1 Introduction
The spread of species outside their original range and their establishment in new areas are currently considered as global processes posing significant challenges for the conservation of native biodiversity. Alien species often represent a threat to natural ecosystem functions because they can alter species relationships and can also affect ecosystem services [1–4].
Coastal ecosystems are particularly sensitive to the introduction of alien species [5–11]. Coastal plants species are stress-tolerant, and often specialized to survive in this environment. As a consequence of this specialization, plant species are not able to establish in different environment, and this makes coastal ecosystems particularly vulnerable to the displacement of native species and to biodiversity loss [12,13].
The genus Acacia includes more than 1350 species [14] distributed throughout South America, Africa, Asia and Australia [15]. Australian species of Acacia are especially significant invaders and have become major invaders in many areas outside Australia [16–19].
In Europe, the most invasive Australian acacias are A. dealbata, A. longifolia, A. mearnsii, A. melanoxylon, A. pycnantha, A. retinodes, and A. saligna [18,20–23]. However, in Europe, the possible effects of invasion have been investigated only for A. dealbata and A. longifolia [20,21,23]. The ability of A. dealbata to spread in new areas appears to depend on its plasticity, in response to environmental and soil changes and on its resistance to fire, rapid vegetative reproduction and allelopathic potential [20]. A. longifolia can modify the chemical and microbial properties of the soil and can consequently affect native vegetation [21]. Seed dispersal mechanism in the secondary distribution areas is unclear too. In the native range, seeds are mainly dispersed by ants [24], while in tropical areas, seeds can be dispersed by frugivorous birds [25]. Some authors hypothesize a myrmecochory dispersal mechanism also in Europe, although this has not been demonstrated yet [24].
According to Celesti-Grapow et al. [26], the most invasive acacia in Italy is Acacia saligna Labill. This species has been introduced in coastal areas for reforestation purposes and for dune stabilization. However, its spread has not been controlled, and it currently occurs in many Italian regions: Liguria, Tuscany, Campania, Basilicata, Calabria, Apulia, Molise, Sicily, and Sardinia. In particular, this species is widespread on the Southern Adriatic coast, and it grows between the Mediterranean scrub and the evergreen forest of the fixed dunes in central and southern Italy [27].
A. saligna is a fast-growing species. It is characterized by both clonal and sexual reproduction; it is well adapted to arid environments and is fire-resistant [1,19,28]. Seeds can remain dormant for a long time, even for decades, forming a persistent soil seed bank [1]. This species has been widely planted outside its natural range, and it has become an invader in several areas, including South Africa, the Middle East, Portugal, France, Spain and Cyprus [1,16,18,20,21,29–32].
Although the invasive potential of A. saligna is now recognized [28,33], the impact of this species in invaded areas is still poorly explored. Studies conducted in the Middle East have shown that introduced A. saligna can modify soil characteristics by increasing the levels of total N and of organic matter, favoring the development of nitrophilous species [29]. On top of this, vascular plant species diversity can be lower in patches under A. saligna trees than in patches outside the tree canopy [16]. In South African Fynbos invaded by A. saligna, plant species richness, species cover and frequency can also decline, and these changes can modify the vertebrate and invertebrate guild structure [25]. However, to our knowledge, the effects of A. saligna on the European Mediterranean coasts have not yet been investigated.
