1 Introduction
The cultivation of olive trees prevails in the Mediterranean countries, where 90% of the world olive trees are concentrated [1]. The most productive regions of olive oil are located in Spain and Italy. Algeria ranks at the eighth position with oil production estimated at 1% of world production [2] and the department of Tizi-Ouzou ranks at the second position in Algeria after Bejaia. The cultivated varieties of olive trees are Olea europea var. chamlal (90%) and O. europea var. azaradj and O. europea var. lemli (10%) (Direction des services agricoles, Tizi-Ouzou). Oil industry generates in a short period (2 to 6 months) huge quantities of seasonal wastes: olive mill waste water (OMWW) or vegetation water and solid olive mill wastes (SOMW) or olive-cake or olive pomace. To get rid of these wastes that amount to 80% of the initial weight of harvested olive (SOMW: 30%, OMWW: 50%) represents a crucial problem for oil-producing countries [3,4], all the more that the world demand for olive oil is continuously increasing [5].
The raw solid waste is a by-product of the first pressing or centrifugation. It is composed of husk of the grinded core (endocarp or pit) (42%), the crushed “kernel” (3%), the olive envelope (epicarp or skin) and the pulp (mesocarp) (21%) and about 25% to 28% of water and a significant quantity of residual oil (9 to 10%), which enhances the rancidness of the SOMW [6,7]. The fact that it contains a lot of raw cellulose (35 to 50%) and phenolic compounds and the link of the proteins to parietal components (75% of nitrogen) makes it a substratum not very used in animal foods, because of its weak digestibility and degradability [7–10].
SOMW are sometimes used, as a combustible, or to extract residual oil, or to “straw” olive trees in order to prevent erosion (but gives rise to soil acidity problems) or finally composting [11–13]. Another suitable way of SOMW valorization is its conversion into edible mushrooms that could be used in country, like Algeria, where demography is much increasing. Indeed, fruiting bodies are of high nutritional value, they contain an important amount of proteins well balanced with essential amino acids, vitamins and minerals and have low sugar and fat contents [14–17]. Pleurotus spp. fall between high-grade vegetables and low-grade meats [18]. Edible mushrooms are recommended by FAO as food, contributing to the protein nutrition of cereal-dependent developing countries [19]. They can be marshaled to aid in solving many problems, like protein shortage, resource recovery, and environmental management [20]. The most used species for the valorization of various rural or agricultural crude wastes is the white-rot fungus Pleurotus spp. (oyster mushroom). Pleurotus spp. is cultivated worldwide, it has favorable organoleptic and medicinal properties, and its growth is vigorous. The interesting aspects of the cultivation of Pleurotus are that it is a relatively simple and low-cost technology with high yield potential [21–24], and it does not require a composted substrate [25,26]. In addition, the production of mushroom is a way to promote employment and social development in rural areas [27,28].
Criteria of good mushroom production are a good yield of fruiting bodies, estimated by Olivier et al. [29] to 10–16% of marketable mushrooms harvested per disinfected and spawned substrate for non-enriched substrates and 14–18% for substrates rich in protein inoculated at the rate of 5–8%, with short incubation period (8–10 days) and fruiting duration (3 weeks).
The life cycle of the oyster mushroom goes through two phases: the vegetative and the fruiting stages. In mushroom cultivation, the vegetative growth ensures dicaryotic mycelium growth. Both stages have different requirements and, according to Olivier et al. [29], they depend on three main factors: the strains themselves, growing medium and environmental factors.
The fungus is saprophytic, then, the growing medium must bring him the organic matter it needs, such as carbon, nitrogen, minerals, and vitamin. The carbon elements represent a major source of energy for the growth of mushrooms. The carbon used by the oyster mushroom is in a complex form, cellulose, hemicellulose, lignin, and pectin. Oyster mushrooms use for their development agricultural wastes rich in cellulose with low nitrogen content. According to Delmas [16], nitrogen requirement of mushrooms seems to be covered by its ability to extract the available forms of nitrogen, even in low quantities. The C/N ratio can be up to 40–50.
