1 Introduction
Nematodes of the superfamily Spiruroidea are mainly stomach parasites in birds and mammals, and are well represented in the helminth fauna of rodents (Murinae) of El Hierro, the smallest and westernmost island of the Canary Archipelago. In a previous study carried out in the island [1], an unusual and intense stomach polyparasitism was observed in the black rat Rattus rattus Linnaeus, 1758, with a high prevalence of four spiruroid species: Mastophorus muris (Gmelin, 1790) (Spirocercidae), Streptopharagus greenbergi Wertheim, 1993 (Spirocercidae), Gongylonema neoplasticum (Fibiger and Ditlevsen, 1914) (Gongylonematidae), and Protospirura sp. (Spiruridae). In the same prospection area, the house mouse Mus musculus domesticus (Schwarz & Schwarz, 1943) also showed stomach infection with M. muris and Protospirura sp.
The transmission and development of spiruroids has been studied in only a few species, with a variety of insects, mostly Coleoptera and Dictyoptera, acting as intermediate hosts [2]. Biological data, including larval morphology and life cycles, have been used to identify spiruroid species found in mammals and birds of North Africa, where tenebrionid darkling beetles of the genera Pimelia and Hegeter have been recorded as natural intermediate hosts, harbouring infective third stage larvae of different spiruroids [3].
Darkling beetles of the species Pimelia laevigata costipennis (Wollaston, 1864) and Hegeter amaroides (Solier, 1835) (Tenebrionidae) are endemic to El Hierro and are abundant in xerophilic environments [4]. One of the aims of this study was to investigate the role played by these endemic darkling beetles as intermediate hosts of the four co-parasitizing spiruroid species found in the black rat of El Hierro. The present paper describes for the first time larval and experimental adult forms of S. greenbergi in the tenebrionid intermediate hosts and definitive murine host, respectively, as well as other detected spiruroid larval types. Molecular characterization of S. greenbergi is also provided for the first time.
Internal transcribed spacer of ribosomal DNA (ITS rDNA) has proved to be valuable for determining the phylogenetic relationships of closely related nematodes [5]. This genetic marker was sequenced since it has previously been used to explore the intra- and interspecific evolutionary variation in spiruroids (Camallanus spp.) [5], and due to the lack of molecular information for S. greenbergi.
2 Material and methods
2.1 Invertebrate collection
The study took place on the island of El Hierro (17°53′–18°09′W and 27°38′–27°50′N) of the Canary Archipelago. Prospection was carried out in a semi-arid stony area with semi-desert vegetation near the coast (76 m a.s.l.) in the surroundings of the ancestral village of Guinea in the northeast part of the island. Darkling beetles of the species P. l. costipennis and H. amaroides (Coleoptera, Tenebrionidae) were hand-collected and analysed (155 and 154 specimens, respectively) in February/May 2010 and February 2011.
2.2 Parasitological techniques
Larval stages of nematodes were obtained by dissection of live coleopterans under a stereoscopic microscope. Morphological and morphometrical studies were performed under the microscope, essentially with specimens in vivo, but also fixed in 70% ethanol after clearing in lactophenol. Identification of Spiruroidea larvae was based on detail morphology and biology of spiruroid nematodes information recompiled in [2]. Adult worms of S. greenbergi preserved in 70% ethanol and obtained from natural murine hosts in a previous study [1], as well as experimental adults fixed in 70% ethanol were cleared with lactophenol and measured under the microscope. Drawings were made with the aid of a camera lucida.
2.3 Experimental rat infection
A white laboratory rat (Rattus norvegicus Sprague–Dawley strain) weighing 225 g was orally infected with 11 isolated third-stage larvae of S. greenbergi, which were imbibed in a mixture of physiological serum and honey with the aid of a Pasteur pipette, and sacrificed 35 days post-infection to detect adult worms. Animal ethics guidelines were strictly followed in accordance the European Communities Council Directive (86/609/EEC; 2010/63/EU).
