1 Introduction
The success of invasive, non-indigenous species relies on interrelated factors including their own characteristics, the recipient community, and the abiotic conditions [1]. It is therefore a challenge to study these species in their native range in order to identify which traits can permit them to become invasive once introduced into a new area. Invasive ants offer a good model for exploring this issue. Of the approximately 11,500 ant species known, about 150 ‘tramp species’ have been transported and introduced into many parts of the world through human activity; some of them proliferated quickly, and have become some of the most devastating invaders ever known [2].
Invasive ant species often form large supercolonies over vast areas thanks to their intrinsic ability to achieve unicoloniality (i.e., no colonial boundaries exist within populations; therefore, there is no intraspecific territoriality). This ability has been biologically and/or genetically demonstrated in certain species [3–5]. Their separation from coevolved parasites, predators, and competitors, known as the ‘enemy release hypothesis’, often allows invasive ants to flourish virtually unchecked [2], while a mutualistic association with hemipterans can permit outbreaks in the densities of both partners [2,6,7]. Finally, the success of most invasive ants is associated with a greater aggressiveness than for native species, which are then displaced or eliminated through a combination of competition and predation [2].
Although numerous studies have focused on the impact of invasive ants in areas where they have been introduced, little is known about their behavior in their native range. To that end, we felt that one of the best ways to understand the mechanisms leading to an introduced ant's invasive success was to study its predatory behavior in its native area.
This study focuses on Pheidole megacephala (F.), an ant native to tropical Africa that has become one of the most successful invasive ant species [2,8,9]. It has long been assumed that it is a proficient predator, since arthropod diversity declines in areas it has invaded [2,8,10], but this has never been corroborated by published data. We hypothesized that particularly efficient generalist predators are likely to be strong competitors in introduced areas where, by depleting prey, they eliminate native species through competition. Because the foraging efficaciousness of a predatory ant species is related not only to its ability to master a wide range of prey, but also to its successful recruitment behavior, we asked the four following questions. (1) Are the workers able to attack and rapidly retrieve their most frequently encountered prey items, including termites? (2) Is the short-range recruitment (a worker discovering food emits a pheromone to recruit nestmates foraging in the vicinity [11]) used by workers effective, and do workers use long-range recruitment (a foraging worker discovering a large food source returns to its nest to recruit nestmates) based on prey size? (3) Is the perception of termite landmarks enough to trigger the recruitment of nestmates? (4) Is the same true for the landmarks of other ant species and does the perception of the latter trigger the recruitment of a greater number of nestmates when a prey item is discovered?
2 Materials and methods
This study was conducted in Cameroon on the campus of the University of Yaoundé on twelve P. megacephala colonies. Because invasive ants have been recorded in very different parts of the world, their identification can be controversial. To avoid any such confusion, we clearly state here that the colonies studied originated from Yaoundé (
Despite the negative environmental and economical impacts of P. megacephala, few studies have been conducted on its biology. Like for most species in the genus, the worker caste is dimorphic, with no intermediary body size between the small minors (approximately 2 mm long; average 0.35 mg) and the big-headed majors (or soldiers; 3–4 mm long; average 1.65 mg). In Pheidole species, minor workers perform most of the tasks, including foraging for food, while the big-headed major caste is specialized in colony defense and food processing and is much less frequently found outside of the nest. Also, workers have an atrophied sting that they use to lay scent trails, but not to master prey or competitors [9]. Pheidole megacephala nests in a wide variety of habitats, such as in the ground, in termitaries or in the crevices of tree bark and, like most other invasive ants, is omnivorous. Its workers harvest seeds, scavenge, prey on invertebrates and small vertebrates, and attend a wide range of hemipterans [12–14].
2.1 Prey capture behavior
We placed plywood planks (
The behavioral sequences were recorded through direct observation from the introduction of the prey into the center of the hunting areas (on the plank of plywood) until their capture and retrieval to the nest. A full repertoire of behavioral sequences was first established during preliminary experiments. Referring to this complete list, we recorded each behavioral act performed vis-à-vis the prey (i.e., detection by contact, antennal palpation, attack, seizure, immobilization, spread-eagling, cutting up, and retrieval) as well as nestmate recruitment (see also [15–17]). This allowed us to build flow diagrams with transition frequencies between each behavioral act. Note that the spread-eagling of prey or alien ants means that several workers attack them simultaneously, each seizing an appendage and then pulling backward [11,18].