For this reason, we analyzed the impact of A. saligna on Italian Adriatic dune ecosystems, which are the most invaded areas of the Italian coast. We selected this region as a representative sample of the dune systems invaded by this species in the Mediterranean coastal environments. In particular, we focused on EC habitats “Coastal dunes with Juniperus spp.” (habitat code: 2250*; hereafter “Juniper dune shrubland”), “Wooded dunes with Pinus pinea and/or Pinus pinaster” (habitat code: 2270*; hereafter “Pinus dune wood”) and “Cisto-Lavanduletalia dune sclerophyllous scrubs” (habitat code: 2260; hereafter “Dune sclerophyllous scrubs”), as they are the only suitable habitats for the establishment and development of bushes and trees plants species, such as A. saligna, in the study area. We investigated the distribution of this alien species in these target habitats and its influence on the total plant species richness and composition. As the presence of an alien plant can have differential effects on particular native groups of species or “plant guilds” [34,35], we identified from the total pool of species two distinct groups that are particularly sensitive to environmental changes [35,36]: focal and ruderal plant species. Focal species are the descriptors of dune habitats, according to the European Directive Habitat 92/43/EEC [37]. These species play a crucial role in determining the structure and functioning of the plant communities; they are particularly vulnerable to disturbance and habitat modification [38,39]. On the contrary, ruderal species tend to occupy disturbed areas [40–42]. We analyzed the impact of A. saligna on these guilds, since we assume that they are good indicators of the conservation status of coastal dune habitats.
2 Methods
The study was conducted in the dune system of the Molise region (Adriatic coast), where A. saligna forms dense stands. We analyzed a coastal strip of 11 km, including three Sites of Community Importance (SCIs): Foce Trigno–Marina di Petacciato (IT7228221), Foce Biferno–Litorale di Campomarino (IT7222216), and Foce Saccione–Bonifica Ramitelli (IT7222217). In particular, the SCI Foce Saccione–Bonifica Ramitelli (IT7222217) hosts the most northern stand of the population of Juniperus oxycedrus subsp. macrocarpa (EU habitat 2250*) along the Italian Adriatic coast [43,44]. As a consequence, the local Juniperus population has a remarkable biogeographic value and its conservation is relevant.
A. saligna was planted in the study area in approximately 1950 along a narrow strip between the Mediterranean scrub of mobile dunes and the Pinus afforestation area located on the fixed dunes. The plantation was established to create a vegetation belt to protect the pine wood from salt spray and the strong salty winds blowing from the sea [45–47].
We used photo-interpretation and a map of EU habitats [43] to identify the fixed dune vegetation of the study area. In a GIS environment, we selected all patches belonging to the following vegetation types: Pinus dune wood (habitat 2270*), Juniper dune shrubland (habitat 2250*) and Dune sclerophyllous scrubs (habitat 2260), which are the only habitats in the study area, where A. saligna individuals could establish and grow. We used these habitats as layers for a random sample, generating 70 random points with ArcGIS 9.2 within the polygon of vegetation that included the selected habitats. Then, we eliminated the points in inaccessible or non-vegetated areas, producing a sample of 55 points.
A GPS (GPSmap 60CSx) was then used to identify the georeferenced points in the field. At each point, we recorded all the vascular plants together with their percentage of cover on the Braun–Blanquet scale [48] in a 4 m × 4 m plot, which was previously estimated to be a sampling area of suitable size for the fixed dune vegetation [49,50]. In each point, we also recorded the percentage cover of the tree layer.
The focal species were successively identified and selected according to the list of diagnostic and characteristic species of the “Italian Interpretation Manual of the 92/43/EEC Directive habitats” [38]. Focal species of other habitats that were accidentally present in the target habitat (2250*, 2260, and 2270*) were not considered. All the other opportunistic species, identified according to Pignatti [42], were classified as “ruderal” species (Supplementary data).
2.1 Data analysis
Random sampling yielded a matrix of 55 relevés × 72 species. We analyzed the matrix with multivariate techniques (PCoA) using species as explanatory variables and the Bray–Curtis dissimilarity index to measure the distance (R software [51]). The ordination scatter diagram allowed us to identify groups of relevés according to the floristic differences and similarities among different EU habitats. To test statistically the differences between these groups, we performed analysis of similarities (ANOSIM; 999 permutation). We then identified plots that were invaded or not invaded by A. saligna. Successively, we explore the distribution of A. saligna relative to the tree coverage of the Pinus dune wood (habitat 2270*) by a logistic regression model, with the dependent variable being the invaded or non-invaded plots and the independent variable being the percentage of cover of the tree layer (canopy cover).