2.4 Molecular analyses
Molecular analyses were carried out to provide the first data for S. greenbergi. Adult worms from natural hosts were preserved in 100% ethanol for DNA extraction. Total genomic DNA was extracted using the Fast DNA (BIO 101 Systems) kit, following the manufacturer's instructions, and the resulting DNA was stored at 4 °C. The ITS rDNA region was amplified with primers TW81 and AB28 following [6]. PCR amplifications were performed in a total volume of 50 μl, including 10× buffer (Bioline, London), 1 μl of each dNTP (10 mM), 2 μl of each primer (20 mM), 0.2 μl of Biotaq polymerase (5 U/ml) (Bioline, London), 1.5 mM MgCl2, and 20 ng of total genomic DNA. PCR conditions were as follows: 5 min at 94 °C followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 45 s, and an extension at 72 °C for 1 min, with a final extra extension step at 72 °C for 10 min.
The amplifications were carried out in a Multigene II Personal thermocycler (Labnet International, Inc, USA). Amplification products were analysed on 1.7% agarose gel and visualized by ethidium bromide staining. PCR products were purified using the UltraClean PCR Clean-up kit (MO BIO, Carlsbad, CA). Sequencing reactions for the TW81 primer were performed in SEGAI (University of La Laguna). A BLAST sequence similarity search (WU-BLAST) of the GenBank database was carried out.
3 Results
3.1 Identification and prevalence of larval stages of Spiruroidea detected in Pimelia laevigata costipennis and Hegeter amaroides
Both tenebrionids, P. l. costipennis and H. amaroides, were found to be infected with different species of nematode larvae. At least three species of spiruroid third-stage larvae were identified based on their morphology/biology: S. greenbergi, M. muris, and Gongylonema type larvae. Pimelia specimens showed a higher rate of infection with these spiruroid larvae than specimens of the smaller darkling beetle Hegeter. The predominant species in both tenebrionids was S. greenbergi, with a higher intensity (1–150 larvae) in Pimelia (Table 1). No significant difference between prevalences of parasitation of February and May for the two populations of beetles were detected (Chi2 test). Gongylonema type larvae were also detected in both hosts, while M. muris larvae were observed only in Pimelia specimens, in both cases at a low intensity (1–3).
Prevalence and intensity of infection with Spiruroidea larvae in the Tenebrionid studied species.
| Total prospections | February 2010 | May 2010 | February 2011 | |||||
| n (%) | r (MI) | n (%) | r (MI) | n (%) | r (MI) | n (%) | r (MI) | |
| Pimelia laegivata costipennis | N = 155 (84♂ + 71♀) | N = 38 (17♂ + 21♀) | N = 59 (33♂ + 26♀) | N = 58 (34♂ + 24♀) | ||||
| Streptopharagus greenbergi | 25 (16.1) | 1–150 (22.6) | 4 (10.5) | 2–51 (15.0) | 12 (20.1) | 1–36 (7.7) | 9 (15.5) | 1–150 (45.9) |
| Mastophorus muris | 3 (1.9) | 1–4 (2.0) | – | – | 2 (3.4) | 1–4 (2.5) | 1 (1.7) | 1 (1.0) |
| Gongylonema type | 7 (4.5) | 1–4 (1.3) | – | – | 4 (6.8) | 1–2 (1.5) | 3 (5.2) | 1 (1.0) |
| Total Spiruroidea | 31 (18.1) | 4 (10.5) | 13 (22.0) | 11 (18.6) | ||||
| Hegeter amaroides | N = 154 (90♂ + 64♀) | N = 97 (63♂ + 34♀) | N = 53 (25♂ + 28♀) | N = 4 (2♂ + 2♀) | ||||
| Streptopharagus greenbergi | 11 (7.1) | 1–5 (2.7) | 10 (10.4) | 1–5 (2.6) | 1 (1.9) | 3 (3) | – | – |
| Gongylonema type | 4 (2.6) | 1–3 (2.0) | 4 (4.1) | 1–3 (2.0) | – | – | – | – |
| Total Spiruroidea | 12 (7.8) | 11 (11.3) | 1 (1.9) | – |
3.2 Biology and morphology of Streptopharagus greenbergi
Identification of spiruroid larvae as S. greenbergi was achieved by obtaining experimental adults from an infected laboratory rat. Two male and one female worms were isolated in the stomach 35 days after infecting the rat with larvae from a parasitized specimen of P. l. costipennis (27.3% infection). The morphology and morphometrics of the experimental worms matched those of S. greenbergi adults previously detected in R. rattus on El Hierro [1], as well as the main features of the original species description based on paratypes obtained in the murid Acomys cahirinus (Deomyinae) from Israel [7]. Comparative morphometric features are described in Table 2.