2.2 Testing the consequences of mass recruitment on groups of termites
To examine P. megacephala's response to termites under natural conditions, we placed pieces of termitaries (about 1 dm3) containing termite workers, soldiers and brood on their territory 3–4 m from each nest entrance (six colonies tested). We tested pieces from five Shedorhinotermes sp. (Rhinotermitinae) and Nasutitermes sp. (Nasutitermitinae) colonies and 10 C. fungifaber (Termitinae) and Macrotermes bellicosus (Smeathman) (Macrotermitinae) colonies. We monitored P. megacephala during two hours following the installation of the piece of termitary to determine if the workers discovered and then attacked it. We broke apart the pieces of termitaries 4 h after the beginning of the experiment to see if any termite individuals had resisted P. megacephala's raids.
2.3 Verifying the existence of short-range recruitment
We used 2-cm-long anaesthetized last instar B. orientalis cockroach nymphs to determine if the workers truly recruit nestmates at short range (four colonies tested). We placed a cockroach nymph 10 cm from a P. megacephala trail (Fig. 1A) and compared the duration in seconds (D1) between the moment when we deposited the prey and the moment when it was first discovered by a worker, which then remained in contact with the prey, and the duration (D2) between its arrival and that of a second worker (50 cases; paired t-test). As most second workers first came into contact with the discovering worker, which had not moved while trying to immobilize the prey, we compared the results to a random approach to see if the second workers accidentally found the first workers or were alerted by them (see details in Fig. 1B). The latter case would, then, indicate the presence of a signal emitted by the first workers to attract the second workers. We hypothesized that this signal could be a chemical that the second workers emit from the end of their gasters. As a result, we also noted on what part of the first workers' body contact between the first and second workers occurred and again compared the results to a random approach (Fig. 1C).
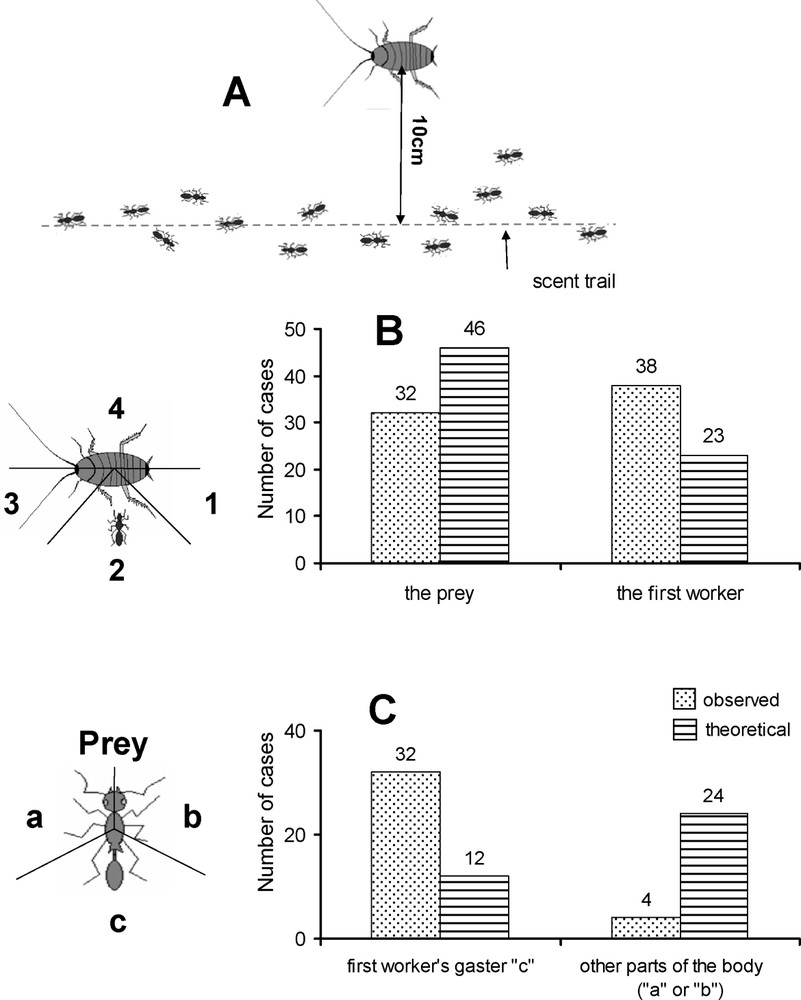
Representation of the experimental setup for testing the occurrence of short-range recruitment. (A) We placed 2-cm-long, numbed cockroaches 10 cm from Pheidole megacephala scent trails. The workers that discovered the cockroaches first positioned themselves at ‘1’, ‘2’ or ‘3’ (never at ‘4’). (B) We verified where the ‘second’ workers positioned themselves when reaching the prey (i.e., in the zone where the first worker was located or one of the other two zones; 70 cases). The difference from a theoretical random distribution was significant (Fisher's exact test: P<0.01). (C) As the ‘second’ workers most often came into contact with the ‘first’ worker attempting to immobilize the prey, we verified in 36 of these cases on what part of the first workers' bodies antennal contact first occurred (i.e., laterally or on the gaster). The difference from a theoretical random distribution again was significant (Fisher's exact test: P<0.001). Masquer
Representation of the experimental setup for testing the occurrence of short-range recruitment. (A) We placed 2-cm-long, numbed cockroaches 10 cm from Pheidole megacephala scent trails. The workers that discovered the cockroaches first positioned themselves at ‘1’, ‘2’ or ‘3’ ... Lire la suite