To analyze the impact of A. saligna on species richness, we compared the invaded and non-invaded plots using rarefaction curves, which are simple tools to compare general trends of species richness (EstimateS v 8.0 [52]). The 95% confidence intervals of the rarefaction curves (ŜMao Tao) were calculated to determine whether species richness was significantly different among data sets.
Finally, to investigate the effect of A. saligna on the identified species guild on the flora of the habitats, we analyzed the frequency of focal and ruderal species (weighted by species cover) in invaded and non-invaded plots and verified the differences with a Chi-squared test (Statistica 7.0).
3 Results
The ordination scatter diagram obtained from the PCoA analysis separated the relevés into two groups according to their floristic composition (Fig. 1). The groups were statistically different (ANOSIM test; R: 0.4613; P < 0.001). One group included the shrubby vegetation of the Mediterranean scrub belonging to the Juniper dune shrubland (habitats 2250*) and the Dune sclerophyllous scrubs (habitat 2260). The other group was primarily represented by the Pinus dune wood (habitat 2270*). This ordination highlights that A. saligna is able to colonize both Mediterranean scrub (habitat 2250* and 2260) and Pinus dune wood (habitat 2270*), although it is more common in the latter vegetation type.
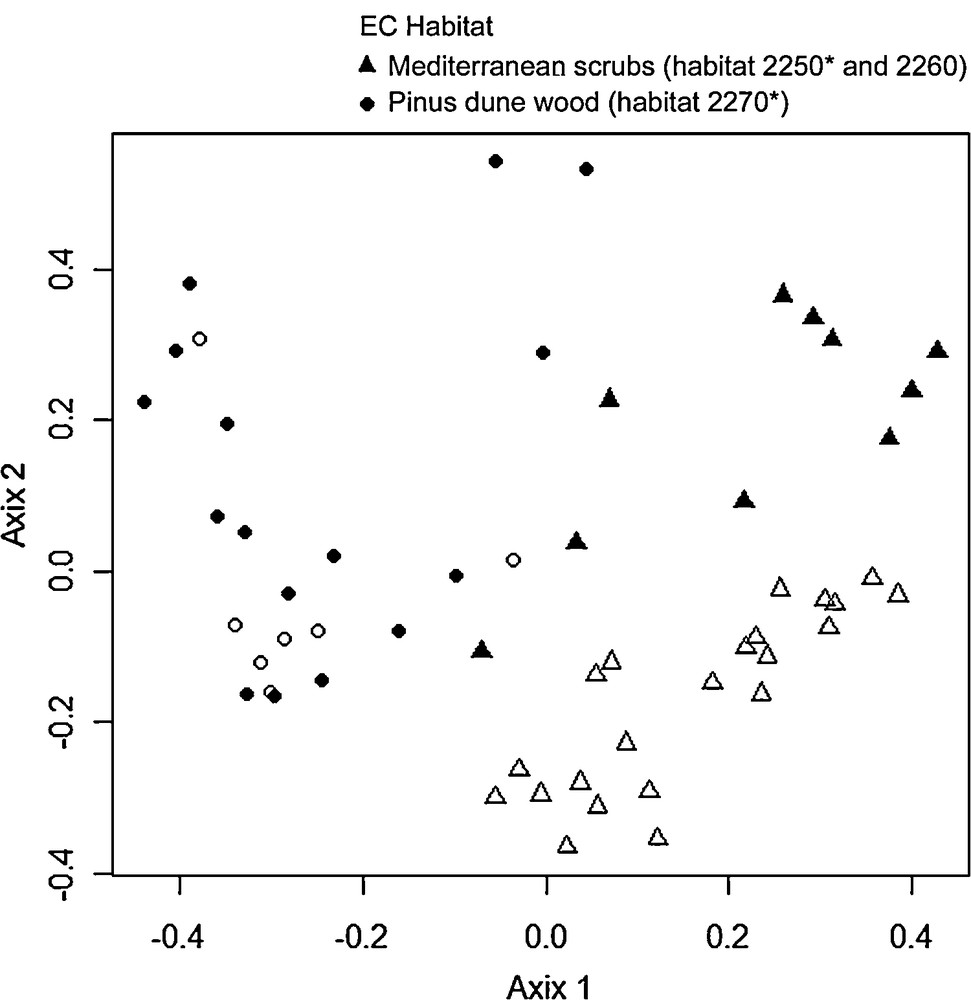
PCoA scatter diagram of sampled plots, using species as explanatory variables. Only the first two axes are represented. Solid symbols represent Acacia saligna invaded plots; empty symbols represent non-invaded plots.
