1 Introduction
Termites and ants, both species-rich eusocial insects, are characterized by their abundance in terms of biomass and number of individuals. Together, they represent up to 33% of the terrestrial animal biomass [1]. The abundance of termites is related to their specialized diet based on cellulose- and hemicellulose-containing substances that are the most common organic compounds on Earth [2,3]. The ability of primitive termites to digest wood is based on the cellulolytic activity of mutualistic flagellates housed in the hindgut, while the bacterial ecto- and endosymbionts of these flagellates play a major role in the metabolism of nitrogen; these termites also produce endogenous cellulase [3–5]. Although they are primarily known for producing their own cellulases, evolutionarily-advanced termites are associated with cellulolytic bacteria; also, Termitinae, an Old World subfamily, cultivate cellulolytic basidiomycetous fungus [3,6,7].
The ant diet is very diverse and many species are either predatory or omnivorous, but their abundance is directly related to herbivory. Leaf-cutting ants cultivate cellulolytic basidiomycetous fungus and bacteria [8,9]; however, the relationship of other ants to herbivory is indirect as they exploit the honeydew produced by the sap-sucking hemipterans they attend (trophobionts). Such a nitrogen-poor diet is associated with the presence of mutualistic endo- and exo-cellular bacteria, the latter developing in the gut lumen [10,11]. For instance, in Camponotine ants, Blochmannia, the first endosymbiotic bacteria ever described [12], has nitrogen-recycling and upgrading properties; it also contributes to the metabolism of sulphur and lipids by the host and improves the immune defence of their host ants [8,13,14]. Furthermore, bacteria from the order Rhizobiales, well known for their ability to fix nitrogen when associated with leguminous plants, have been noted in the gut of ‘herbivorous’ ants as have the genes involved in atmospheric nitrogen fixation [15–17].
The present study focuses on myrmicine ants of the genus Melissotarsus that have an extremely specialized set of behaviours. Both workers and gynes hollow out a network of galleries in the bark of their host trees. Workers walk with their middle pair of legs pointing upwards and in contact with the ceiling of the galleries, so that they stagger when placed on a flat surface. As a consequence of their nearly constant digging, the queens and oldest workers have well-worn mandibles, and they plug the gallery openings with silk they secrete themselves. Also, Melissotarsus workers tend Diaspididae for their flesh as members of this Hemipteran family do not produce honeydew [18–22]. Furthermore, due to the huge size of their colonies and the fact that they dig galleries in the bark where they tend Diapididae (up to 1,585,000 individuals per tree attending 556,000 Diaspis), they are considered pests in the management of forest and tree crop plantations [19–21]. The formation of these huge colonies is possible through particularities in the Melissotarsus biology. Above all, colonies have numerous egg-producing physogastric queens, each situated at the centre of a zone ca. 1 m in diameter in the bark of the host tree (oligogyny). Prior to this, these queens took part in a dynamic process wherein they first participated in digging galleries, a worker-like task. After swarming, newly-inseminated females are accepted into a colony, but they do not produce eggs until they have the opportunity to dominate their own section of the colony and become physogastric. Also, the workers produce chorioned, viable eggs and can elude the queen's influence. Finally, intraspecific aggressiveness between colonies is low, so that as they spread beneath the bark, the colonies merge, forming huge colonies over vast areas [21].
The Melissotarsus workers (and young queens) dig the living tissues situated around the cork cambium (from the inside to the outside: the outer layers of the secondary phloem, the phelloderm, the cork cambium and the still tender cork cells). Meanwhile, layers of cork continue to form all around the excavated area, so that older galleries are found in the hard, most peripheral part of the bark [20,21].
This mode of digging soft tissues is similar to that of certain borer and miner insects that, while doing so, ingest the cells’ cytosol plus very small particles (i.e., individual cells or even parts of secondary membranes [23]). Note that the smaller the insects, the smaller the particles they bite off [23,24]; Melissotarsus workers are very small (less than 2 mm in length). Furthermore, reducing the tissues into very small particles, or comminution, creates new surface areas for enzymatic activity [3]. The size of the ingested particles is important in ants, because adults are rather liquid feeders due to the existence of two filtering apparatuses, the first situated after the infrabuccal pocket, the second constituted by the proventriculus. For instance, particles more than 10 μm in diameter cannot reach the crop of Camponotus pennsylvanicus (Formicinae) workers, and only particles of 1 μm or less pass through the filtering proventriculus bulb and reach the midgut and the hindgut [25]. Yet, in most myrmicine ants, the proventriculus bulb is absent, and the digestive tract simply narrows at the proventriculus, permitting the ants to feed on solid food [26,27] so that many myrmicine ants are granivorous. Like in Melissotarsus, workers from these species wear down their mandibles while milling seeds [28,29] and certain of them secrete amylases in their midgut and hindgut in addition to the amylases produced by the maxillary and labial glands [30] and so are able to feed directly on starch.