2.4 Influence of prey or competing species' landmarks on recruitment behavior
We tested four colonies to see if termite and ant landmarks alone are enough to trigger long-range recruitment in P. megacephala scouts. Prior to each experiment, we placed two brand new or ‘clean’ sheets of A4 paper 5 m from a P. megacephala nest entrance, and counted the number of workers on these sheets of paper 15 min after they were initially discovered by a scout (pre-controls). For the experiments where the Cubitermes termites were the potential prey, we placed a 3–4-cm3 piece of termitary devoid of termites at the center of a clean sheet of paper, and placed a piece of dirt of the same volume to serve as a control at the center of another sheet of paper. For the tests using ants (Pachycondyla soror (Emery) and Plectroctena minor Emery), 48 h prior to the start of our observations, ‘experimental’ sheets of paper were installed in the foraging arenas of artificial nests where an ant colony was in brood, so that the workers deposited landmarks on them; clean sheets of paper served as controls. We compared the results using the Kruskal–Wallis test followed by a post hoc test (Dunn's test).
We also noted that when P. megacephala foraged on food sources situated in areas where colonies of Oecophylla longinoda Latreille had deposited landmarks (they are easily visible and contain a territorial pheromone lasting for several months; [11,19]), the proportion of soldiers recruited appeared high [7]. Note that both species are dominant and can compete for habitat and resources [12]. We compared the recruitment behavior of four P. megacephala colonies after scouts discovered food placed in “neutral areas” (clean sheets of A4 paper) vs. “marked areas” (sheets of A4 paper previously left for 48 h on the territory of an O. longinoda colony). In addition, we also tested the influence of ‘prey size’ on the number of individuals recruited to retrieve the eggs of a Bombycidae species frequent in Yaoundé. To collect the eggs, we placed captured female moths in plastic boxes lined with Bristol board. Within 24 h they had laid eggs that adhered to the board. This allowed us to cut out sub-circular pieces of board bearing various quantities of eggs. These pieces of board were then placed at the center of the sheets of paper. As a result, this experiment compared egg clusters of different sizes (with 6, 12 or 24 eggs) placed on neutral areas vs. those placed on areas marked by O. longinoda, an ant species known to compete with P. megacephala. We noted the total numbers of recruited individuals 15 min after the scouts discovered the egg clusters, as well as the percentages of soldiers at the food item. The influence of the size of the egg clusters on the number of nestmates and percentage of soldiers recruited was compared using the Kruskal-Wallis test. A post hoc test (Dunn's method) was then performed to isolate the groups that differed from the others. We tested the influence of the Oecophylla landmarks on the recruitment pattern by using a Mann–Whitney test (a separate analysis was performed for each egg cluster size).
Statistical tests were performed using Statistica® 5.0 or GraphPad Prism 4.0 software.
