1 Introduction
Saudi Arabia is characterized by its wide biodiversity due to the prevalence of various environments. About one hundred lizard species were recorded in the kingdom [1]. The Saudi sandy environment includes one species only known as Diplometopon zarudnyi, belonging to the family Trogonophidae, which lies under suborder Squamata [2]. Generally, most of reptiles in Saudi desert areas are characterized by a short breeding season [3]. Papenfuss [4] demonstrated that the incubation period in worm lizards from the time of oviposition till hatching ranged from 2 to 3 months. It was found that the fat bodies were fluctuated with the reproductive activity where they were minimal during the peak of reproduction, and then they were increased in size just before hibernation [3]. Studies on reptilian reproductive cycles in the Arabian Peninsula are still scanty when compared with those in other countries. Arnold [5] reported that the breeding season of Acanthodectylus schmidti in the eastern area of the peninsula was considered longer, since it lasted from January till June. Papenfuss [4], using worm lizards, reported that Bipes tridactylus showed only one reproductive cycle yearly, whereas Bipes canaliculatus had one reproductive cycle each 2 years. Colli and Zamboni [6] studied the ecological and reproductive aspects of Amphisbaena alba and found that the egg mass was 8–16 eggs which was higher than that recorded in most other worm lizards.
In Argentina, Vega [7] demonstrated that the breeding season of worm lizard Anops kingii lasted from late winter (July) till the beginning of summer (December), and both sexes reached sexual puberty with the same body length (SVL 155 mm), whereas egg mass was 2–4 eggs. To the best of our knowledge, reproductive cycles of D. zarudnyi are not well fully studied in the Riyadh region of Saudi Arabia, therefore this research was undertaken to provide the necessary information of reproductive biology of D. zarudnyi by tracking the changes in gonadal sizes throughout the months of the year and by the determination of the breeding season and of other related aspects.
2 Materials and methods
A total number of 130 specimens of adult worm lizard D. zarudnyi (70 males and 60 females) were collected from the study area and other zones from central region of Saudi Arabia (Fig. 1) by monthly field trips. Ten specimens of the lizard (males and females) were obtained monthly over a one-year duration. Fig. 2 shows the phenotypic features of D. zarudnyi. The collected samples were then transferred to reptilian laboratory in Zoology Dept., College of Science, King Saud University, where they were killed immediately by freezing. Body weight and dimensions were recorded before dissecting the lizards, testes and ovaries were excised, weighed and fixed in Bouins fluid (10%). Paraffin sections (5–7 μm thick) were prepared according to [8], and then stained with hematoxylin and eosin. Measurements of seminiferous tubules were carried out by using Cool Scope (Nikon, Japan); the volume of each testis was estimated according to the formula from [9]:
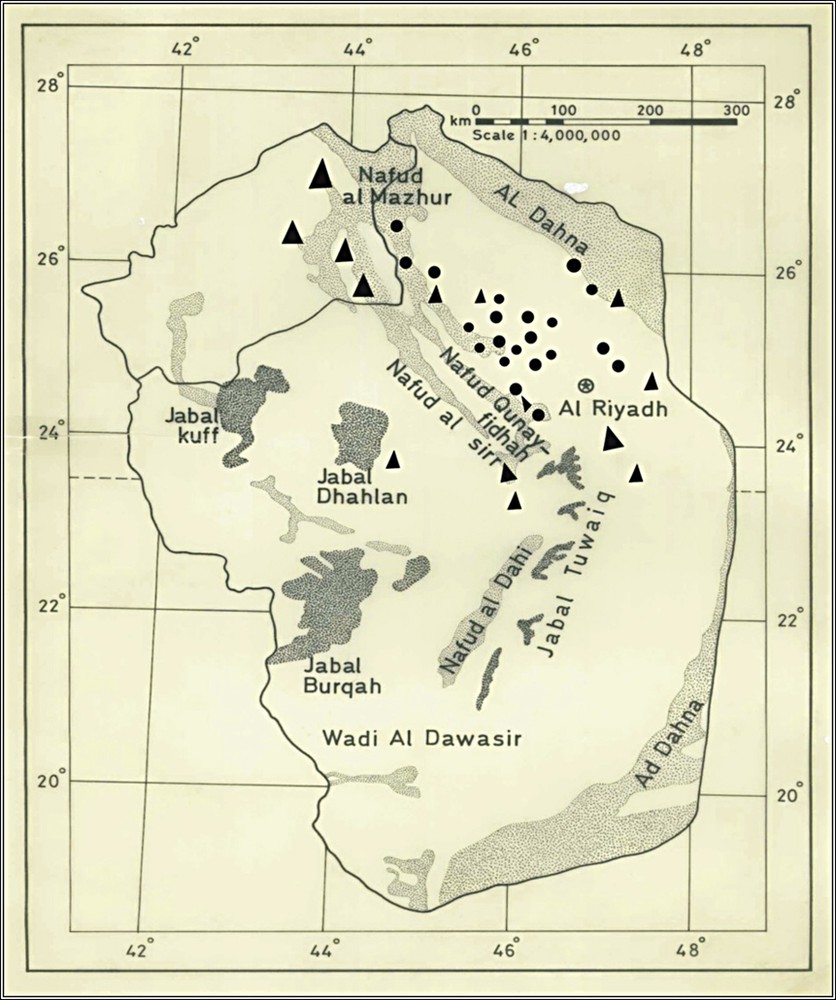
(Color online) map showing the distribution of Diplometopon zarudnyi in the study area (Riyadh, Saudi Arabia). • Current study, previous studies.