Oyster mushrooms need evenly oxygen for breathing and for degradation of lignin [30], minerals as potassium, phosphorus, magnesium, calcium, zinc… and vitamin (thiamine). The main problem for growing mushrooms is the contamination by molds of the genus Trichoderma (Velázquez-Cedeño [22]) and the genera Penicillium and Aspergillus [31]. A pH value from 5 to 6, which is favorable for the growth of Pleurotus ostreatus (pH 5–6), is also beneficial for the development of Trichoderma; so, Laborde and Delmas [32] recommend a pH of 6–7 and Philippoussis [25] recommended a pH of over or equal to 7.5. The optimum growth temperature is 25 °C and the fruiting temperature is about 15 °C [29]. Oyster mushroom requires a humidity of 80–85% in the incubation period and 80–90% during fruiting. Fruiting requires light, preferably of daylight type.
In the present work, the potential of the local strain was compared with that of a commercial strain of P. ostreatus for its ability to produce fruiting bodies by using SOMW as a substrate. The aim was to determine if SOMW could be valorized near the place of their production by generating human food and a complementary source of income.
2 Material and methods
2.1 Fungal strains
Two strains of oyster mushrooms were used: A local strain of Pleurotus ostreatus (Jacq.: Fries) Kummer (LPO) isolated in 1993 under our care in Oued-Aissi (Tizi-Ouzou, Algeria) from tissue culture of the fruiting bodies [identification of LPO was performed using morphological characters in 1993 and confirmed in 2007 by the method of Internal Transcriber Spacer (ITS)] and a commercial strain of P. ostreatus (P535) (CPO) coming from the “Royal Champignon” company (France) in 1993. The strains are kept in tubes on PDA (potatoes–dextrose–agar) at 4 °C, and sub-cultured every 3 to 4 months.
The quality of the strains after storage was determined by measuring the diameter of the mycelia formed along two perpendiculars axes of LPO and CPO on PDA, after incubation for 8 days at 25 °C. Ten replicates per strain were used.
2.2 Spawn preparation
We used the method consisting of moistening barley grains (90 to 100 mL water/100 g of washed and drained barley grains) and then, sterilized during 1 h at 120 °C in an autoclave; each bag, containing 100 g of sterilized grains, was inoculated by using the mycelium colony developed completely on 30 mL of PDA in a 9 cm Petri dish [31]. The barley grains used are taken from the harvest of the year. They were purchased from a farmer of Boghni (Wilaya of Tizi-Ouzou, Algeria).
The multiplication of the spawn was achieved from the first spawn made by inoculating grain bags with 10 g of the spawn (Fig. 1), according to the method used by Velázquez-Cedeño [22].

Spawn of CPO (left) and LPO (right).
2.3 Estimation of mycelial ability to colonize pasteurized SOMW
Raw solid olive mill wastes was gathered from a modern olive mill (three phases) in Oued-Aissi (Tizi-Ouzou, Algeria). The water content (29.8%) was increased with 350 mL of distilled water/kg of solid waste and pasteurized by thermal processing in a steam cooker during 1 h (Fig. 2) [31,33]. Samples of 40 g of pasteurized SOMW were placed in a Petri dish to estimate the mycelial growth of the two P. ostreatus strains (Figs. 3 and 4). In order to enable ventilation, five small holes were made on the dish covers with a red-hot needle. Each dish was inoculated with three grains of spawn placed at the center, with ten replicates per strain. The mycelial growth ability was measured (in cm2) as the area filled by the mycelium on the third and sixth days of incubation at 25 °C.

Traditional steamer (Couscoussier).

Experimental apparatus for evaluating the mycelial growth in Petri dish of CPO and LPO on SOMW (without spawn, left, with spawn, right).
