1 Introduction
The Cerrado (Brazilian savanna) biome is characterized by a prolonged dry period and plant dormancy in the winter season, after which the vegetative apices reinitiate development [1]. The new leaves are exposed to a dry environment just when they are devoid of structural traits to reduce desiccation risks. The occurrence of colleters and the production of highly hydrophilic acidic polysaccharides by these structures appear to represent an important mechanism for water retention in young and desiccation-vulnerable organs, mainly during the expansion stage [2].
Celastraceae is a family widely distributed in tropical and subtropical regions, and is represented in the Cerrado by a number of species that produce edible fruits and can present medicinal or ornamental value [3–5]. Among these Cerrado species, Tontelea micrantha stand out by its edible fruits, and the commercially valuable oil extracted from its seeds has anti-inflammatory properties [6].
The mature leaves of T. micrantha demonstrate a number of morphological characters associated with protection against water losses. Young leaves, however, are exposed to an extremely dry environment during their initial stages of expansion, but the structures that will be avoid dehydration in fully mature leaves are still under development (Mercadante-Simões, unpublished data).
In addition to the role of colleters in protection against excessive transpiration, it will be important to consider their relevance as useful characters in the taxonomy of some families [7–9], as well as their possible value to understand phylogenetic relationships [10].
In light of the ecological importance of colleters to plants that inhabit hot and dry environments like the Cerrado, the potential taxonomic value of these structures and the absence of any previous report of their occurrence in the order Celastrales, the present work describes the colleters found in T. micrantha leaves and discusses about their functional aspects.
2 Materials and methods
Vegetative branch apices of T. micrantha (Mart. ex Schult.) A.C. Sm. were collected in an area of Cerrado in the municipality of Montes Claros, Minas Gerais State, Brazil (16°52′15′′S, 44°00′58′′W). The collections were undertaken at the end of the dry season, a period that coincides with the emission of new leaves. Voucher specimens were deposited in the BHCB Herbarium, Department of Botany, Universidade Federal de Minas Gerais, Belo Horizonte, MG, under number 144623.
The numbers of colleters per leaf and their biometric data were obtained by examining 20 leaves from five individual plants. Fragments of the vegetative apices and young leaves (about 20% of their final size) were fixed under vacuum conditions in Karnovsky solution [11] and subsequently embedded in 2-hydroxyethyl-methacrylate (Leica Microsystems, Heidelberg, Germany). The sections (5 μm thickness) were obtained using a rotary microtome and stained with Toluidine Blue O, pH 4.7 [12,modified]. Histochemical tests were performed using: ruthenium red to identify acidic polysaccharides [13]; Alcian Blue [14] and tannic acid [15] to stain mucilage; Sudan IV to identify lipids [14]; Lugol reagent to detect starch [16]; 10% ferric chloride to detect phenolic substances [16]; and NADI reagent to identify essential oils and resins [17]. Photographic documentation was performed using an Olympus B × 41 light microscope with a digital camera coupled.
2.1 Electron microscopy
For electron microscopy studies samples of young leaves (about 20% of their final size) were obtained in the Cerrado and immediately processed. For scanning electron microscopy (SEM), these samples were fixed in a 2.5% glutaraldehyde solution (0.1 M phosphate buffer, pH 7.2), dehydrated in an ethanol series, dried to their critical point using CO2, mounted on aluminum stubs and gold coated [18] using a MED 010 Balzers Union apparatus. The samples were analyzed with a Quanta 200 Scanning Electron Microscope (FEI Company, Eindhoven, Netherlands) with digital image capture at 12–20 kV.
For transmission electron microscopy (TEM), the samples were prepared using conventional methods [19]. These samples were fixed in Karnovsky's solution [11] for 24 h, post-fixed in 1% osmium tetroxide (0.1 M phosphate buffer, pH 7.2) and dehydrated in a graded acetone series for embedding in Araldite resin. Ultrathin sections (50 nm) were contrasted with a saturated solution of uranyl acetate and lead citrate. The sections were examined using a Philips CM 100 TEM (Philips/FEI Corporation, Eindhoven, Netherlands) at 80 kV.