We hypothesised in this study that while digging galleries, a quasi-constant task for certain individuals to the point that their mandibles become worn, Melissotarsus workers ingest a part of the host tree bark. So, we wondered if, to satisfy their energetic needs, these ants are able to degrade oligosaccharides, heterosides, and even the structural polysaccharides of plant material.
2 Materials and methods
2.1 Collecting biological material
We collected samples of ants on the campus of the University of Yaoundé I (03° 53’ N; 11° 30’ W) and in the old secondary forest at Kala (03° 59’ N; 11° 28’ W) from two safoo trees (Dacryodes edulis; Burceraceae) and two mango trees (Mangifera indica; Anacardiaceae) for the M. beccarii and M. weissi colonies, respectively (one tree species per site). To do so, we peeled away the bark at the base of the trees with a machete. The debris plus live ants were hand-collected and placed into plastic bags for further study in the laboratory.
2.2 Research on enzymatic activities
The detection of enzymatic activity was carried out on crude extracts of biological material following a protocol very similar to that described by D’Ettorre et al. [31]. Each sample (1 g of M. beccarii or M. weissi worker gasters, respectively) was ground in 5 mL of distilled water using an Ultra Turrax T18 micro-tissue grinder. This was then centrifuged at 15,000 rpm for 20 minutes and the supernatant dialyzed against distilled water for 24 hours in a Naturin membrane (Ets Soussana, 92 Orly, France) to remove reducing sugars. The dialyzed solution constituted the enzymatic extract.
Seventeen specific and synthetic plant material substrates (from Sigma Chemicals; St Louis, MO, USA) were used in the enzyme assays (see [31]): five natural oligosaccharides (i.e., maltose, sucrose, cellobiose, lactose, and gentiobiose), five synthetic heterosides (i.e., ONP β-galactoside, ONP α- and ONP β-glucoside, ONP β-xyloside, and ONP N-acetylglucosamine), and seven polysaccharides (i.e., starch, xylan, lichenan, carobe galactomannan [GM carobe], carboxymethylcellulose [i.e., CMC or microcrystalline cellulose], pullulan, and laminarin).
Enzymatic activity was assayed according to the methods described by Rouland et al. [32]. Each enzyme solution was incubated with substrates at 37 °C in a Mac Ilvain buffer at pH 5 for 30 min (oligosaccharides and heterosides) or 60 min (polysaccharides).
The reducing sugars produced by the hydrolysis of the polysaccharides were assayed using the Somogyi–Nelson microdosage technique [33]. The glucose released from the oligosaccharides was determined through the glucose oxidase method [34]. The heterosidasic activity was assayed by measuring the amount of released para-nitrophenol according to the method described by Mora and Rouland [35]. The quantity of glucose liberated from the different substrates was determined through spectrophotometry (UV-VIS 2650; Labomed, Inc) at 505 nm for the oligosaccharides, at 400 nm for the heterosides, and at 650 nm for the polysaccharides. The activity was expressed as the amount of glucose equivalent (μg G) per minute per milligram of ant gaster (wet weight; this represents the gasters of ca. 22 workers). For each Melissotarsus species, two colonies were studied and, for each colony and each substrate, three enzymatic assays were conducted.
3 Results and discussion
Enzymatic activity was noted in all cases, but to different degrees. In M. beccarii, the oligosaccharidasic activity was high for sucrose and maltose (Fig. 1A), while M. weissi was characterized by a high α-glucosidasic activity, followed by an N-acetyl glucosaminic activity. Other oligosaccharides and heterosides were also degraded, but at a low rate (like for M. beccarii; Fig. 1B). Finally, although low, a polysaccharidasic activity was noted in both ant species, particularly for lichenan and xylan (Fig. 1C).
