communities ideal systems for the study of changes of tenebrionids ensembles in a short space.
In this study, we investigated if the tenebrionid beetles forming an ensemble on a Mediterranean coastal dune zonation showed variations in spring community organization (relative abundances and species diversity) in different, but spatially strictly associated, biotopes [11] defined by different plant communities.
2 Material and methods
2.1 Study area and data collection
Sampling was done along three vegetation transects on a coastal sand dune system in Central Italy, near Montalto Marina (Latium region) during spring (May 2012). As outlined in previous ecological investigations [12], the study area is characterized by a good conservation status and the three vegetation transects share similar physical settings. Coastal zones are under severe human pressures, which may alter profoundly the structure of insect communities [8]. To minimize the possibility that observed patterns are the result of anthropogenic influence we selected our sampling areas among the less disturbed coastal zones in Latium.
Each vegetation transect included three plots. Each plot belongs to a different biotope as defined by European Commission [13,14] (in parentheses EC codes): Embryonic shifting dunes (2110); Shifting (white) dunes along the shoreline with Ammophila arenaria (2120); Malcolmietalia dune grasslands (2230). The three biotopes were identified in the field following the dominant and diagnostic plant species as indicated by the Habitat Directive Interpretation Manual [15]. Given that a natural stress gradient develops along the sea-inland profile and gives rise to a vegetation zonation in a short space, these three biotopes are typically disposed perpendicularly to the seashore and virtually contiguous. In particular, the biotope corresponding to EC habitat 2110 is closer to the sea, the biotope corresponding to EC habitat 2120 follows inland and the biotope corresponding to EC habitat 2230 is the most inland [16,17].
For the present study, in each transect, the three biotopes (EC habitats 2110, 2120 and 2230) were sampled using square plots of 2 × 2 m placed at distance of about 10 m. Within each plot, beetles were collected by hand for a standard time of 20 minutes. A total of 12 trained students were involved in beetle sampling. Students were randomly divided into three groups of four students. In each transect the three biotopes were sampled simultaneously by four students per plot. Students groups also rotated among transects. In this way, a student group, which sampled one biotope in the first transect, sampled another biotope in the second transect, and another biotope again in the third transect. All three biotopes were therefore sampled by all three groups, albeit in different transects.
In each plot, students were instructed to first collect all beetles that moved on the ground, to reduce the risk that escaping animals abandoned the plot before being sampled. Then, the four students moved from the four angles of the square towards the centre collecting all animals they were able to find. Insect sampling was done by conscientiously searching for beetles on the grounds, under leaves, and sieving sand at the base of the plants. To establish an adequate sampling time, we conducted a preliminary sampling in a 2 × 2 m plot placed in a biotope corresponding to the EC 2120 habitat. This preliminary sampling section was conducted by two expert entomologists who collected beetles until no further individual was found. This preliminary sampling required about 10 minutes. Thus, 20 minutes with four persons was considered a sufficient time to collect virtually all tenebrionid individuals occurring in a plot.
To minimize the sacrifice of animals, large sized tenebrionids (Pimelia, Erodius, and Tentyria) were placed into tubes, identified by an expert taxonomist, and released close to the sampling plot after all plots of the same site were sampled. Small sized tenebrionids were instead identified in the laboratory.
A further sampling performed during the summer (July 2012) confirmed that the highest activity and diversity of tenebrionids beetles on coastal dunes are mainly concentrated in spring [8], probably because of more suitable climatic conditions (lower temperature and higher soil moisture during spring).
2.2 Data analysis
We used a main effect ANOVA to investigate the first order effects of plant association, site and species identity on tenebrionid abundances. Numbers of individuals were square root transformed before ANOVA to normalize their distribution. This preliminary analysis allowed us to test if mean abundances varied significantly among transects. Because we were interested in the habitat-tenebrionid relationships, and there was no significant effect of transect, we pooled species numbers collected in the same biotope of the three transects.
To investigate if there was some association between species and habitat (i.e. if species occur with different proportions in different biotopes) we applied a χ2 test to a species × biotope contingency table pooling data from the three transects. After this overall χ2 test, we used single species χ2 tests to assess if species abundances in the three biotopes deviated significantly from a uniform distribution.