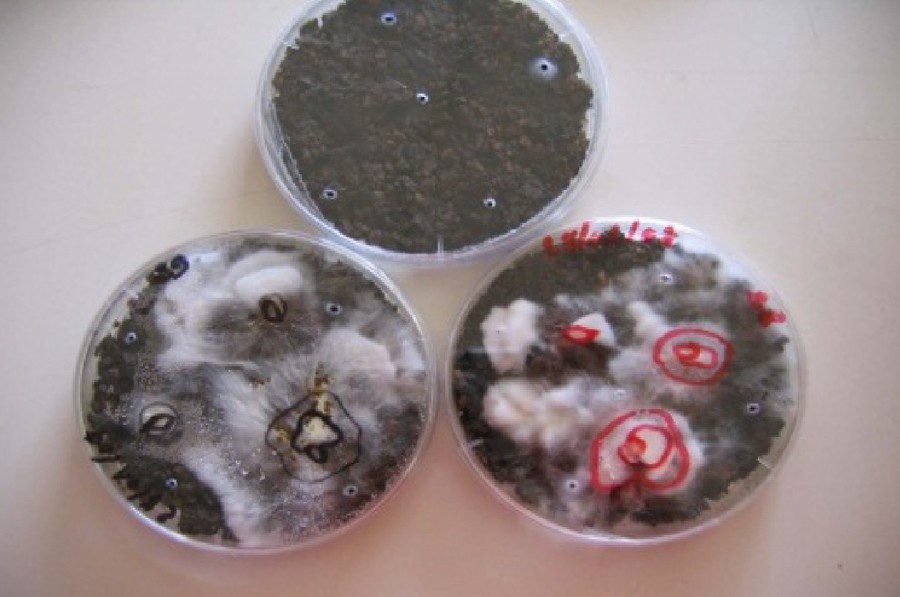
Top: Petri dish with SOMW. Bottom: mycelium of CPO (at the right) and LPO (at the left) after 3 and 6 days of incubation (inner line = limits of spawn, intermediate line = mycelium after three days of incubation, outer line = mycelium after six days of incubation).
2.4 Cultivation in bags and yields
Pasteurized solid olive waste and pasteurized solid olive waste supplemented (SOMWS) (88%) with 2% of calcium carbonate and 10% of wheat straw (WS) were distributed in plastic bags of 2 kg each (Fig. 5). Wheat straw had been previously chopped in pieces of 1 to 3 cm of length, then, soaked in water for the night and afterwards drained for two to three hours to allow the excess water to drop. Five bags were prepared for each strain cultivated on the SOMW and ten bags for each strain cultivated on the SOMWS (The difference in the number of the bags is due to the needs of another experiment. SOMW and SOMWS were wetted with 350 mL of tap water per kilogram of substrate.

Preparation of SOMWS (88% SOMW + 10% WS + 2% CaCO3).
Wheat straw was purchased from the straw market of Oued-Aissi (Tizi-Ouzou, Algeria). The chemical compositions of SOMW, WS and SOMWS are given in Table 1.
Chemical composition of WS, SOMW and SOMWS in % of dry weight.
| SOMW | WS | SOMWS | |
| Moisture | 29.80 ± 0.25 | 6.59 ± 0.16 | 16.38 ± 0.32 |
| Dry weigh (%ww) | 70.20 ± 0.25 | 93.41 ± 0.16 | 83.62 ± 0.32 |
| Ash | 1.95 ± 0,09 | 6.06 ± 0.59 | 2.18 ± 0.17 |
| Organic matter | 97.23 ± 0,13 | 97.40 ± 0.20 | 93.51 ± 0.64 |
| Organic carbon | 56.39 ± 0,08 | 54.24 ± 0.37 | 56.49 ± 0.11 |
| Cellulose | 33.42a | 40.80c | ND |
| Hemicelluloses | 15.12a | 31.70c | ND |
| Lignin | 22.1a | 10.00c | ND |
| Nitrogen | 1.06b | 0.38c | ND |
| Phosphorus | 0.113b | 0.08d | ND |
| Potassium | 0.833b | ND | ND |
| Calcium | 0.820b | 0.25d | ND |
| pH | 6.80 ± 0.06 | 6.92 ± 0.08 | 7.65 ± 0.05 |
The bags were aseptically sowed near the flame of two Bunsen burners, with the prepared spawn at the rate of 7% (w/w). The incubation duration was 30 to 35 days at 25 ± 1 °C and 70–80% of relative humidity in air. On the fifteenth day of incubation, the bags were perforated with a very tin needle. When the primordia began to appear, the bags were removed and to enhance fructification, the climatic conditions of the cultivation room were changed. The room was cooled with frozen water (4 °C) and by putting melting ice near the cultivation blocks shaped by the mycelium grown around the SOMW and the SOMWS. The temperature was thus kept at 20 °C and ambient moisture till 95% by means of a humidifier. The fruiting bodies were harvested daily at their commercial stage of maturity. The number and the weight as well as the size of the caps and the length and width of the foot of each fruiting body were recorded and the average yields and the biological efficiency (BE) were calculated. Yields and BE are two way to express the productivity, i.e. the conversion of the substrate into fungal biomass.