3 Results
3.1 Distribution, structure, and ontogenesis
The vegetative apices of T. micrantha show leaf primordia emerging in pairs from the apical meristem, resulting in an appressed vernation and giving rise to leaves with involute margins (Fig. 1A and B). This overlapping of the leaves creates chambers shielding the leaf primordia of the posterior node (Fig. 1B) and immerses them in secretions produced by the colleters. The abundant secretion is observed to be hyaline and viscous, and spread out over all the surfaces of the expanding leaves and the apical meristem (Fig. 1A–D).
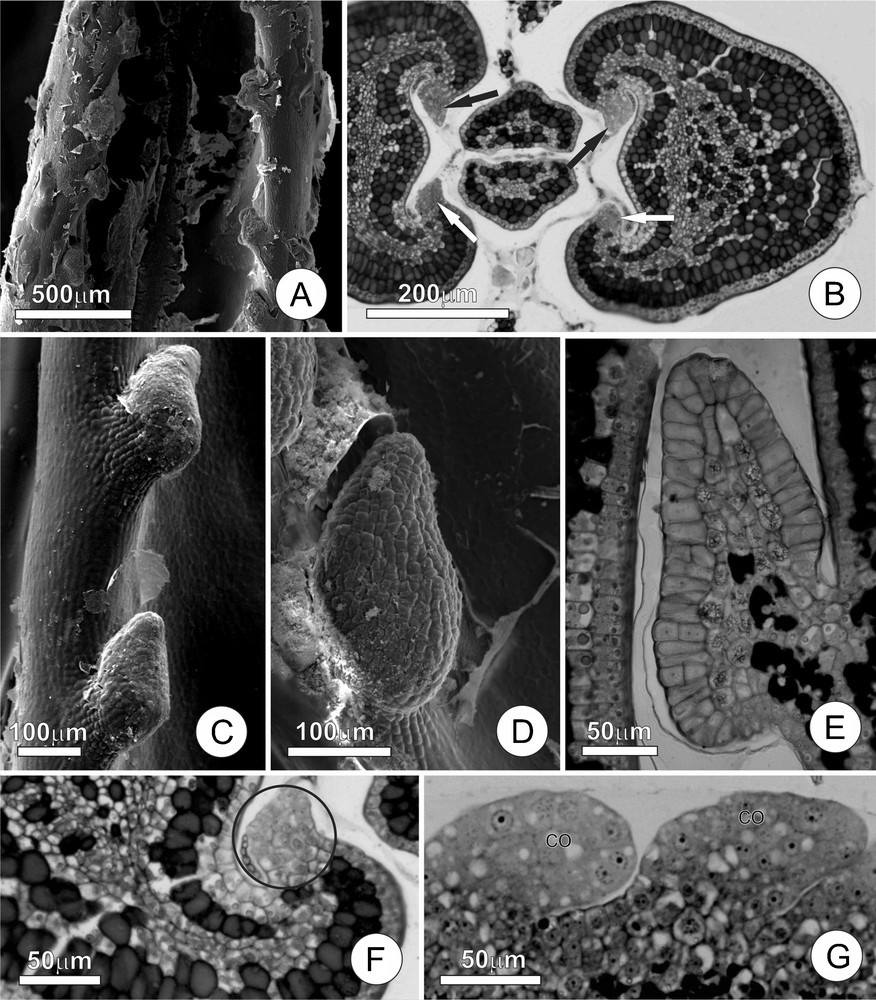
Leaf colleters of Tontelea micrantha. A. Young leaf at the beginning of expansion, showing the leaf colleters and secretion residues. B. Transversal section of the stem apex, demonstrating colleters at the beginning of their development (arrows) in a chamber, with secretions enveloping the leaf primordia. C and D. Detail of a colleter, note the short stalk and the secretion residues. E. Longitudinal section of a completely differentiated colleter; note cells in the central column containing druses and phenolic compounds, as well as the secretory epithelium composed of palisade cells. F and G. Colleters in their initial stages of ontogenesis; note the participation of the ground meristem and the protoderm. (Co) colleter.