Variations in community structure parameters among the tenebrionid ensembles of the three biotopes were investigated using Shannon diversity index: , where ni is abundance of species i and n is the overall abundance. H ranges from 0 (one species dominates the community completely) to high values for communities with many species, each with few individuals [18]. For comparative purposes we also used both Margalef richness index (Mg = (S − 1)/ln(n), where S is the number of species) and Berger-Parker dominance index (number of individuals in the dominant taxon divided by n) [19].
To compare diversity indexes of tenebrionid ensembles of different biotopes, a bootstrapping procedure was applied in pairwise tests. The two samples in a pairwise comparison A and B were initially pooled. Then, 1000 random pairs of samples (Ai, Bi) were taken from this pool, and a diversity index was calculated for each replicate pair with the same numbers of individuals as in the original two samples. The probability of obtaining the observed difference by random sampling from a unique parental population was calculated as the number of times that the absolute difference of the indexes of a replicate pair exceeded or equaled that of the original samples. A P (equal) < than 0.05 was assumed to indicate a significant difference in diversity index between the two samples. Calculations were done using PAST [19].
3 Results
We found an association between species abundances and biotopes (χ2 = 23.436, df = 10, P = 0.009), which indicates that species occur with different proportions in the three sampled biotopes (Fig. 1). Separate χ2 tests for uniform distributions revealed that Pimelia bipunctata (χ2 = 10.5, df = 2, P = 0.005) occurred with different abundances in the three biotopes. A significant value was also found for Trachyscelis aphodioides (χ2 = 6, df = 2, P = 0.05) but the low number of sampled individuals suggests some caution in accepting this result. Also there is an indication that abundance of Erodius siculus varied among biotopes (χ2 = 5.30, df = 2, P = 0.071). Ammobius rufus showed similar abundances in the three biotopes (χ2 = 4.17, df = 2, P = 0.124), and the other species were not tested because of the small number sampled individuals.
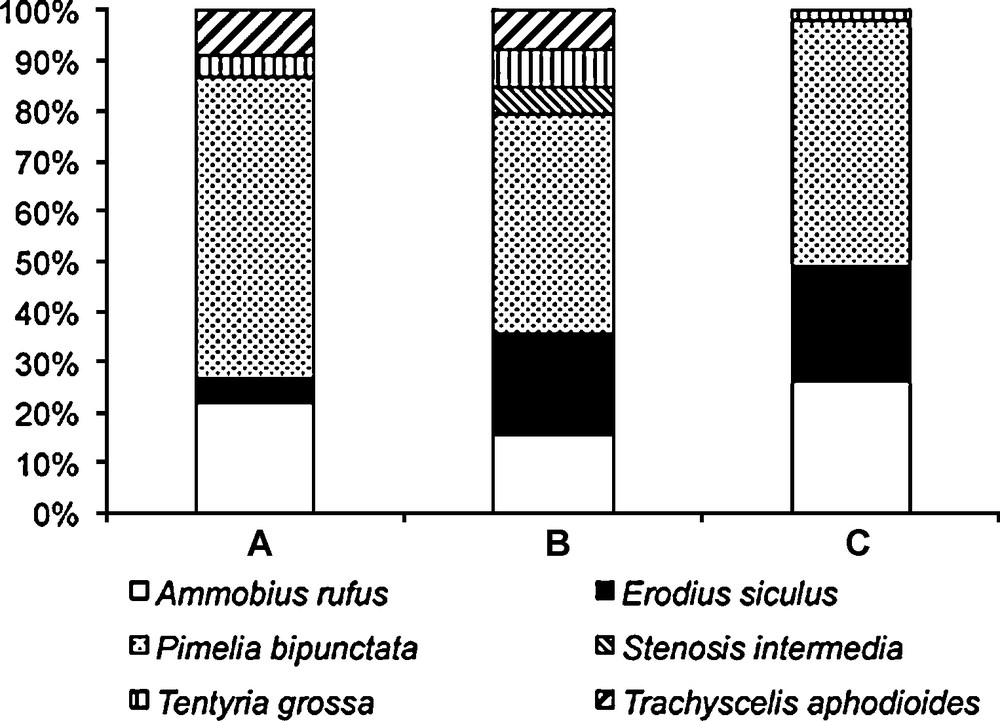
Proportion of tenebrionid species among three biotopes on a Mediterranean dune during spring. The biotopes correspond to three different European Commission (EC) habitats (A – habitat 2110, B – habitat 2120, C – habitat 2230). Number of sampled individuals: n = 68 for EC habitat 2110, n = 39 for EC habitat 2120, and n = 53 for EC habitat 2230.