These tests were performed between mid October and late December 2009.
2.5 Statistical analysis
The whole results were submitted to a multifactor variation analysis. ANOVA was completed by the Newman–Keuls test when there were differences between the average values (STATISTICA, version 5.1).
3 Results
Both commercial strain and local strains kept in collection since 1993 were of good quality with average diameters of the mycelium colony on PDA after incubation for 8 days at 25 °C being 8.3 ± 0.24 cm and 7.9 ± 0.39 cm, respectively. The difference between the stains was not significant (P = 0.05).
The spawn was obtained after incubation for 8 to 10 days and it was of good quality with barley grains, well coated by a white root-like mycelium.
The ability of the two strains to colonize pasteurized SOMW was not significantly different (Table 2). It is noteworthy that the average mycelial growth rate between day 0 and day 3 was 0.5–0.6 cm2 per day, whereas it was 1.05 cm2/day between day 3 and day 6. This shows that the pasteurized SOMW is a good substrate for P. ostreatus.
As regards for the fruiting bodies (Figs. 6–8), the flushes were not well delimited; the fruiting bodies were formed continuously on both substrates SOMW and SOMWS. The addition of wheat straw (10%) and calcium carbonate (2%) had significantly enhanced the yields of the two strains. The different results are gathered in Tables 3 and 4. On the SOMW alone, there was no significant differences between the strains for most of the parameters, but on average, CPO tend to produce higher percentages of small fruiting bodies than LPO. On SOMW with 10% of WS and 2% of CaCO3 (SOMWS), the yields of the local strain of P. ostreatus were significantly higher (P = 0.05) than those of the commercial strain P535. However, fruiting bodies formed by CPO had caps with biggest average diameter.

Fructification of LPO on SOMW.

Fructification of CPO on SOMW.

Fructification of LPO (a) and of CPO (b) [33].
Fruit body yields according to weight, size of caps, length and width foot intervals of CPO and LPO on SOMW and SOMWS.
| Character | Limit value | SOMW | SOMWS | ||||||
| CPO | LPO | CPO | LPO | ||||||
| Nb | % | Nb | % | Nb | % | Nb | % | ||
| Fruit bodies weigh | 2–4.99 g | 45 | 49 | 52 | 48 | 47 | 17 | 87 | 22 |
| 5–9.99 g | 32 | 35 | 33 | 31 | 57 | 20 | 110 | 27 | |
| 10 g and more | 15 | 5 | 23 | 18 | 176 | 63 | 203 | 51 | |
| Diameter's cap | < 5 cm | 62 | 67 | 52 | 48 | 92 | 33 | 168 | 42 |
| 5–9.99 cm | 27 | 29 | 54 | 50 | 171 | 61 | 188 | 47 | |
| 10 cm and more | 3 | 3 | 2 | 2 | 17 | 6 | 15 | 4 | |
| Foot's length | < 2 cm | 12 | 13 | 22 | 20 | 44 | 16 | 93 | 23 |
| 2–4.99 cm | 68 | 74 | 74 | 68 | 136 | 49 | 155 | 39 | |
| 5 cm and more | 12 | 13 | 12 | 11 | 100 | 36 | 152 | 38 | |
| Foot's width | < 1 cm | 78 | 85 | 87 | 80 | 66 | 24 | 126 | 31 |
| 1–1.99 cm | 9 | 10 | 21 | 19 | 185 | 66 | 241 | 60 | |
| 2 cm and more | 5 | 5 | 0 | 0 | 29 | 10 | 33 | 8 | |
| Total | 92 | 108 | 279 | 400 | |||||
| Nb of repetitions | 5 | 10 |
Cultural characters of CPO and LPO on SOMW and SOMWS.