(Color online) photo showing the phenotypic features of Diplometopon zarudnyi.
Testes diameters were measured by using a Vernier caliper. The spermatogenic stages of the seminiferous wall were determined according to the method of [10]. Concerning ovaries, the size changes throughout the months of the year were recorded using a Vernier caliper; additionally, the period where ovaries were overloaded with ova was also recorded versus the period of the year with the least number of ova. During dissection, fat bodies were removed from individuals of both sexes and weighed.
3 Results
3.1 Reproductive cycles
The sexual activity of D. zarudnyi occurred throughout a short period of the year, i.e. during April, May, and June. This activity followed a completion of hibernation and improved climatic conditions. Reproduction was quiescent throughout the rest of the year. Testes weight was increased during reproductive activity in which spermatocytes and spermatids were seen in the spermatogenic epithelium, also the lumen of most cases was filled with a mass of spermatozoa. The maximal expansion of seminiferous tubules was observed in May (Figs. 3–6).

Monthly variations in testes size in mm3 of male worm lizard Diplometopon zarudnyi (vertical lines represent
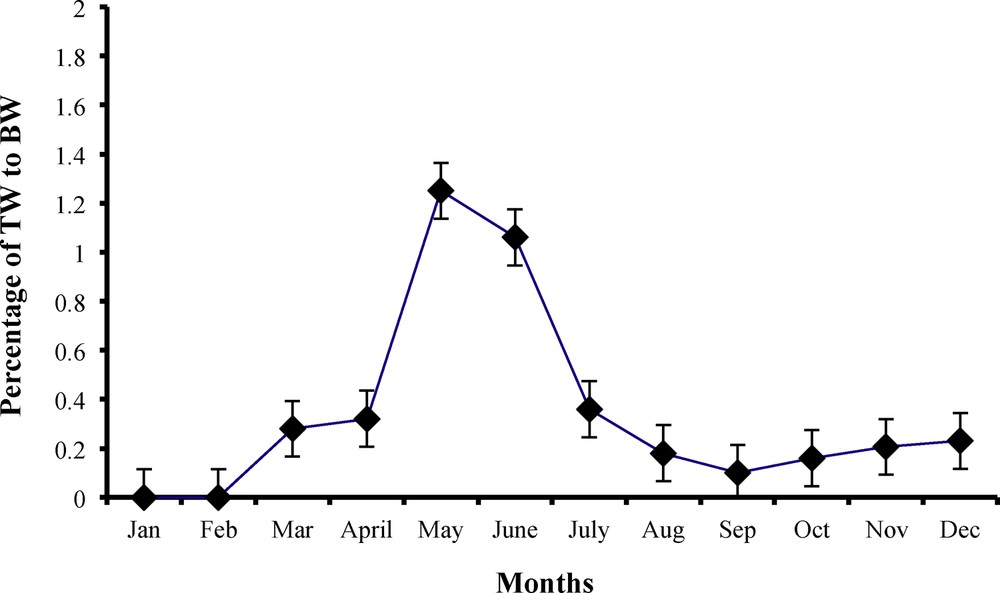
Monthly variations in testes weight (TW in g) in relation to body weight (BW in g) of male worm lizard Diplometopon zarudnyi (vertical lines represent
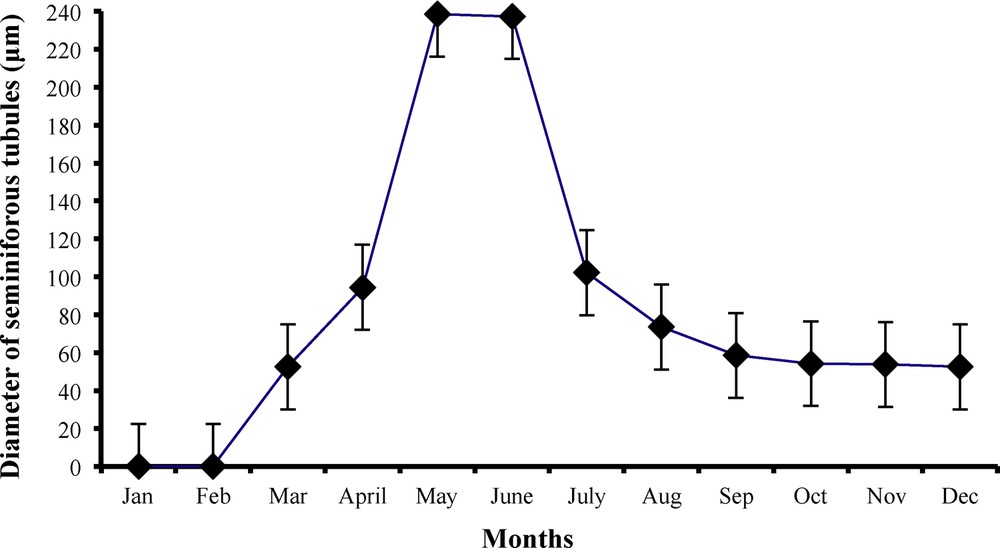
Monthly variations in seminiferous tubules diameter (in mm) of male worm lizard Diplometopon zarudnyi (vertical lines represent
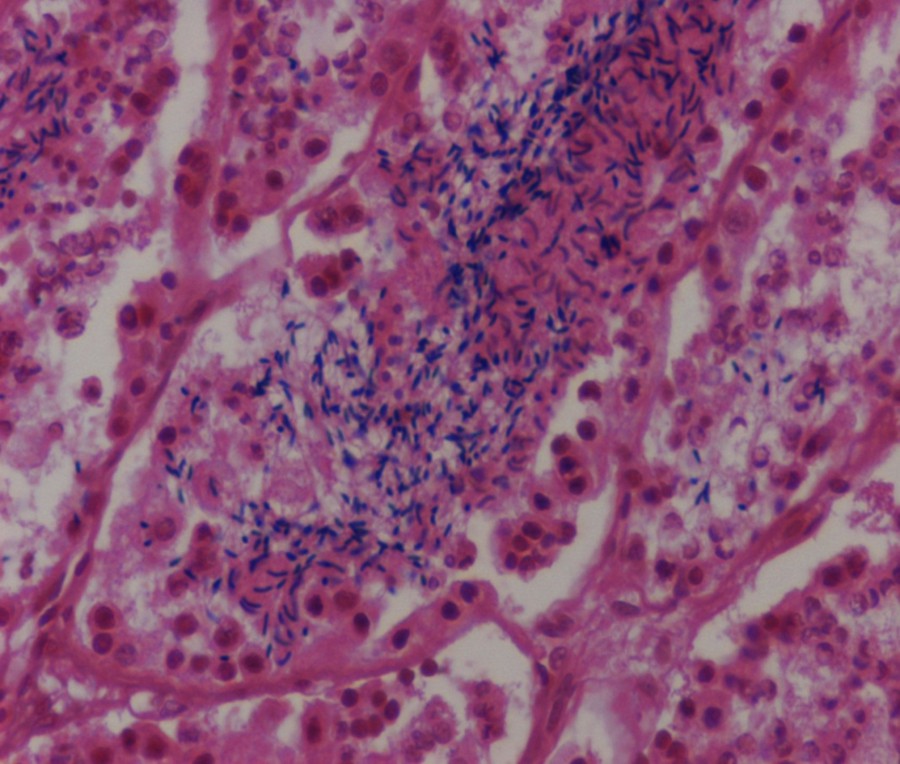
(Color on line) seminiferous tubules of worm lizard Diplometopon zarudnyi testis during May, showing the lumens that are full of spermatozoa (magnification: 200 ×).
Ovaries were overloaded with eggs during April, May, and the beginning of June. Copulation occurred by the end of May. Specimens collected at the beginning of July were characterized by the disappearance of the eggs. Moreover, females were not available at that time, which evidences the process of oviposition, for which hatchability was expected in mid-September. This is also another clue that females are oviparous species. The newly born juveniles were seen during the middle of October. They were characterized by smaller body sizes and pale color; however, they resemble their parents in phenotypic appearance and movement.