The colleters, found in small teeth along the margins of the young leaves (Fig. 1C and D), are claviform and occur in amounts that vary according to the leaf size (approximately 30 per leaf); they are about 200 μm long at maturity. The secretory phase of these colleters initiates in very young leaves (approximately 10% of their final size) and extends until the end of the leaf expansion phase. When young, the colleters are hyaline, but they gradually turn brown as the leaf expands. At the end of the leaf expansion phase, the colleters accumulate phenolic substances, become necrotic and abscise from the leaves, leaving discrete and practically imperceptible scars on the mature leaves.
The colleters of T. micrantha have short stalks and an elongated secretory portion that accounts for most of their total length (Fig. 1E). The stalk is approximately two to four cells long and is covered by cubical and vacuolated non-secretory cells. The central axis of the secretory portion (Fig. 1E) is projected above the stalk, and is composed of isodiametric parenchyma cells with a conspicuous nucleus, and a large vacuole containing druses and phenolic substances; there are no vascular elements in the central axis. The secretory epithelium is predominantly uniseriate, and is composed of radially elongated palisade cells, sometimes with inconspicuous intercellular spaces; very few cells demonstrated periclinal divisions. The secretory cells have cuticle and thin cell wall, dense cytoplasm, a large nucleus, and reduced numbers of vacuoles. No rupturing or pores in the cuticle are noted, but they probably occur to release secretion.
The ontogenesis of the colleter starts with a small group of protoderm initials and ground meristem cells (Fig. 1F and G). The multiplication of the protoderm cells by anticlinal divisions increases the colleter surface. The ground meristem initials proliferates through a series of divisions in many planes, forming a dome that progressively increases in volume until taking on the dimensions and characteristic shape of a mature colleter.
3.2 Ultrastructural aspects
The cells of the secretory epithelium (Fig. 2A–D) has dense cytoplasm and large population of organelles, notably dictyosomes, plastids, endoplasmic reticulum and mitochondria. The cuticle (Fig. 2A), although thinner if compared with the other regions of the leaf blade, appeared intact. Golgi apparatus is conspicuous (Fig. 2B–D), with numerous dictyosomes from which large numbers of vesicles are formed. Vesicles are observed fusing (Fig. 2C) with vacuoles or with the plasma membrane, which becomes visibly sinuous. Plastids (Fig. 2B) are not very numerous and have dense stroma and large starch grains; their internal membrane systems are only poorly developed. The endoplasmic reticulum (Fig. 2D) is composed of a web of dilated tubular cisterns that permeated the cytoplasm, frequently associated with few ribosomes. The mitochondria have prominent internal cristae (Fig. 2B–D) and are very numerous.