| Character | SOMW | SOMWS | ||
| CPO | LPO | CPO | LPO | |
| Incubation period (days) | 30 | 30 | 30 | 30 |
| Total duration of the culture (days) | 65 | 65 | 65 | 65 |
| Substrate surface colonized by the mycelium | Totality | Totality | Totality | Totality |
| Aspect of the mycelium | White, root-like | White, root-like | White, root-like | White, root-like |
| Number of sporophores | 92 | 108 | 280 | 400 |
| Average weight sporophores (g) | 8.74 ± 11.8 a | 6.71 ± 5 a | 17.82 ± 15.29 b | 13.81 ± 11.77 c |
| Extreme limit value of the weight (g) | 2–66.76 | 2.02–21.5 | 2.01–107.67 | 2.02–74.82 |
| Average diameter of the cap (cm) | 4.63 ± 1.90 d, f | 5.10 ± 1.51 d, e | 6.04 ± 2.17 g | 5.58 ± 2.09 e, g |
| Extreme limit value of the diameter of the cap (cm) | 1.75–12.50 | 2.50–10 | 1.75–16.50 | 1.53–13 |
| Average length of the feet (cm) | 3.16 ± 1.29 h | 2.65 ± 1.39 h | 3.08 ± 1.47 i | 2.72 ± 1.31 i |
| Extreme limit value of the length of the feet (cm) | 0.5–6 | 0.5–7 | 0.5–11 | 0.4–9 |
| Average width of the feet (cm) | 0.64 ± 0.55 j | 0.62 ± 0.63 j | 1.12 ± 0.51 k | 1.08 ± 0.48 k |
| Extreme limit value of the width of the feet (cm) | 0.2–3 | 0.15–1.8 | 0.5–3.5 | 0.2–3 |
| Yield (g sporophores/kg initial substrate) | 80.39 ± 63.26*l | 72.43 ± 25.84*l | 248.58 ± 71.15**m | 276.24 ± 85.45**n |
| Yield (g sporophores/kg dry substrate) | 114.52 | 103.18 | 297.73 | 330.51 |
| Biological efficiency (% fresh weight mushroom/DWsubstrate) | 11.45 | 10.32 | 29.77 | 33.05 |
| (Yield on SOMWS)/(Yield on SOMW) | 2.60 | 3.20 |
* Average of five replications.
** Average of ten replications.
4 Discussion
The capacity of a mushroom to grow on a lignocellulosic substrate is related to the vigor of its mycelium as well as to its capacity to activate physiological mechanisms necessary to adequately exploit the medium [38]. If fructification characters are among the criteria for determining strain selection; particular interest must be placed on the strain capacity to invade a given substrate; the first important stage in the cultivation of a mushroom on a solid substrate is the speed of the hyphal colonization [38]. In fact, the initial colonization speed is an important character because of competition in case of the presence of antagonistic microorganisms, but the growth of a mushroom must be slow and dense, in order to enable the mycelium to exploit the nutrient resources of the lignocellulosic substrate [27]. PDA has been used by numerous searchers. It was an adequate medium for up-keeping mycelium of both P. ostreatus strains studied here, i.e. LPO and CPO. The present records of mycelia diameters are higher than those obtained previously with the same strains (CPO: 8.27 ± 0.24 cm vs 7.31 ± 0.73 cm; LPO: 7.87 ± 0.39 cm vs 6.36 ± 0.85 cm) [31]. They suggest a physiological adaptation of the strains to this medium and a selection during the regular multiplication on PDA since 1993. It is confirmed when also compared to data obtained by Gibriel et al. [39] after 7 days of incubation (8.6 cm) and those of Alam et al. [40], with an optimal growth of P. ostreatus on PDA of 7.70 cm in 10 days of incubation at 25 °C.