3 Results
3.1 Prey-capture behavior
Pheidole megacephala workers detected all prey by contact. They were able to master and singly retrieve termite larvae and workers, as well as the largest and heaviest Tetramorium males (Fig. 2). Note that, although the workers have an atrophied sting, their attacks were very rapid, with the workers retrieving termite larvae and workers just after seizure by pulling them backward or lifting them. To control larger prey (>10 mm), the discovering worker recruited nestmates at short or long range. In the latter case and only for termite soldiers, the discovering workers abandoned the prey, laying a scent trail back to the nest. Numerous nestmates rapidly left the nest and followed the trail to the termite soldiers. For the relatively large cockroaches, some of the workers recruited at short range returned to the nest to recruit more individuals. In the end, these prey were always spread-eagled. Only rarely were the largest prey items cut up on the spot (15% of the winged Camponotus females but 62% of the cockroaches), with small pieces being retrieved by single workers, while other individuals or large prey pieces were collectively retrieved (Fig. 2). As a result, most prey were captured within a few minutes of their introduction (but note that four out of the 100 termite soldiers were abandoned, and eight out of 50 cockroaches escaped).
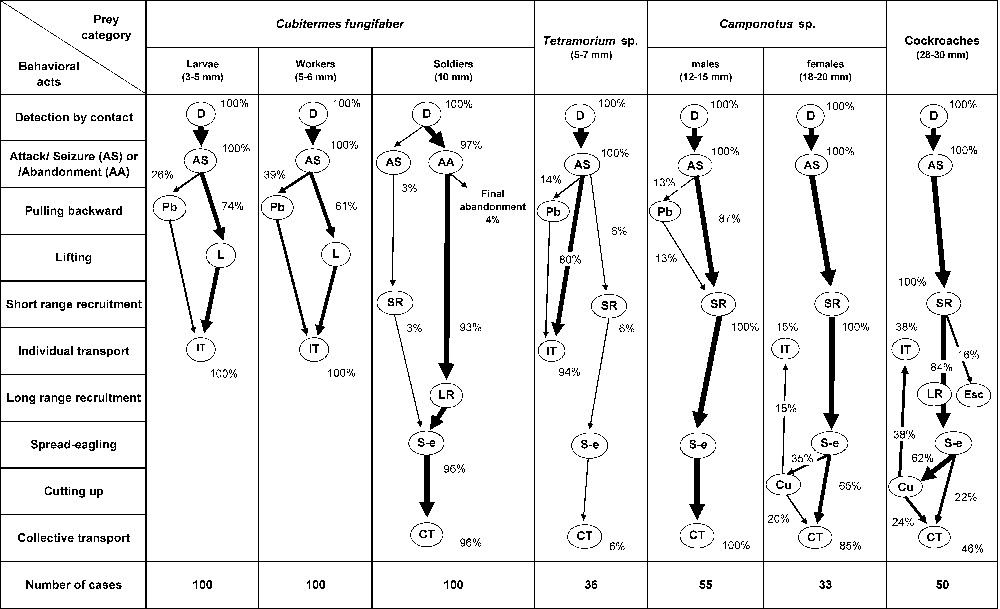
Diagrams representing the sequences of behavioral events observed during attempts by Pheidole megacephala workers to capture termite larvae, workers and soldiers; winged ants; and cockroaches. Esc = the prey escaped.
3.2 Testing the consequences of mass recruitment on groups of termites
The termites quickly obstructed openings in the pieces of termitaries with dirt and secretions. When discovering these pieces of termitaries on their territories, foraging P. megacephala scouts returned to their nests to recruit several dozen nestmates (long-range recruitment). They rapidly captured the termites patrolling outside of the piece of termitary, spread-eagled the Macrotermes soldiers (and sometimes the soldiers and workers of other species), and then retrieved them. Later, some recruited workers made holes in the pieces of termitaries and within four hours all of the termites were captured in all cases. Most termite workers and Nasutitermes soldiers were retrieved by single P. megacephala individuals, rarely two, while the largest soldiers of the other termite genera were cooperatively retrieved by two to 10 workers. As a result, 4 h after the beginning of the tests, all termite individuals from the 30 tested pieces of termitaries were captured and retrieved.