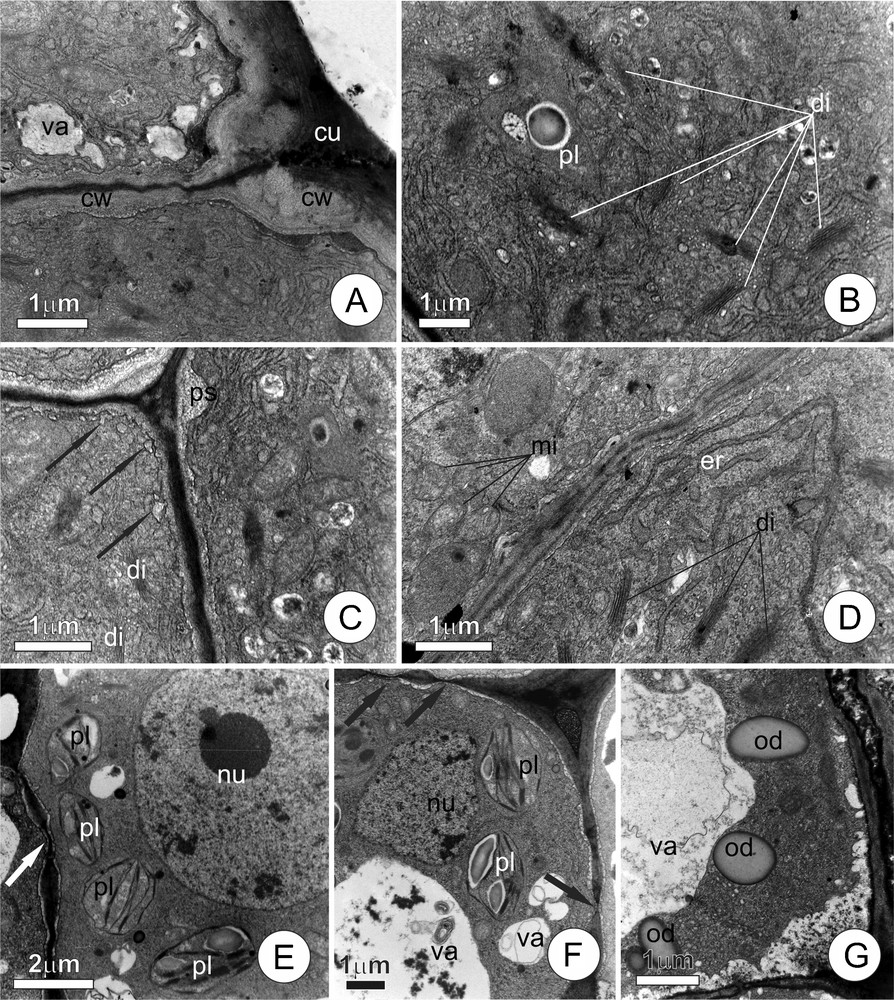
Ultrastructural aspects of the cells of leaf colleters of Tontelea micrantha. A–D. Epithelial secretory cells. A. Distal portion of the secretory cells, note dense cytoplasm and cell wall with cuticle on the external periclinal face. B. Detail of the cytoplasm, showing plastids and numerous dictyosomes liberating vesicles. C. Peripheral cytoplasm, arrows indicate the fusion of vesicles with the plasma membrane; note the initiation of the amplification of the periplasmatic space. D. Cytoplasm with numerous dictyosomes, mitochondria, and endoplasmic reticulum. E–G. Cells of the central axis; note the nucleus and large vacuoles; primary pit fields (arrows); plastids with well-developed internal membrane systems and starch grains; lipidic reserves. (cu) cuticle; (cw) cell wall; (di) dictyosomes; (er) endoplasmic reticulum; (mi) mitochondria; (nu) nucleus; (od) oil droplet; (ps) periplasmatic space; (pl) plastid; (va) vacuole.
The central axis of the colleter (Fig. 2E–G) is characterized by the presence of cells with a conspicuous nucleus, well-developed vacuoles, many primary pit fields, and cytoplasm with few organelles, in which plastids predominate. The cells contain reserve substances, including starch grains within the plastids, and oil droplets dispersed throughout the cytosol.
Colleters of distinct ages (Fig. 3) demonstrated a clear sequence of events culminating in the liberation of the secretion to the plant surface. The presence of peripheral vacuoles (Fig. 3A) with fibrillar content is notable in certain cells, while others demonstrate the same type of material but in the intercellular, periplasmic, and subcuticular spaces (Fig. 3B–D). In some cells, the periplasmic space distended by the accumulation of fibrillar material, occupies most of the cell lumen, compressing the protoplast in the central portion of the cell and giving it a lobed outline (Fig. 3C). The presence of fibrous material in the subcuticular spaces is likewise very noticeable, causing the displacement of the cuticle and creating many ample spaces (Fig. 3D). Some of the colleters (Fig. 3E) show intense proliferation of the rough endoplasmic reticulum in the cytoplasm, to the point that they become the predominant cell organelle; the protoplast of these cells is also seen to be very electron-dense.