The pasteurized SOMW is a good substrate for P. ostreatus, since the average hyphal speed of LPO and CPO on SOMW in Petri dish was 3.99–4.37 mm/day during the three first days and of 5.78 mm/day during the next three days. These data on a complex medium like olive pomace are higher than those found by Zervakis et al. [27] (3 mm/day) on PDA, a simple medium.
As regarding spawn, the inoculation rate of 10% used for the production of the spawn was chosen to allow the rapid development of the fungus and prevent mold contaminations (Penicillium, Aspergillus). Indeed, the incubation time was reduced to one week, instead of three to four weeks. We have not tested the rates above 10%. A higher rate of inoculation (> 10%) of the spawn will probably enhance the mycelial growth and reduce the incubation period, but the spawn will be too expensive and non-attractive economically, since the barley grains used are those used for human consumption. In addition, with the production of large amounts of spawn, there is a risk of overheating in the oven. Indeed, the excess heat emitted by the fungal activity may kill the mycelium. The inoculation rate of 7% used for the production of mycelium on the two substrates was chosen also to allow rapid development of the fungus and prevent mold contaminations. We have previously tested the rates of 1%, 2%, 3% and 5%, every time we were faced with serious problem of mold contaminations. We have not tested rates above 7%, since Olivier et al. [29] recommended a maximum 8% rate, with a special packaging of the inoculated substrate in the form of tubes of small cross section so as to allow the rapid evacuation of heat. Contrary to the spawn, which is conditioned in pockets of 500 g to 1 kg, bags of cultivation mushroom used in mushroom farms weigh 20 to 25 kg, so the risk of overheating is too high. Actually, the usual rate of inoculation used in mushroom farm is around 5%. Bhatti et al. [41] studied the growth and the yield of P. ostreatus on WS affected by different spawn rates (1% to 10%). They concluded that spawning at a rate of 7% is the best dose for obtaining early and high yielding (BE = 45.4%) with a maximum number of flushes and fruiting bodies per bag. Zhang et al. [42] had tested three levels of spawn (12%, 16%, and 18%). They suggested the use of 16% rate spawn, but they emphasized the need for further studies before to be used (16% spawn) for large-scale cultivation.
The main aim of our work was to check whether the LPO strain isolated in Oued-Aissi could develop on raw SOMW without any supplementation. Since wheat straw is the principal raw material for edible mushroom substrate preparation in Europe and America [43], we have examined in the second time whether the supplementation of the SOMW with 10% of wheat straw and 2% of calcium carbonate could enhance the productivity of the two strains. In both cases, the whole colonization of the substrate by the mycelium needed on average 30 days to be achieved. Fruiting duration was variable according to the bags (30 to 35 days) and the flushes were not well delimited. It is a characteristic of Pleurotus spp. contrary to Agaricus bisporus, the bottom mushroom that has marked flushes [17]. The behaviour of the local strain is similar to that of the commercial strain. In term of performance, SOMW is not completely satisfactory, but in terms of quality of fruiting bodies and in terms of taste, we got the most beautiful and the most soft-fruiting bodies. We had even consumed the foot that is usually tough. Indeed, the yields of the two strains of oyster mushroom were weak, since under ideal growing conditions, 1 kg of well-colonized substrate should yield about 1 kg of marketable mushrooms [25]. The yields are low when compared with the results obtained on coffee-grounds with biological efficiency of 31.7% for CPO and 19.7% for LPO [31].