3.3 Verifying the existence of short-range recruitment
In this study we demonstrate that short-range recruitment can cover a distance of at least 10 cm (Fig. 1A). First, the duration (D1) between the moment when we deposited a prey item and the moment when the first worker discovered it was significantly longer than the duration (D2) between the latter and the arrival of a second worker (mean ± SE;
3.4 Influence of termite or competing ant species' landmarks on recruitment behavior
In the first series of experiments we noted that pieces of termitaries or ant landmarks alone are enough to trigger long-range recruitment by P. megacephala scouts. The scouts first antennated the pieces of termitaries or the sheets of paper marked by the ants, and then returned directly to their nest to recruit nestmates in most cases (Fig. 3). Consequently, the number of P. megacephala workers present 15 min after the scouts discovered the sheets of paper was significantly higher on those with pieces of termitaries or those with ant landmarks than all control cases (Fig. 3). Among the latter, or sheets of paper used as true control cases plus those serving during pre-experiments, the numbers of workers were always low.
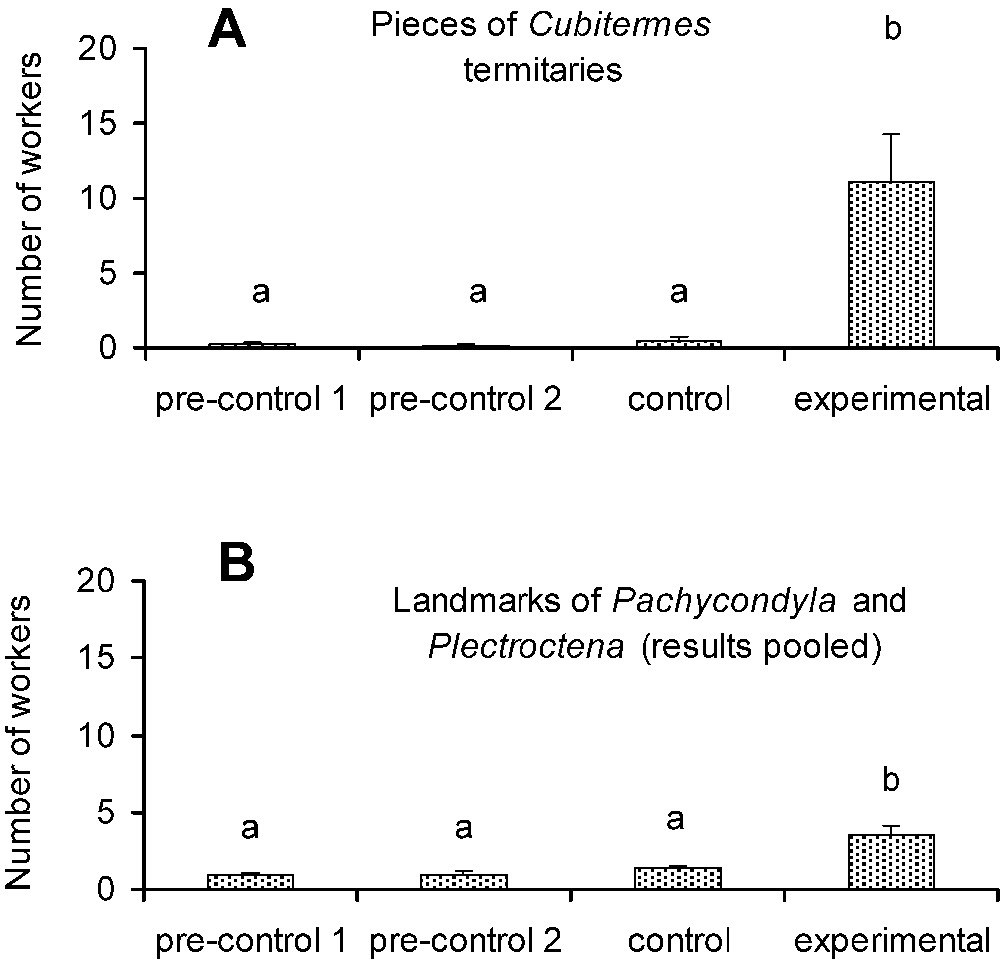
Testing the influence of landmarks on the recruitment behavior of Pheidole megacephala scouts. (A) Number of recruited individuals according to the different situations. ‘Pre-controls’ corresponds to ‘clean’ sheets of paper, ‘controls’ to sheets of paper with a piece of dirt about 3 cm3 deposited at their centers, and ‘experimental cases’ to sheets of paper with a piece of Cubitermes termitary about 3 cm3 placed at their centers (N=10 in each case). (B) Same experimental setup, but with the ‘experimental’ lot corresponding to sheets of paper bearing the landmarks of colonies of Pachycondyla and Plectroctena (results pooled). Here, both pre-control and control lots correspond to ‘clean’ sheets of paper. Global comparisons, A: Kruskal–Wallis tests:
Testing the influence of landmarks on the recruitment behavior of Pheidole megacephala scouts. (A) Number of recruited individuals according to the different situations. ‘Pre-controls’ corresponds to ‘clean’ sheets of paper, ‘controls’ to sheets of paper with a piece of ... Lire la suite
In the second series of experiments, when P. megacephala scouts discovered the egg clusters, they palpated and tried to bite the eggs and then returned to their nests, laying a recruitment trail. The number of recruited individuals significantly increased with the size of the egg clusters and was higher on marked areas in two out of three cases (Fig. 4A), while the percentage of recruited soldiers did not vary significantly (Fig. 4B). The recruited soldiers opened the moth eggs with their powerful mandibles, permitting workers to imbibe the contents. Additionally, the soldiers sometimes gripped the eggs so tightly that they pried them from their substrate and thus were able to retrieve them.