Comparative morphometric characterization of adults of Streptopharagus greenbergi.
| Present study | Wertheim, 1993 | ||||||||
| Experimental adults 35 days p.i. in Rattus rattus | Natural adults in Rattus rattus from El Hierro (Canary Islands) | Paratypes in Acomys cahirinus (Israel) | |||||||
| ♂ n = 2 | ♀ n = 1 | ♂ n = 10 | ♀ n = 10 | ♂ n = 7 | ♀ n = 4 | ||||
| Exp 1 | Exp 2 | Exp 1 | X | Range | X | Range | Range | Range | |
| Length (mm) | 10.89 | 9.86 | 17.25 | 11.4 | 10.0–13.0 | 23.6 | 21.0–30.0 | 9.77–13.5 | 15.2–18.3 |
| Width at cephalic end | 37.9 | 35.0 | 37.9 | 37.9 | 30.7–43.5 | 46.1 | 41.0–51.2 | 28–48 | 30–48 |
| Width at oesophagus end | 215.0 | 184.0 | 245.0 | 236.0 | 190.0–260.0 | 287.9 | 245.0–312.5 | 180–220 | 220–223 |
| Width at equatorial level | 220.0 | 205.0 | – | 259.0 | 235.0–280.0 | – | – | 180–230 | – |
| Width at cloaca level | 121.4 | 118.0 | – | 152.8 | 125.0–210.0 | – | – | 83–108 | – |
| Width at vulva region | – | – | 265.0 | – | – | 323.8 | 262.1–383.1 | – | – |
| Width at base of tail | – | – | 105.0 | – | – | 126.5 | 100.0–155.0 | – | – |
| Ant. end–left CP (D) | 161.9 | 189.7 | 185.0 | 135.9 | 73.4–177.1 | 176.8 | 146.7–215.1 | 140–190 | 122–158 |
| Ant. end–right CP (D) | * | 374.4 | * | 360.2 | 334.0–389.6 | 367.6 | 335.1–420.0 | 245–324 | 225–308 |
| Ant. end–nerve ring (D) | 300.0 | 253.0 | 350.0 | 271.8 | 242.9–299.5 | 275.4 | 255.0–330.2 | 218–248 | 205–300 |
| Ant. end–excretory pore (D) | * | 346.6 | * | 334.5 | 275.8–379.5 | 373.6 | 355.7–405.0 | 276–288 | 260–382 |
| Pharynx's length | 101.2 | 91.0 | 131.5 | 117.0 | 105.0–128.0 | 127.5 | 120.3–138.2 | 104–143 | 110–122 |
| Oesophagus's length | 1683.0 | 1480.0 | 1920.0 | 2125.2 | 1709.8–2863.4 | 2623.4 | 2235.1–2987.0 | 1720–2280 | 1970–2400 |
| Muscular oesophagus's length | 283.4 | 250.0 | 275.0 | 366.5 | 225.0–445.0 | 425.4 | 315.0–530.3 | 237–305 | 270–310 |
| Tail's length | 180.0 | 195.0 | 200.0 | 185.6 | 150.0–260.0 | 246.0 | 210.0–275.0 | 160–200 | 168–190 |
| Left spicule's length | 1150.0 | 1110.0 | – | 1103.6 | 780.9–1292.1 | – | – | 780–895 | – |
| Right spicule's length | 325.0 | 310.0 | – | 323.5 | 339.6–386.3 | – | – | 255–326 | – |
| Gubernaculum | 40.5 | 43.0 | – | 41.2 | 30–51.2 | – | – | 38–42 | – |
| Ant. end to vulva (D) | – | – | * | – | – | 7054.0 | 6375.7–7776.5 | – | – |
| Eggs length | – | – | 32.0 | – | – | 37.7 | 35.8–38.4 | – | 34–41 |
| Eggs width | 17.5 | 21.6 | 20.5–23.0 | 20–26 |
3.2.1 Morphology of experimental and natural adult worms
Adult S. greenbergi worms are small, slender and fusiform and present the typical cuticular ornamentation of the species, with numerous short, interstrial ridges only in the anterior part of the body (Fig. 1B), being absent in front of the anus. Mouth lips have six prominent lobules. Two asymmetrical longitudinal alae originate a few μm posterior to the cervical papilla. These are very clear in the experimental female: the left alae reaches a maximum width of 15–25 μm and originates 41 μm behind the cervical papillae and 553.7 μm from the cephalic end; the right alae reaches a maximum width of 15–17 μm and originates 244.8 μm from the cephalic end (Fig. 1A). The pharynx is thick-walled, cylindrical and curved in the middle, and the oesophagus is long, clearly divided into an anterior muscular and a long posterior glandular part. The nerve ring is situated at the mid-level of the muscular oesophagus.