Most of the invaded plots were located in areas of lower tree coverage, suggesting that A. saligna grows better in open woods. The logistic regression model (extraction method = forward conditional; −2 log likelihood = 27.6) allowed correct classification of 68.8% of the cases (χ2 = 12.1, P < 0.001), and showed that the presence of A. saligna was significantly influenced by the percentage of canopy cover (score 0.051, P = 0.005; Fig. 2). The ecological equation was:
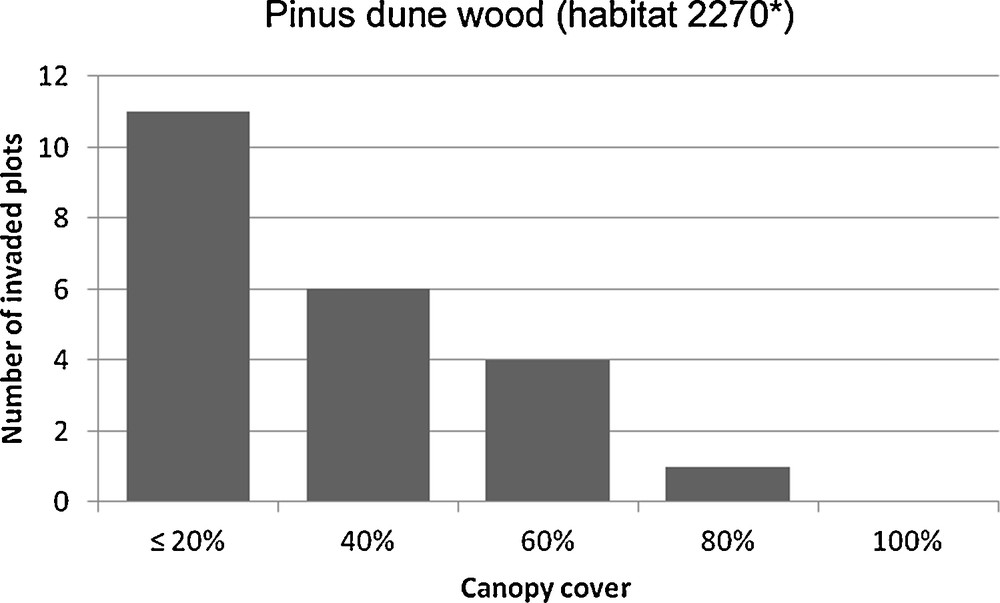
Total number of Acacia saligna invaded plots in relation to canopy cover percentage classes. The presence of Acacia saligna decrease when the canopy cover is higher.