The yields are very weak when compared with results of different researchers like those of Mane et al. [44] on 28 combinations of five agricultural wastes with Pleurotus sajor caju; cotton stalks with WS gave high BE (79.61%). Upadhyay et al. [45] studied the effect of nitrogen supplementation in wheat straw on the yields of P. ostreatus var. florida and had recorded with mustard cake the lowest BE (53.2%) and highest BE with cotton seed cake (94.6%). Yildiz et al. [46], with cultivation of P. ostreatus on 33 combinations of agro-industrial wastes, had found that the most suitable combination (leaves of European aspen + waste paper) gave a biological efficiency of 82.1%. Zervakis et al. [1] on raw SOMW obtained a BE of 46% and on SOMW mixed with 12.5% OMWW a BE of 36% with cultivation of P. eryngii. The highest BE (125%) was recorded on coffee-pulp with P. ostreatus IE-38 by Velázquez-Cedeño et al. [47].
The conversion of hard lignocellulosic components of solid wastes by oyster mushrooms is surely due to their ability to produce a high level of cellulases and hemicellulases and of phenoloxydases [48]. Sherief et al. [49] have noticed that dipping of rice straw and sawdust in heated water at 70–80 °C sterilized the substrates but also contributes to a slight decrease in the ligneous material. Pasteurization by using a cooking steamer probably helped detoxification of SOMW as the boiling water in the steamer became dark.
In our case, the perforation of the cultivation bags had been done relatively late, after 15 days of incubation, while Sheriff et al. [49] made ventilation holes after three days of incubation. This might have an impact on the growth of the mycelium of LPO and CPO, since the holes allow air renewal and passage of moisture to the mycelium. This period of 15 days was chosen because we had long been faced with mould contaminations that had caused serious losses. The risk for LPO and CPO is the accumulation of carbon dioxide and water shortage. However, according to Olivier et al. [29], during mycelial growth, carbon dioxide is not a problem for Pleurotus that supports up to 20–25% and this rate is negative for fungal competitors, but it is harmful to the fruiting bodies. In addition, for the safety of the staff in the culture room, the rate should not exceed 1%.
The present results obtained about LPO strain are close to those of Velázquez-Cedeño et al. [47], with 50% of fruit bodies having a cap diameter ranging between 5 and 9.9 cm. The CPO strain showed more fruit bodies with little caps (< 5 cm) (65%) and about ten bulky mushrooms (>10 cm). In general, the size of LPO fruit bodies can be compared to those obtained by Mane et al. [44] on soya straw. The CPO ones were slightly smaller. The mushrooms (LPO and CPO) were, however, of good quality. They were creamy-white and very tender, particularly those of small size. The number of fruiting body obtained for CPO and LPO were over the numbers obtained by Islam et al. [19] with P. flabellatus on different substrates. The highest pileus diameters of LPO and CPO fruit body were larger than those obtained by these authors on a mango substrate (7 cm) but, contrariwise, the lowest diameters of the pileus of the fruiting bodies of LPO and CPO were too low (respectively 2.5 and 1.75 cm for LPO and CPO, vs 4 cm).
According to the results of Mane et al. [44], the larger the cap, the longer the foot. We have found out the opposite: the more the cap was large, the more the stipe was short and conversely. The stipe length is a lighting indicator during fructification: the more the stipe is long, the more it misses lighting [46]. Laborde and Delmas [32] related that with excess humidity (90–100%), fruiting bodies of P. ostreatus had a narrow cap with a very long stipe and, in the presence of 75–80% of moistness; fruiting bodies are normal, with a large cap and a short foot. We had noticed that when the stipe is too long, it becomes tough and the mushroom is not marketable.