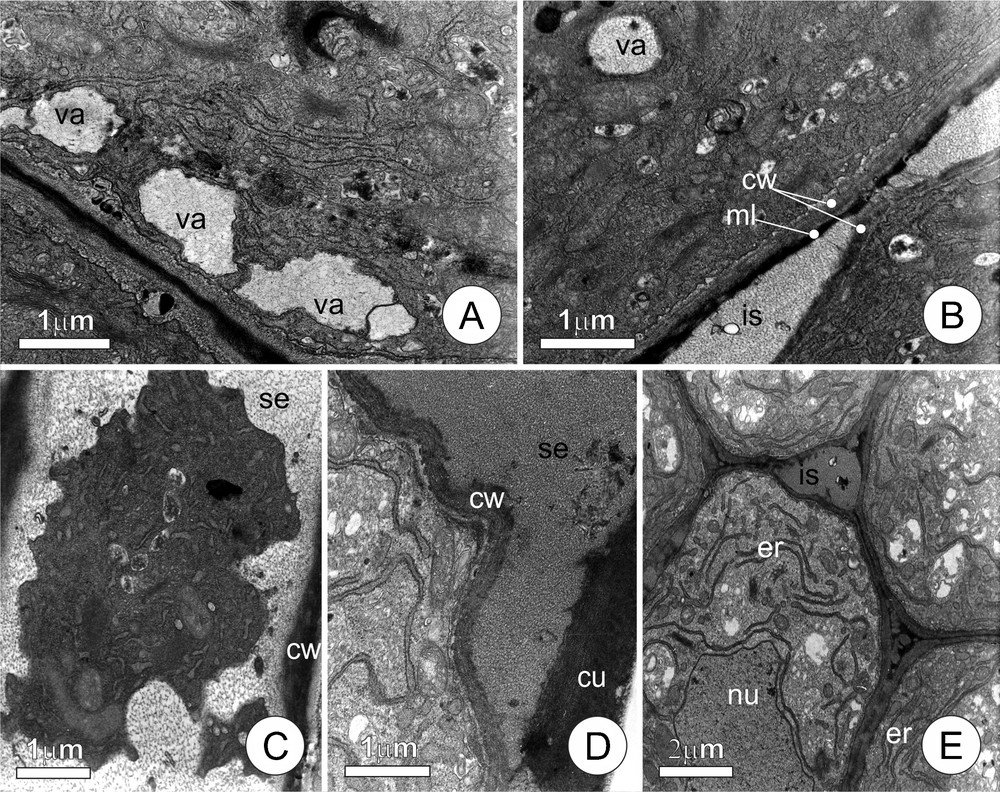
Ultrastructural aspects of the secretory cells of leaf colleters of Tontelea micrantha, demonstrating details of the secretion process. A. Vacuoles forming by the fusion of secretion vesicles originating from the dictyosomes and positioned near the plasma membrane. B. Secretion accumulated in the intercellular space (note middle lamella between adjacent cells). C. Protoplast showing a lobed contour, pressed by the accumulating fibrous materials (secretion), in the periplasmatic space. D. Secretion liberated into the subcuticular space. E. Final phase of the secretory process; the conspicuous endoplasmic reticulum suggests the production of lytic enzymes. (cu) cuticle; (is) intercellular space; (ss) subcuticular space; (ml) middle lamella; (nu) nucleus; (cw) cell wall; (er) endoplasmic reticulum; (se) secretion; (va) vacuole.