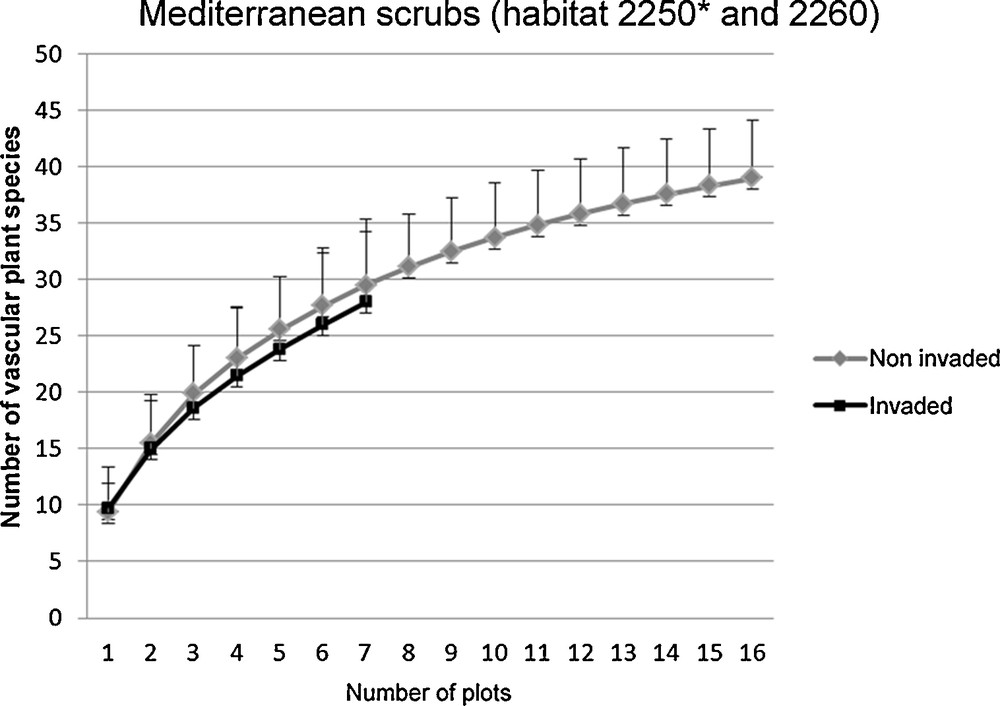
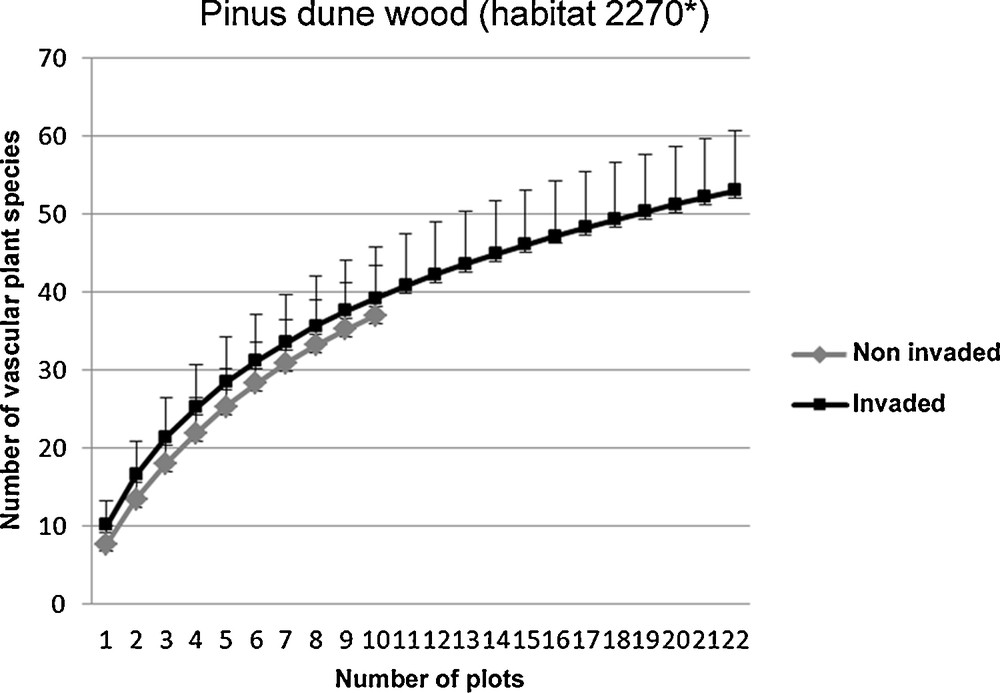
The rarefaction curves (based on the overlapping confidence intervals for ŜMao Tao) show similar species richness values in invaded and non-invaded plots (Figs. 3 and 4). However, we found significant differences in the identified guilds. In the Pinus dune wood plots (habitat 2270*), we found a higher frequency of ruderal species in the invaded plots and a higher frequency of focal species in the non-invaded plots (Chi-squared test; χ2 = 0.06; P < 0.001; Fig. 5). Conversely, in the Mediterranean scrub (habitats 2250* and 2260), we observed no differences in the frequency of the ruderal species or the focal species between the invaded and non-invaded plots (Chi-squared test; χ2 = 25.97; P = 0.8; Fig. 6).