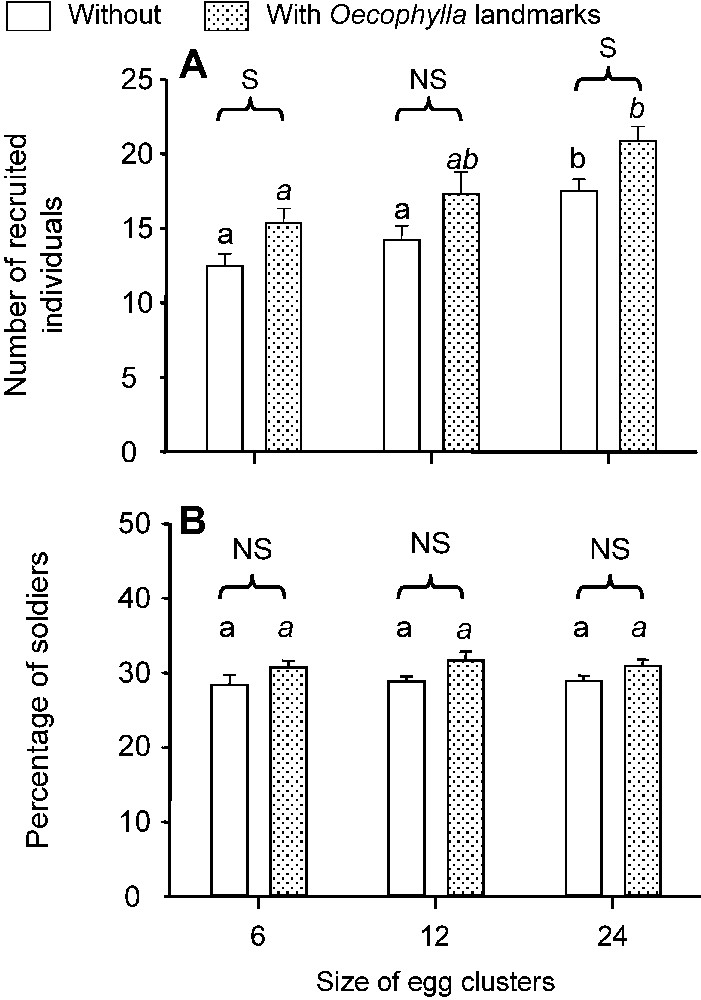
(A) Number of recruited individuals according to the presence vs. absence of Oecophylla landmarks (N=12 in each case). Global comparisons while testing the effect of the size of the egg clusters, Kruskal–Wallis tests:
(A) Number of recruited individuals according to the presence vs. absence of Oecophylla landmarks (N=12 in each case). Global comparisons while testing the effect of the size of the egg clusters, Kruskal–Wallis tests:
4 Discussion
We have seen here that in their native range P. megacephala can capture many different types of prey either alone or in a group. The ability of P. megacephala workers to singly capture and retrieve prey several times their size (and more than nine times their weight), including some Cubitermes soldiers, is astonishing, knowing that they possess an atrophied sting [9]. In comparison, ponerine ants about 8 mm long (four times as long as P. megacephala workers) almost always use their venomous sting when preying upon Cubitermes workers, and even 19–23-mm-long Pachycondyla tarsata (F.) workers sting these prey in 40% of the cases [20,21]. Consequently, although it has not yet been proven beyond a shadow of a doubt, we cannot rule out that the success of P. megacephala workers might be explained by the possibility that they produce offensive chemical compounds as is known in Pheidole biconstricta Mayr, whose pygidial gland produces a volatile component that repels other ant species and a viscous component that is applied directly onto the enemy, causing irritation [22] (see also [9]).