4 Discussion
4.1 Colleter distribution, structure, and ontogenesis
This work presents the first report of the occurrence of colleters in T. micrantha. Besides their functional importance, these structures may also have taxonomic value in Celastraceae, because of their presence in other genera and species (Mercadante-Simões, unpublished data). The occurrence of colleters in this family is so spread that suggests the presence of colleters as a possible synapomorphy in Celastraceae. Colleters may have significant taxonomic importance, which could aid future phylogenetic analyses, as pointed by Robbrecht [20]. So, it will be very interesting to widen the search for these structures. The relevance of colleters to understand phylogenetic aspects was recently pointed by Silva et al. [10] in Myrtaceae.
The secretion produced by colleters relates to the protection of shoot apices and young leaves against excessive transpiration; this assumption is reinforced by a series of structural evidence and seems to be the main role of colleters [21]. Diverse morphological characters have been observed in Cerrado plants that can contribute to reducing water losses under severe climatic conditions. The occurrence of colleters, however, had been thought to be restricted to just a few species, such as Mandevilla spp. [22], Caryocar brasiliense [23], and Hymenaea stigonocarpa [24] – although this may likewise reflect the scarcity of detailed morphological studies undertaken with secretory structures in this biome.
The fact that colleters become functional shortly after being formed and then become senescent with leaf expansion, as was observed in T. micrantha, reinforces the relationships of these structures with protecting the shoot apices against dehydration. In the mature leaves, the small scars that we observed at the leaf margins are the result of colleter abscission and suggest the formation of an abscission zone, as reported in Cariniana estrellensis [21]. According to Paiva [21], it was noted that the formation of a colleter abscission layer would be expected on durable plant organs such as leaf blades, which has been corroborated by observations of other species of Celastraceae (Mercadante-Simões, unpublished data).
The presence of colleters along leaf margins has been reported in other taxa of diverse angiosperm families [21]. According to this author, there appears to be a strong correlation between the placement of colleters and ptixys on leaf blades that are rolled up onto themselves (as in the involute type, for example). The presence of colleters along the leaf margin and slightly involute ptixys in T. micrantha corroborates the statement that as the placement of the colleters in this species appears to facilitate the distribution of the mucilaginous secretion over the entire leaf surface.
The leaf colleters of T. micrantha are the standard type, according to the classification proposed by Thomas [25]. Colleters of the standard type are characterized as emergencies due to their mixed origin from the protoderm and ground meristem. The secretory activity of this type of colleter is restricted to their epithelial cells, while the central parenchymatous cells appear to act in the transference of secretion precursors [23] that are originated in the innermost tissues of the organs in which the colleters are inserted.
The presence of calcium oxalate crystals is common in plant secretory tissues and is reported to colleters of Psychotria nuda [26]. It is known that the calcium excess in the cytosol induces the formation of callose that can block plasmodesmata, making symplastic transport slower [27], similar to the blockage of phloem sieve elements [28]. As such, the presence of calcium oxalate crystals indicates the removal of excess calcium from the cytosol, as suggested by Paiva et al. [29]. This process prevents the blockage of symplastic transport. In T. micrantha colleters, the presence of intracellular calcium oxalate crystals is restricted to the cells of the central axis, which reinforces the hypothesis that those cells really act in transferring secretion precursors.
The starch grains and oil droplets in the colleter secretory cells of T. micrantha probably represent a transient energetic reserve. This strategy facilitates uninterrupted energy source for mucilage synthesis and secretion during their relatively short period of the secretory activity.
The presence of a thin cuticle covering the secretory epithelium of the colleters, as observed in the present study, constitutes a characteristic feature of colleters, corroborating reports from many different taxa. There are some evidence that the secretion is released after cuticle rupture, possibly due to the pressure generated by the secretion accumulation in the subcuticular space. However, we did not observe ruptures or cuticle pores, which may indicate the possibility of cuticle regeneration.