The weight of basidiomata of CPO was over that of LPO but the numbers of basidiomata of LPO was higher than that of CPO. Indeed, the greater the weight of the basidiomata, the lower the number of basidiomata; this has been equally observed by Zervakis et al. [27]. Supplementation of the solid olive mill waste with wheat straw and calcium carbonate enabled us to improve the yields of the two strains. In fact, addition of 10% wheat straw and 2% of calcium carbonate had multiplied the number of caps of LPO and CPO, respectively, by 3.7 and 3.0 and the yield of LPO by 3.2 and CPO by 2.6 in the same operating conditions, even so wheat straw is considered as a poor source of nitrogen (0.5 to 0.8%) [48]. Nitrogen is an essential element for the cellular functions for growth and various metabolic activities, particularly protein and enzyme synthesis. Wheat straw contains slightly more than 50% of carbohydrates (54.24% of DW in the WS we used), making it one of the best substrates [50]. The presence of straw could also have improved the structure of the substrate. In fact, the straw provides channels to oxygen inflow and through the substratum [12]. The fruiting bodies have the same quality as on the SOMW for the two strains, but even if the local strain had a higher yield, CPO presented pileus with significantly higher average diameter and weight. The yields of LPO and CPO on SOMWS could be compared to the yields obtained by Mane et al. [44] on soya straw, but the caps can be compared to those obtained by these authors on a mixture of cotton stalks and wheat straw for CPO as well for the diameter of the pileus as for the length of the foot. Concerning the other cultural characters taken into account, there were no significant differences (P = 0.05). These results agree with those of Iqbal et al. [51], and it is known in Europe that Oyster mushroom is commonly grown on pasteurized straws of wheat [52–54]. Furthermore, the addition of calcium carbonate improved the pH of the SOMW (7.65 vs 6.80).
As part of another work, a trial was conducted between 15 October 2012 and December 2012 (olive oil season: the same period as in present tests but different years) on the SOMW supplemented with 2% calcium carbonate (SOMWCC) and WS also supplemented in the same way (WSCC). SOMWCC and WSCC were conditioned in bags of 500 g of wet substrate inoculated with LPO or CPO spawn at 7% rate. The holes were made at the end of the first incubation week. Six repetitions for each treatment had been done.
The dry weights of SOMWCC and WSCC were respectively (24.66 ± 0.08% WW) and (56.47 ± 0.13% WW). The pH of SOMWCC was (7.67 ± 0.27) and that of WSCC was (7.30 ± 0.02). Unfortunately, the culture was not conducted on WS alone.
The biological efficiency of CPO on SOMWCC (23.30%) and on WSCC (21.41%) had been multiplied respectively by 1.95 and 1.87 compared to the results obtained on SOMW alone. The biological efficiency of LPO on SOMWCC was 69.55% and that on WSCC 31.63%. The productivity was 6.74 times larger on SOMWCC and 3.06 times larger on WSCC than on SOMW. The best yields we observed were those of the local strain on olive pomace supplemented with 2% of CaCO3. These results highlight the importance of the pH in the culture substrate for the two studied strains. LPO seems to prefer neutral to slightly alkaline pH, even if the used SOMW had a high pH (6.80 vs 5.2–6.0 [1]). Calcium carbonate provides also the mycelium with calcium. The results reveal that the SOMW is a good growing substrate when supplemented with 2% of CaCO3; indeed, according to Philippoussis [25], the pH of the substratum must be adjusted with limestone to about 7.5 or higher to provide selectivity against the green mould.
Wheat bran may be used as an organic source of nitrogen and shall probably contribute to further improve the yields of the two strains.
To conclude, we can say that the cultivation of P. ostreatus is promising in Algeria close to the facilities of olive oil production. The LPO strain presents abilities to grow on raw SOMW, such as the commercial strain (CPO) do and the mushrooms produced are tender and have tasty aspects, but the yields remain low. The supplementation with 10% of wheat straw and 2% of calcium carbonate had improved the yield of the two strains, particularly that of LPO, and the trial with supplementation of SOMW with only 2% of calcium carbonate confirmed that olive pomace is a good substratum for the cultivation of oyster mushroom. But further improvement of the substrate formulation might be expected. Cultivation of LPO and CPO on cereal straws and other agro-industrial wastes can be considered outside the olive season for a better profitability of culture rooms.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We wish to thank Mr. Hamid Madiou and Ms. Samia Mebrek for help in statistical analysis.