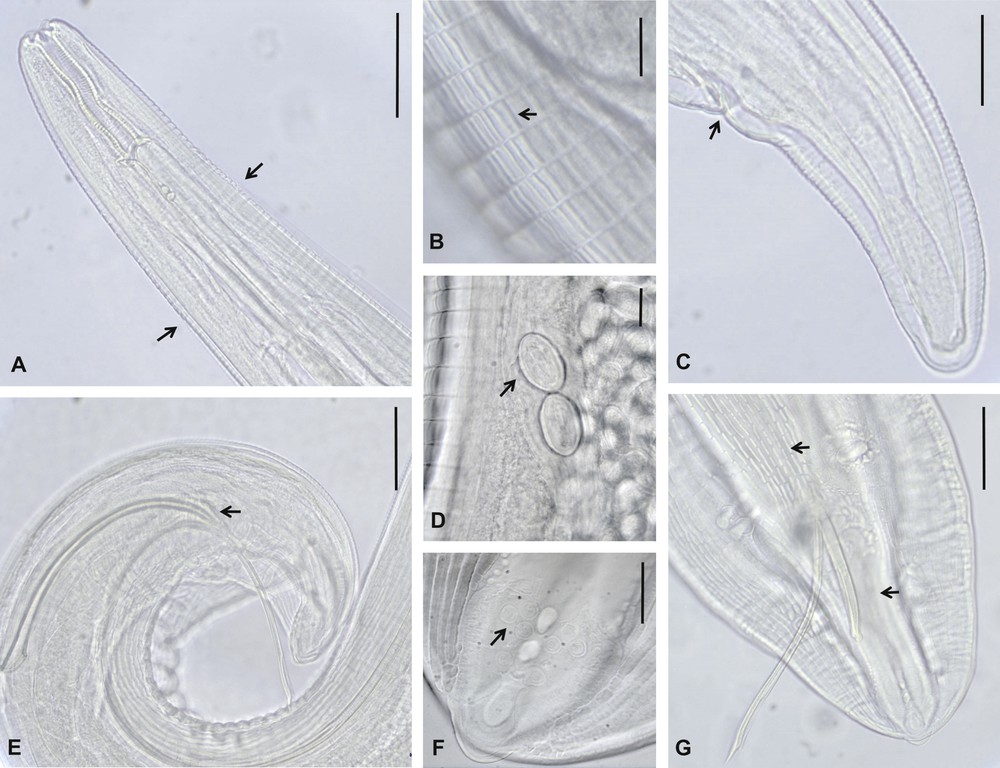
Morphological features of Streptopharagus greenbergi adults from experimental and natural hosts of El Hierro. A–D. Females. A. Anterior end, showing the start of lateral longitudinal alae (arrows). B. Cuticular interstrial ridges in the anterior part of the body. C. Caudal end and anus (arrow). D. Eggs in the vagina. E–G. Males. E. Spicules and gubernaculum (arrow). F. Caudal papilla. G. Caudal end showing cuticular longitudinal ornamentation (upper arrow), absent between the cloaca and caudal end (down arrow). Scale bars: B, D, F = 20 μm; C, G = 50 μm; A, E = 100 μm.
3.2.1.1 Male
Spicules are unequal and dissimilar: the right spicule is broad, short and round-pointed; the left spicule is slender, winged and sharp-pointed (Fig. 1E, G). Caudal alae and sensorial papilla display the same appearance and distribution as detailed in the original description: the area rugosa has longitudinal ornamentation, which is absent in the zone between the cloaca and caudal end (Fig. 1F, G).
3.2.1.2 Female
Eggs of females are elliptical, thick-shelled and were already embryonated in the experimental female (Fig. 1D).