It is very frequent in the genus Pheidole for several recruited workers to spread-eagle intruders [18]. While hunting in a group, spread-eagling relatively large prey, associated with short- and/or long-range recruitment, permits P. megacephala to be a very efficacious predatory species. This behavior is also seen in several dominant, arboreal ant species [11,15,16] and in Paratrechina longicornis (Latreille), another invasive ant [17]. When several foragers patrol or ambush in the same area, short-range recruitment is an efficient way to avoid losing large, mobile prey, since nestmates are rapidly attracted to the emitting individual.
Although it may seem obvious that P. megacephala colonies would raid termites, this has never before been demonstrated. Therefore, we specifically studied the predatory behavior of P. megacephala to see if it preys on only a limited number of termite subfamilies or if it preys on all termite species (including those with well-developed abilities to defend their colonies). The fact that it did raid the pieces of termitaries belonging to all of the tested species and preyed upon all of the termite individuals shows that P. megacephala is an effective termite predator. During most of their 100 million year-long coexistence, ants and termites have probably been engaging in a co-evolutionary arms race, the ants acting as predators and the termites as the prey, which are continually forced to develop new means of resistance. As termites represent a very large proportion of overall insect abundance and biomass, they serve as a proteinic resource for many ant species, particularly some ponerine ants that are so specialized as termite predators that they organize periodic raids without, in most cases, destroying the entire termite colony [11]. These ants may suffer greatly from being in competition with P. megacephala, particularly in areas where P. megacephala has been introduced and has developed huge colonies. Also, the presence of P. megacephala could make it difficult for occasionally predatory ants to satisfy their nitrogen requirements.
This study points out that P. megacephala's success is related to the efficaciousness of the recruitment behavior of its scout workers. Because the perception of the landmarks of termites or other ants (that serve as kairomones) was enough to trigger long-range recruitment, this may have allowed the scouts to remain safe by avoiding aggressiveness on the part of their prey or competitors. This, in fact, increases their success because it is not necessary for a scout worker to come into physical contact with termite individuals to trigger long-range recruitment. Also, recruiting nestmates after having detected the landmarks of other ant species may increase their ability to compete with them.
Like in Formica schaufussi Mayr [23], P. megacephala scouts appear to assess prey mass in terms of its ability to resist retrieval, because they elicit recruitment only for relatively large prey. Furthermore, we noted that competition influences the number of P. megacephala workers recruited at long range, with greater numbers recruited in the presence of the landmarks of Oecophylla, a competing ant species (see also [24]). However, the proportion of recruited big-headed soldiers did not increase in areas with Oecophylla landmarks, while this was the case for large sugary food sources [7]. This difference is probably due to the fact that prey are retrieved rapidly, while gathering a large sugary food source is a long process and requires soldiers to protect and transport liquid food.
In conclusion, in addition to the already known causes of P. megacephala's ecological dominance in areas where it has been introduced (including its intrinsic ability to achieve unicoloniality; the absence or rarity of enemies; its associations with hemipterans), we have shown in this study that the predatory behavior of this species in its native range can also contribute to its dominance when introduced into new areas. Indeed, P. megacephala workers are able to attack and quickly retrieve a wide range of prey, including termites, employ efficacious short- and long-range recruitment, and respond effectively to the presence of termite prey and the landmarks of competing ants. These abilities are likely prerequisites to its ability to have a high impact on native ants in areas where it has been introduced.
Acknowledgements
We are grateful to Barry Bolton for the identification of the ants, and to Andrea Dejean for proofreading early versions of the manuscript. CSM would like to thank Stefan P. Cover and Edward O. Wilson for many helpful discussions regarding Pheidole. This research was supported by a project from the French ‘Ministère des Affaires étrangères’ (CORUS program, research agreement No. 02 412 062).