Shannon diversity peaked in the biotope corresponding to EC habitat 2120 (H’ = 1.52), being significantly higher in this biotope than in those corresponding to EC habitats 2110 (H’ = 1.13, P = 0.042) and 2230 (H’ = 1.11, P = 0.009), whereas the biotopes corresponding to EC habitats 2110 and 2230 were no significantly different (P = 0.925) in their tenebrionid diversity.
Margalef richness index peaked in the biotope corresponding to EC habitat 2120 (Mg = 1.365), being significantly higher in this biotope than in EC habitat 2230 (Mg = 0.76, P = 0.014) but not with respect to EC habitat 2110 (Mg = 0.948); the biotopes corresponding to EC habitats 2110 and 2230 were marginally different (P = 0.047) in their tenebrionid richness. Berger-Parker dominance index decreased from EC habitat 2110 (BP = 0.603) to EC habitat 2230 (BP = 0.491) to EC habitat 2120 (BP = 0.436), but no significant difference was detected.
4 Discussion
The three dune biotopes, the object of the present study, host spring tenebrionid communities with similar species composition and overall abundances. This confirms that tenebrionids found in the three coastal dune habitats form a single ensemble. However, tenebrionid species are differently associated with different biotopes along the coastal zonation, with some species occurring with different proportions among the three biotopes, showing some habitat selection. Because species relative abundances varied among biotopes, biotopes with different plant associations also differ in tenebrionid community structure, although all biotopes share similar values of species richness and overall abundances. Thus, the same basic tenebrionid ensemble can lead to different community organizations even in very close biotopes. Preliminary data on the vegetation structure of the three biotopes investigated here indicate that those corresponding to EC habitats 2110 and 2230 have a lower vegetation cover (40–50%) than that corresponding to EC habitat 2120 (60–80%). This seems correlated with the highest tenebrionid diversity found in the EC habitat 2120. Previous research showed that tenebrionids of coastal environments tend to be distributed across a variety of plant communities, from the seashore (EC habitat 1210) to the high maquis (EC habitat 2260), but with different abundances, thus showing more or less strict preferences for particular dune habitats [8,20]. Therefore, communities that are composed of the same species may have different structural parameters if a given species is represented with different abundances in different biotopes. In general, the EC habitats 2110 and 2230 seem to host tenebrionid communities with similar diversity, whereas the EC habitat 2120 seems characterized by a differently organized community.
It has been recently demonstrated that biotopes separated by less than 10 km, but with strong differences in soil characteristics, host tenebrionid communities with similar species composition but very different community structures, and local selection mechanism has been invoked, suggesting that soil characteristics regulated species abundances [21]. Our study suggests that a local selection process may also operate at a much smaller spatial scale, leading to differences in species proportions (and community diversity) even among very close biotopes (coastal dune habitats separated by few meters). The driving factor(s) responsible for such differences in dune biotopes are still elusive. However, the higher plant cover and more complex vertical structure typical of the habitat 2120 [22], together with a probably higher litter production may be all key factors promoting a higher tenebrionid diversity.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
We would like to thank Francesca Bellotti, Cristina Berardi, Francesca Bernardini, Lavinia Germani, Cristina Mantoni, Silvia Manzini, Gianluca Poeta, Federico Romiti, Giovanni Luca Scardaci, Massimiliano Tini and Fabiana Velletrani for their help with the fieldwork. We are grateful to two anonymous referees for their suggestions.