3.2.2 Morphology of Streptopharagus greenbergi larvae
Larvae of S. greenbergi are mostly free in the haemocoel but can also be encapsulated. Only two of the 565 larvae isolated from Pimelia specimens were encapsulated immature second-stage larvae, the rest being infective third-stage larvae, some of them still encapsulated (Fig. 2E, D). All the 29 larvae detected in parasitized specimens of Hegeter were free and third-staged. The capsule is subspherical, crushable and sticky, around 0.40–0.45 mm in diameter, and contains one coiled larva.
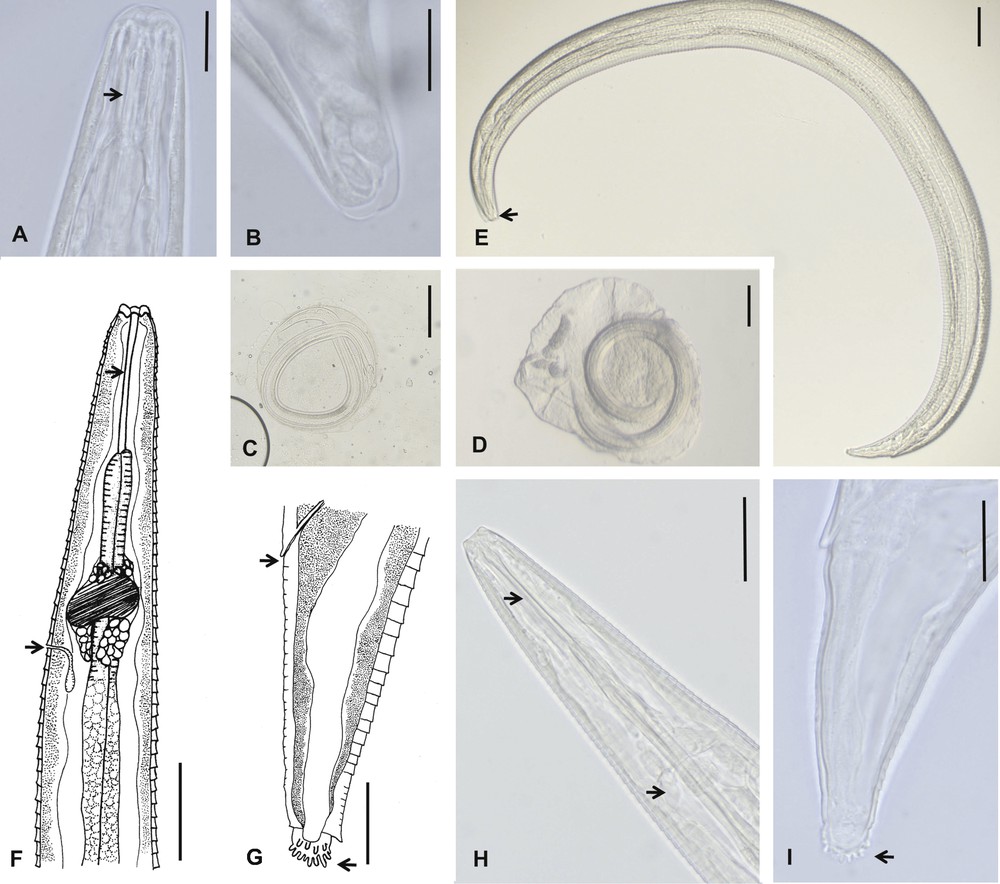
Morphological features of Streptopharagus greenbergi larvae from intermediate hosts, Pimelia laevigata costipennis and Hegeter amaroides, of El Hierro. A–C. Second-stage larvae. A. Anterior end, showing a short pharynx (arrow). B. Smooth truncated caudal end. C. Encapsulated larva. D–I. Third-stage larvae. D. Encapsulated larva. E. Body larva, mouth (arrow). F and H. Anterior end showing cylindrical pharynx (upper arrows) and excretory pore and nerve ring respectively (down arrows). G and I. Posterior end, showing anus (upper arrows) and digitalised ending (down arrows). Scale bars: A, B, G = 20 μm; E, F, H = 50 μm; C, D = 100 μm.
3.2.2.1 Second-stage larva
Description of second-stage larva (in vivo) (Fig. 2A, B): body 1.2 mm long and 92 μm wide; pharynx 42.8 μm; oesophagus 375 μm; excretory pore 179 μm from anterior end; tail length 55 μm; caudal end smooth and somewhat truncated.