3.1.1 Male reproductive cycle
The male reproductive cycle was much more obvious compared with that of the females. The variability in male sexual activity could be classified into the following four phases.
3.1.1.1 Quiescence phase
This stage extended from October till March; the testes were atrophied and had a declined volume (
Monthly variations in testicular parameters of Diplometopon zarudnyi males.
| Months | Number of animals | |||||
| January | 0 | – | – | – | – | – |
| February | 0 | – | – | – | – | – |
| March | 2 | 0.018 | 0.28 ± 0.003 | 5.14 ± 0.005 | 0.362 ± 0.07 | 52.5 ± 0.005 |
| April | 9 | 0.019 | 0.32 ± 0.04 | 7.37 ± 1.11 | 0.301 ± 0.07 | 94.41 ± 2.4 |
| May | 13 | 0.125 | 1.25 ± 0.1 | 76.2 ± 5.12 | 1.02 ± 0.11 | 238.42 ± 3.9 |
| June | 15 | 0.087 | 1.06 ± 0.09 | 51.8 ± 4.84 | 0.51 ± 0.04 | 237.3 ± 6.2 |
| July | 7 | 0.018 | 0.36 ± 0.1 | 5.1 ± 1.02 | 0.27 ± 0.05 | 102.18 ± 2.9 |
| August | 10 | 0.01 | 0.18 ± 0.01 | 5.3 ± 0.98 | 0.328 ± 0.04 | 73.5 ± 1.05 |
| September | 7 | 0.006 | 0.1 ± 0.011 | 6.7 ± 1.01 | 0.34 ± 0.04 | 58.57 ± 1.3 |
| October | 3 | 0.01 | 0.16 ± 0.05 | 9.5 ± 1.5 | 0.3 ± 0.08 | 54.17 ± 0.8 |
| November | 3 | 0.019 | 0.206 ± 0.003 | 5.3 ± 0.04 | 0.106 ± 0.006 | 53.75 ± 0.7 |
| December | 1 | 0.01 | 0.23 | 0.82 | 0.09 | 52.5 |
3.1.1.2 Starting activity phase
This stage began in April, when testis volume and relative weight had obviously increased (
3.1.1.3 Maximal activity phase
This stage occurred throughout May and June, when all measured testis parameters were the highest (
3.1.1.4 Regression phase
This phase extended from July till September, where all testis parameters approximately resemble those of the quiescence phase, with disappearance of spermatocytes and spermatozoa (Fig. 3).
3.1.2 Female reproductive cycle