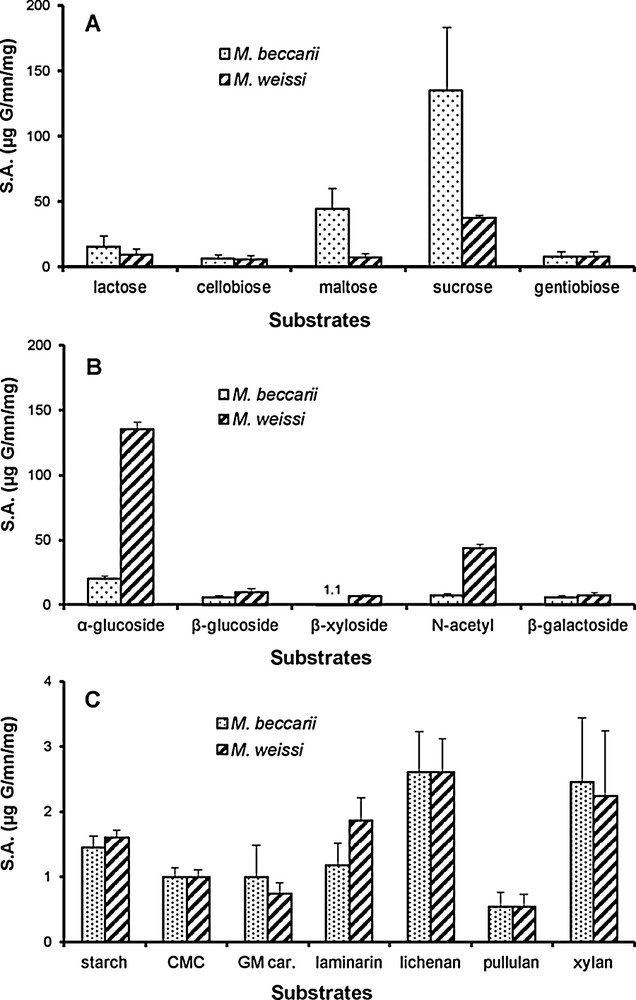
Oligosaccharidasic (A), heterosidasic (B) and polysaccharidasic (C) activities of the digestive tract of Melissotarsus weissi and M. beccarii. Specific activity values (S.A.) are expressed as μg of glucose released per minute per mg of ant gaster (wet weight) (means ± SE; N = 6 in each case).
We need to remember that secondary growth in dicotyledonous tree stems is firstly due to the vascular cambium producing the secondary xylem toward the interior and the secondary phloem toward the exterior. As the diameter of the stems increases, the epidermis is stretched and a layer of cortical cells becomes merismatic through dedifferentiation, forming a layer of “cork cambium” that produces the phelloderm toward the interior and the periderm, made of cork cells, toward the exterior. The “bark” includes everything to the exterior of the vascular cambium (from the phloem to the periderm) [36].
In this general context, it is likely that nesting and feeding behaviours are related in Melissotarsus because the workers dig their galleries through the live tissues situated around the cork cambium of their host tress [18–22]. Indeed, we demonstrate that they possess the enzymatic equipment necessary to degrade oligosaccharides, heterosides and even at least a part of the polysaccharides that they eventually absorb in the form of solutions and tiny particles while digging. They have therefore the capability of assimilating different carbohydrates from the cytoplasm and the nucleus of the corresponding cells plus at least a part of the different membranes. In a way, this shows a kind of convergence with the habits of primitive termites that spend most of their life cycle in the piece of wood that also serves as a food source [37].
Although the level of enzymatic activity was low, we show that Melissotarsus workers can degrade plant polysaccharides (Fig. 1C) as has been noted for the workers of leaf-cutting ants of the genera Atta and Acromyrmex, whose level of enzymatic activity is also generally very low [31,38] and in granivorous ants. It is indeed known that several ant species, particularly granivores, produce their own enzymes to degrade starch; these amylases are produced mostly by the maxillary and the labial glands, as well as, for certain species, by the midgut and the hindgut [31,39]. Furthermore, Went et al. [40] suggested that the adults of three Messor species also produce a protopectinase that dissolves the middle lamella (a pectin layer) of the seed embryos, but this remains to be demonstrated. Leaf-cutting ants of the genera Acromyrmex and Atta are obligatorily associated with a Lepiotaceae fungus which they grow on leaves harvested by the workers. In this mutualistic association, the fungus displays a high polysaccharidasic activity, whereas the ants degrade oligosaccharides and heterosides; yet, a notable enzymatic activity has been shown for Acromyrmex crassispinus workers on starch [31].
It would be particularly interesting to determine in future studies if Melissotarsus ants are able to degrade plant polysaccharides other than starch thanks to endogenous enzymes, and/or if a mutualistic association with endo- and exo-cellular bacteria exists as is known in ants tending sap-sucking Hemiptera [10–12]. The use of the recently-developed technique based on polysaccharide microarrays probed with antibodies and carbohydrate binding [41] could facilitate further analyses. Furthermore, a stable isotopes approach would allow us to verify if Melissotarsus adults and larvae feed on plant tissues and/or on Diaspididae.
4 Conclusion
It is likely that Melissotarsus workers, in addition to the degradation of oligosaccharides and heterosides, are also able to degrade plant polysaccharides. Because this has been shown using basic techniques, studying these systems through metagenomic or proteomic approaches should contribute to our knowledge of the different cellulose-degrading enzymes that insects have developed over the course of evolution.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We are grateful to Andrea Dejean for proofreading the manuscript. Financial support for this study was provided by the French Ministère des Affaires étrangères (CORUS programme research agreement 02 412 062).