3.2.2.2 Third-stage larva
Description of third-stage larva (mean and extreme values of 10 specimens in toto) (Fig. 2E–I): stocky body, 1.7 (1.7–1.8) mm long and 84.2 (78.4–88.6) μm wide; thick cuticle with deep transverse striae, spaced at 5.5 (5.1–6.1) μm and with a jagged appearance, so that the posterior end of the stria is mounted on the anterior end of the next element; absence of lateral alae; buccal cavity with six prominent lobules; subcylindrical pharynx with thick walls, 63.8 (50.6–68.3) μm; oesophagus, 519.2 (495.9–544) μm, divided into two portions: the anterior short and muscular, 141.2 (113.9–164.5) μm, and the posterior long and glandular, 378.0 (336.5–402.3) μm; nerve ring 135.4 (129.0–141.7) μm from the anterior end; excretory pore just behind nerve ring, 192.4 (180.6–205.1) μm from the anterior end; genital primordium small and rounded, 718.9 (675.4–756.0) μm from the posterior end; rectum short, surrounded by two rectal glands; tail length 73.5 (68.3–78.4) μm; caudal end ornamented with a subspherical tuft with a consistent number of about 20 irregularly arranged minute digits.
3.3 Morphology of other Spiruroid larvae
3.3.1 M. muris larva
Third-stage larvae are encapsulated (capsule 0.35–0.40 mm of diameter) in the haemocoel (Fig. 3A–C). Their main measurements in vivo are: length 0.95–1 mm; width 55–60 μm; pharynx slender 60–62 μm; oesophagus 330–360 μm; excretory pore 150–160 μm from the anterior end; tail length 85.4–89.1 μm; caudal end ornamented with 7–8 uniform digits. The striated cuticle has two lateral alae and the pharynx is extended to the mouth, which presents a double ventral and dorsal protuberance.
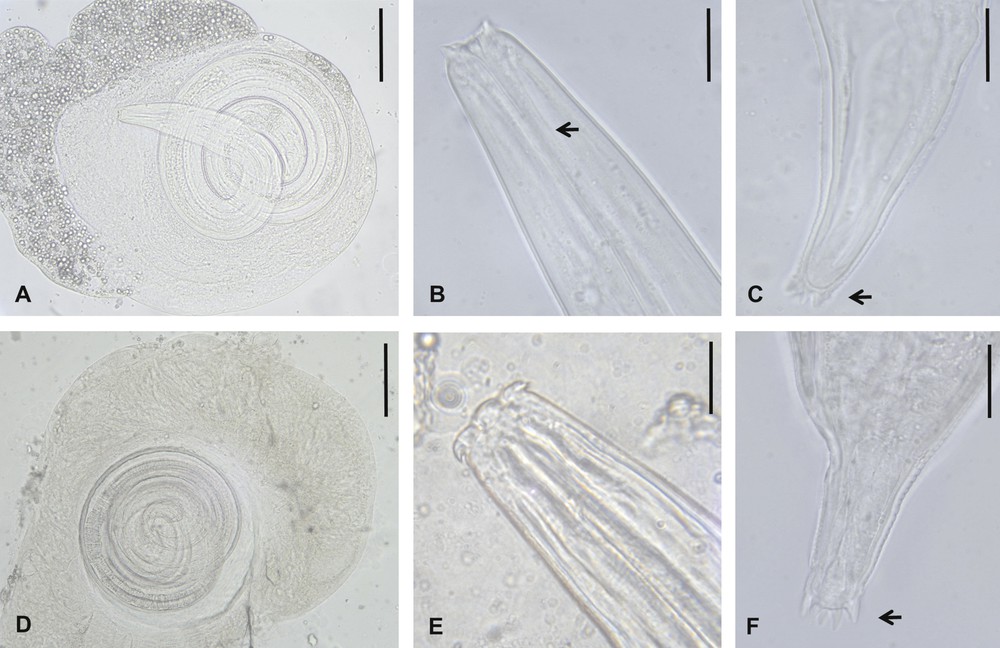
Morphological features of other spiruroid third-stage larvae from darkling beetles, Pimelia laevigata costipennis and Hegeter amaroides, of El Hierro. A–C. Mastophorus muris larva. A. Encapsulated larva. B. Anterior end, showing slender pharynx (arrow). C. Digitalised caudal end. D–F. Gongylonema type larva. D. Encapsulated larva. E. Anterior end. F. Digitalised caudal end. Scale bars: B, C, E, F = 20 μm; A, D = 100 μm.