The dense protoplast of the secretory epithelial cells of the colleters of T. micrantha suggests the occurrence of intense metabolic activity, as reported by Lüttge [30] as a marker for secretory cells. It has frequently been reported that the secretory cells of senescent colleters become vacuolated and accumulate phenolic compounds at the end of their functional lifetimes, as we observed in T. micrantha colleters.
4.2 Ultrastructural aspects
Plant secretions are usually chemically complex and, according to Paiva and Martins [31], it is possible to relate the chemical nature of secretions with populations of certain organelles present in the secretory cells. In T. micrantha, the occurrence of numerous dictyosomes in the secretory cells is compatible with the predominance of acidic polysaccharide secretion. Ultrastructural analyses also indicated that the flocculated material seen in the vesicles, vacuoles, and intercellular spaces is a typical component of the mucilaginous secretion, as reported to other structures involved in acidic polysaccharide synthesis [31,32,33].
The fusion of Golgi vesicles to the plasma membrane, with the consequent liberation of the secretion products in the periplasmatic space, constitutes a mechanism of granulocrine secretion, a recurrent process in colleters [2,34]. The fusion of Golgi vesicles to each other in T. micrantha leads to the formation of vacuoles containing secretions that in turn fuse with the plasma membrane in a continuous process of vacuole formation and secretion release, similar to that reported by Paiva and Martins [31] in Ipomoea cairica.
The release of secretion to the periplasmatic space results in the retraction of the protoplast of the colleter secretory cells of T. micrantha, very similar to the process observed in the secretion of pectic substances [31,35]. The accumulation of secretions in the periplasmatic space in T. micrantha appears to be transitory and cyclic, as the secretion forced against the cell wall end up crossing that barrier and permeating the intercellular spaces, and continue on to the plant surface by passing the cuticle.
After the secretory phase, the epithelial cells of the colleters of T. micrantha increase their rough endoplasmic reticulum which may be associated with the synthesis of lytic enzymes. This indicates the beginning of colleter senescence, similar to described by Thomas and Dave [36] in Mitragyna parvifolia and by Denardi et al. [37] in the secretory trichomes of Connarus suberosus.
4.3 Functional aspects
The data obtained in the present study, and the high transpirational demands that occurs in the Cerrado biome during the dry season, when T. micrantha produces its new leaves, suggests a protective role for these colleter secretions against leaf desiccation. This protection would be especially important in young leaves whose xylem cannot yet supply elevated water demands [2].
According to Curtis and Lersten [38], resin is produced in the serrate leaves of Populus deltoides (Salicaceae); this resin forms a thin coat over the entire leaf and probably has an effect similar to a cuticle in retarding transpiration from epidermal cells. Similarly, the secretion produced by the leaf colleters of T. micrantha spread over the leaf blade and, due to their hygroscopic nature, helps to protect the leaves against excessive transpiration and may even act to retain humidity from the atmosphere that can subsequently be absorbed by the leaf tissues.
The possibility that colleters at leaf margin which occur widely in Celastraceae is reinforced by the observation of this same colleter positioning in 16 genera of this family (Mercadante-Simões, unpublished data). In describing the leaf anatomy of species of Celastrus, Elaeodendron and Euonymus, Pant and Kidwai [39] reported that each marginal tooth in the leaf terminated in a glandular tip indicates the presence of colleters in those genera. As such, our results indicate the relevance of additional studies of the leaf margins of other Celastraceae species in order to recognize the pattern of distribution of colleters in this family.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
The authors would like to acknowledge the Cemel (UFMG) and Center of Electron Microscopy, Instituto de Biociências, UNESP for providing the equipment and technical support for experiments involving electron microscopy. The authors would like to thank D.M.T. Oliveira for helpful comments. This work was supported through a research grant from the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq [308589/2011-4] and Fapemig [PCRH and PPM]. Thanks also to Ildeci Fonseca for the help in field work.