Rarefaction curves for all vascular plant species in Acacia saligna invaded and non-invaded plots of the Juniper dune shrubland (habitat 2250*), and Dune sclerophyllous scrubs (2260). The differences in species richness were not significant.
Rarefaction curves for all vascular plant species in Acacia saligna invaded and non-invaded plots of the Pinus dune wood (habitat 2270*). The differences in specie richness were not significant.
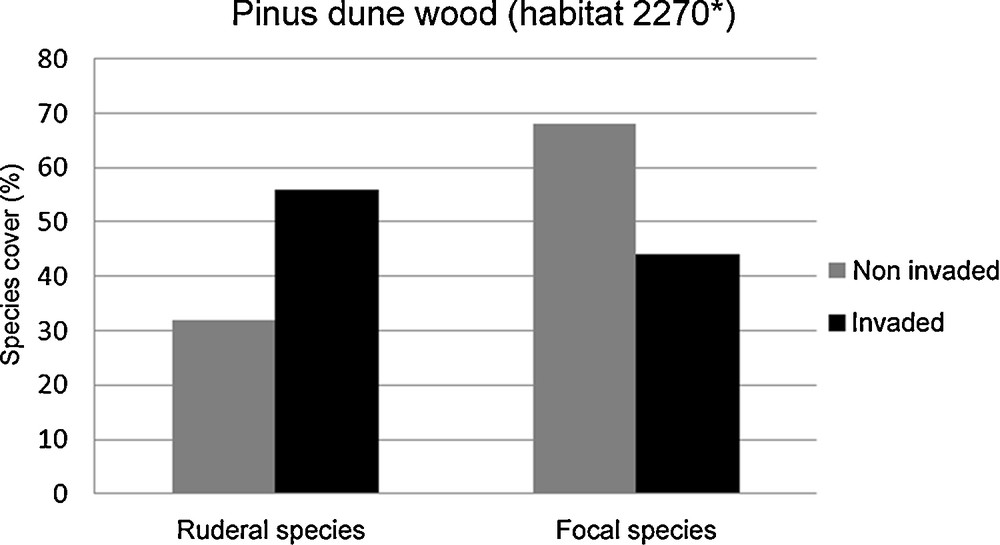
Frequency of ruderal species and focal species between Acacia saligna invaded and non-invaded plots of the habitat 2270*. Ruderal species: 56% in invaded plots; 32% in non-invaded plots. Focal species: 44% in invaded plots; 68% in non-invaded plots.

Frequency of ruderal species and focal species between Acacia saligna invaded and non-invaded plots of the habitat 2250* and 2260. Ruderal species: 23% in invaded plots; 24% in non-invaded plots. Focal species: 77% in invaded plots; 76% in non-invaded plots.
4 Discussion
A. saligna is considered to be an invasive alien in several different countries. It can form dense stands due to its great ability to survive in harsh environments and to resprout after cutting or fire [1,19]. Our results showed that A. saligna is able to colonize the Mediterranean scrub (habitat 2250* and 2260) as well as wooded dunes with Pinus pinea and/or Pinus pinaster.