3.3.2 Gongylonema type larva
Third-stage larvae are encapsulated (capsule 0.9–1.2 mm in diameter) in abdominal muscular fibres (Fig. 3D–F). Their main measurements in vivo are: length 2.3–2.5 mm; width 76–90 μm; pharynx 45–48 μm; long oesophagus, 1055–1080 μm; short tail, 61–73 μm; caudal end rounded and surrounded by a circle of seven to eight small digits. The cuticle is striated.
3.4 Molecular identification of Streptopharagus greenbergi
A fragment of the ITS1 was sequenced for the S. greenbergi adult worm. The sequence obtained was deposited in the GenBank database (accession number KC512904). The BLAST analysis showed similarity with 75 bp of sequences of other spiruroid sequenced for this region, Gongylonema pulchrum Moulin, 1857 (i.e. AB646106.1), specifically at position 1–38 with 97% identity, and at 268–347 with 86% identity.
4 Discussion
Taking into account that S. kuntzi Myers, 1954 has been established as a synonym of S. greenbergi [7], this spirocercid has been previously found in 12 species of rodents (murid and gerbillid) of Southern Israel and the Sinai Peninsula of Egypt [7,8]. S. greenbergi has also been recorded in R. rattus of other islands of the Canary Archipelago besides El Hierro (Tenerife, Gran Canaria and Lanzarote) (Feliu, personal communication). Compared with paratypes of the original species description, S. greenbergi adults of El Hierro show the same characteristic morphological features, but also some morphometric differences (Table 2). The left spicule of males is slightly longer in both natural and experimental adults of El Hierro. The females from natural host are of a greater size, and the eggs of the only obtained experimental female are somewhat smaller, but this may be due to the short mature phase of the worm.
Seven species of Streptopharagus have been recorded from rodents and nine from primates [8], but biological data have only been provided for three species [3,9–11]: S. lerouxi Quentin, 1965, a parasite of Cricetids in the Congo; S. kutassi (Schulz, 1927) Chabaud, 1954, a parasite of Gerbillids and Sciurids in Iran and Ukraine – Central Asia and reported in R. Rattus of Pityusic Islands (Balearics, Spain); and S. pigmentatus (Linstow, 1897), a parasite of Old World monkeys (Cercopithecids and Hylobatids), being particularly common in the Japanese macaque. The darkling beetle P. angustata Fabricius, 1775 has been recorded as an intermediate host for S. kutassi in Mauritania, and dung beetle species (Scarabioidea) for S. pigmentatus in Japan [2].
The most well known description of Streptopharagus infective larvae to date is of S. kutassi, which were detected after an accidental infection of P. angustata specimens collected in Mauritania and maintained in the laboratory. Their specific identification was confirmed by obtaining experimental adults from an infected laboratory rat [3]. These larvae were already showing the sinuous pharynx of the adult form, but this typical morphological feature of Streptopharagus species is not necessarily present in the larval stage, as we noted in S. greenbergi of El Hierro. Thus, we think that the morphology of our larvae matches the description of spiruroid larvae detected in the tenebrionid H. tristi (Fabricius, 1792) of the Canary Islands [3] and identified as a new species of the genus Ascarops, Ascarops joliveti Chabaud, 1954 (Spirocercidae). No adult forms of the genus Ascarops have been reported in the Canary Islands, so the author based the creation of this species exclusively on morphological features of the detected larvae, which did not present a curved pharynx. It is significant that the adult morphology of the genera Ascarops and Streptopharagus (Spirocercidae, Ascaropsinae) is sometimes insufficient to differentiate them, as evidenced by the initial identification of S. kutassi as A. kutassi Schulz, 1927.
The species M. muris is a common parasite of rats and other rodents worldwide, and is prevalent in the rat and house mouse on El Hierro, as well as the other islands of the Canary Archipelago (Feliu, personal communication). Morphological features of the larvae detected in P. l. costipennis on El Hierro match those described for M. muris larvae by other authors who obtained larvae from the experimental infection of different groups of insects that can act as viable intermediate hosts (Coleoptera, Dyctioptera, Orthoptera, and Dermaptera) [2,9,12]. Spontaneous infection of fleas (Siphonaptera) and phlebotomies (Diptera) with this mastophorine from rodent hosts has also been detected in France [2].