In particular, in our study area, this alien species is more invasive in the Pinus dune wood (habitat 2270*) than in Mediterranean scrub (habitats 2250* and 2260). It is probable that A. saligna began to spread from the original plantation towards the inland stands of Pinus dune wood and, toward the coastline, to the Mediterranean scrub (habitat 2250* and 2260). This colonization process occurs although the latter habitats appear to be more resistant to alien invasion. In fact, Mediterranean scrubs are close to the sea and grow on less mature and sandy soil. The shorter distance from the beach entails a stronger influence of the sea, a higher drought and mobility of the soil, which create harsher condition to A. saligna seedling germination and survival. Moreover, soil-heating favors seed germination in A. saligna [53]. We hypothesize that the germination of this species can be contrasted in the Mediterranean scrub (habitat 2250* and 2260) because this vegetation has a dense structure that shades the soil. It is likely that a lower solar irradiation entail a lower soil temperature, which could impair A. saligna seed germination. To support this hypothesis, we observed that in the Pinus dune wood (habitat 2270*), A. saligna invades sites where the tree canopy is open, with a higher solar irradiation at the soil level (such as wood gaps). So, we suggest that these features decrease the propagation potential of A. saligna in the Mediterranean scrub habitats.
Although we did not find any effect of A. saligna on total species richness, we observed significant results if species belonging to particular guilds were considered. In the invaded plots of the Pinus dune wood (habitat 2270*), we found an increase in ruderal grass species, typical of disturbed environments with a significant decrease in focal species. Previous studies demonstrated that other invasive Acacia species proliferate in disturbed areas, such as abandoned fields or after disturbance events (e.g. fire, soil movement) [19,33]. Other quoted studies have found that in Fynbos shrublands, forbs represent the major growth form in areas that have been invaded by A. saligna for a long time. In light of our findings, it is possible that the presence of A. saligna is favored by the presence of more open, disturbed sites. However, this species can also promote the proliferation of ruderal species. Acacias are N-fixing species and produce substantial amounts of litter (including leaves, twigs, flowers, and fruits). The litter can modify the properties of the soil by enhancing the N pool and the organic matter content [23,25,29]. The vegetation of coastal dunes is sensitive to soil modification caused by litter accumulation from invasive plants [54–56]. Native species of semi-fixed and fixed dunes are, in fact, adapted to grow in poor soils [57], and soil modification as surrogate for disturbance can alter the turnover of species, favoring ruderal species, which are often nitrophilous [58]. In agreement with this finding, A. saligna is related to an increase of ruderal species in the coastal dunes, such as the small annuals Bromus madritensis, Geranium purpureum, Oryzopsis miliacea, and Parietaria officinalis, which grow in mesotrophic soils on coastal sand substrate [38]. We suggest that A. saligna could establish in open and disturbed areas where ruderal species already occur; otherwise, it could also be the cause of the increase of ruderals in the understory flora of Pinus dune wood (EC habitat 2270*) by habitat modification. However, further studies are needed to elucidate this, also investigating the population structure in dense and open areas.
We observed that A. saligna affected only focal and ruderal species, not the total pool of species. This finding can depend on the time of colonization by the invading species. Long time invaded areas show a stronger impact of alien species on the native flora [59]. Since A. saligna has been introduced relatively recently (approximately 1950), it is possible that currently only specific guilds have been affected by the more or less recent colonization process. Thus, it is possible that our findings represent only an early stage of the A. saligna invasion process, whereas other effects could be observed at a later stage. In fact, although no significant differences were recorded for the plant species guilds of Mediterranean scrub, we cannot exclude changes in the near future.
These results may also have useful implications for coastal ecosystem management. In fact, it was noted that A. saligna invasive processes represent an important threat to the conservation of EC priority habitat 2270* in Italian sand dunes [38]. For this reason, the monitoring and management of this invasive species should be thoroughly planned.
Finally, we could highlight that Acacia species management in invaded areas has recently become a relevant research topic. The main strategies to control Acacia species spread are eradication or biological control [60,61]. However, it seems that species removal is often not enough to eliminate this invader successfully [19]. It has been suggested that management plans should combine the species removal with the reduction of the seed bank and native species planting in order to contrast the species spread effectively [19,33,53].
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.