The spiruroid genus Gongylonema has about 25 known species, recorded worldwide in many wild and domestic mammals and birds. About 50 species of arthropods such as darkling and dung beetles and cockroaches have been recorded as natural or experimental intermediate hosts of these nematodes [2].
Morphological studies carried out on Gongylonema infective larvae have allowed the separation of species that are very similar in the adult stage, based on different morphological features of larvae such as size, length of pharynx and structure of caudal extremity [2]. In mammal-parasitizing species, four groups of larvae are described: types G. pulchrum, G. neoplasticum, G. problematicum and G. brevispiculum [13,14], but none of them exactly match the larvae detected in our study.
G. neoplasticum adults have been observed in R. norvegicus and larvae in Periplaneta americana (Dyctioptera) in a German Zoo [15]. These larvae have been described from cockroaches experimentally infected in France [16], but they differ from our larvae, principally in being much smaller (1–1.1 mm) and having a clearly bifurcated caudal extremity.
In the Canary Islands, two species of Gongylonema have been previously recorded: G. neoplasticum, only in R. rattus on El Hierro, and G. brevispiculum, only in M. m. domesticus on the eastern islands (Lanzarote, Fuerteventura and La Graciosa), where the prevalence of infection can reach 40–50% (Feliu, personal communication). Taking into account that the only species previously detected in rats with a high prevalence in the prospected area of El Hierro is G. neoplasticum [1], it could be logical to think that the larvae observed in Pimelia and Hegeter specimens in this study belong to this gongylonematid, despite discrepancies with the morphological data available in the literature. In addition, studies conducted in the Iberian Peninsula have never found G. neoplasticum in Arvicolinae [17], the hosts in which this gongylonematid was originally recorded and larvae obtained and studied [16].
Infection of both darkling beetles of El Hierro with S. greenbergi larvae was observed in all the studied periods, which suggests that the life cycle of this spiruroid is well established in the semi-arid biotope of Guinea. The highest seasonal incidence of rat parasitation by helminth nematodes in a previous study in the same enclave [1] was found in autumn, while no significant data are available for animals captured in spring. However, the presence of infected beetles in February indicates that a notable level of rat parasitation in spring is feasible. Furthermore, the regular weather patterns on El Hierro throughout the year and constant presence of the endemic darkling beetles would suggest that the life cycle of S. greenbergi and the other detected Spiruroidea, M. muris and Gongylonema type species, could be completed in all seasons.
At present, the only nucleotide sequence (HM067977.1) of the genus Streptopharagus published in the GenBank database is a partial region of the 18S rDNA gene, and belongs to an unidentified species. Hence, our study provides the first characterization based on an ITS1 sequence for Streptopharagus sp., and the first molecular data for the species S. greenbergi. These data could be useful for further studies on biogeography, phylogenetic analyses, etc.
Our experimental biological study provided new knowledge about the life cycle of the spiruroid S. greenbergi. This nematode presents encapsulated and non-encapsulated larvae, whose morphology is studied for the first time, that develop within the haemocoel of darkling beetles, principally in the species P. l. costipennis and to a lesser extent in H. amaroides. Obtaining experimental adults allowed the identification and morphometrical characterization of the species. Furthermore, the first nucleotide sequence for S. greenbergi is provided, which is also the first ITS1 sequence for the genus Streptopharagus.
This study demonstrates that both studied species of darkling beetles are suitable intermediate hosts of other spiruroid nematode parasites of rats, such as M. muris and Gongylonema type species.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
This study was supported by the Spanish project CGL2009–07759/BOS (Management and Knowledge transfer Programs) of the Ministry of Science and Innovation. We thank to all the “Excmos. Cabildos Insulares” of the Canary Islands the permission to collect invertebrates in the area under their jurisdiction. The authors greatly acknowledge Dr. Pedro Oromí (Department of Animal Biology, Faculty of Biology, University of La Laguna, Tenerife, Canary Islands, Spain) for his assistance in taxonomic identification of Coleoptera, and Dr. Carlos Feliu (Laboratory of Parasitology, Faculty of Pharmacy, University of Barcelona, Spain) for valuable comments and suggestions.