Four phases of ovarian activity of D. zardunyi were identified as follows.
3.1.2.1 Quiescence phase
This phase extended from October till February, when the relative ovarian weight was minimal and the diameter
Monthly variations in ovarian parameters of Diplometopon zarudnyi females.
| Months | Number of animals | |||||
| January | 0 | – | – | – | – | – |
| February | 0 | – | – | – | – | – |
| March | 3 | 0.032 | 0.63 ± 0.003 | 1.2 ± 0.02 | 0.14 ± 0.01 | 0.5 |
| April | 8 | 0.07 | 0.94 ± 0.18 | 2.6 ± 0.06 | 0.46 ± 0.08 | 1.1 ± 0.03 |
| May | 7 | 0.057 | 0.87 ± 0.14 | 3.3 ± 0.07 | 0.49 ± 0.09 | 1.9 ± 0.017 |
| June | 7 | 0.06 | 0.85 ± 7.5 | 3.1 ± 0.05 | 0.43 ± 0.08 | 1.7 ± 0.002 |
| July | 7 | 0.03 | 0.5 ± 0.13 | 0.5 ± 0.005 | 0.31 ± 0.12 | – |
| August | 8 | 0.04 | 0.48 ± 0.08 | 0.5 ± 0.004 | 0.29 ± 0.07 | – |
| September | 13 | 0.026 | 0.42 ± 0.03 | 0.52 ± 0.003 | 0.34 ± 0.02 | – |
| October | 4 | 0.017 | 0.27 ± 0.03 | 0.53 ± 0.007 | 0.226 ± 0.02 | – |
| November | 2 | 0.01 | 0.27 ± 0.005 | 0.56 ± 0.005 | 0.073 ± 0.005 | – |
| December | 1 | 0.009 | 0.24 | 0.55 | 0.312 | – |
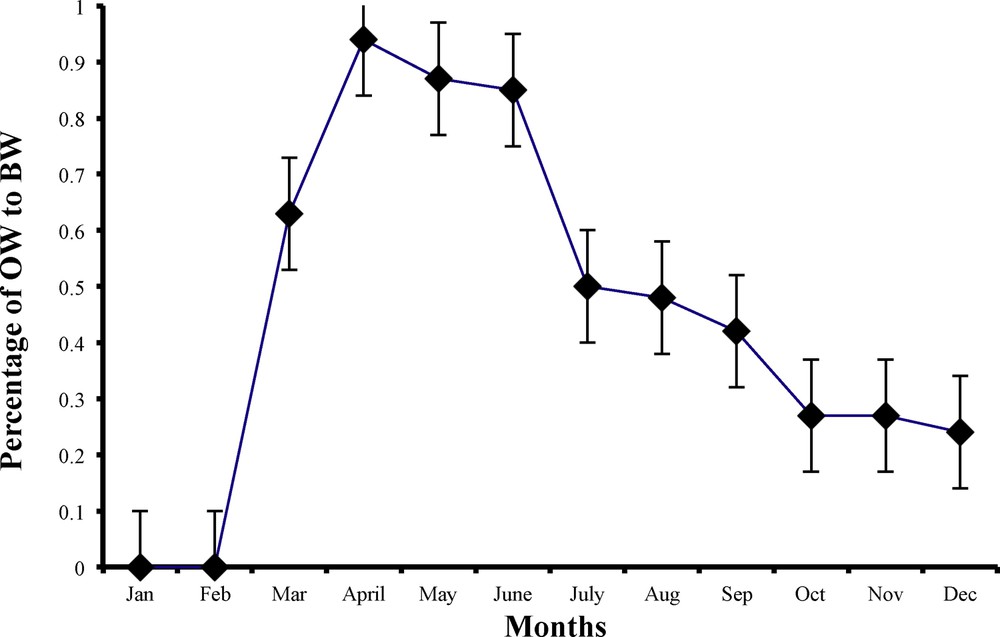
Monthly variations in ovary weight (OW in g) in relation to body weight (BW in g) of female worm lizard Diplometopon zarudnyi (vertical lines represent
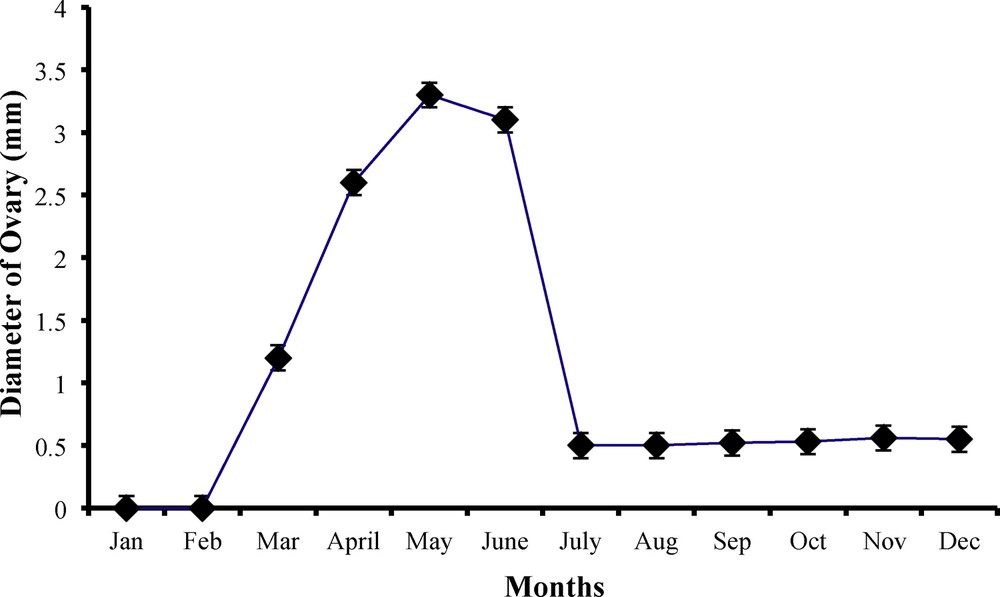
Monthly variations in ovaries diameter (mm) of female worm lizard Diplometopon zarudnyi (vertical lines represent
3.1.2.2 Start of the activity phase
The beginning of ovarian activity was observed to occur in March, when eggs were seen on the ovary. Both relative ovarian weight (
3.1.2.3 Maximal activity phase
The ovarian activity was the highest throughout April, May and June, when ovarian parameters were greater and each ovary was loaded with 6–9 eggs, with an average egg diameter of 1.5 mm (Table 2 and Fig. 9).
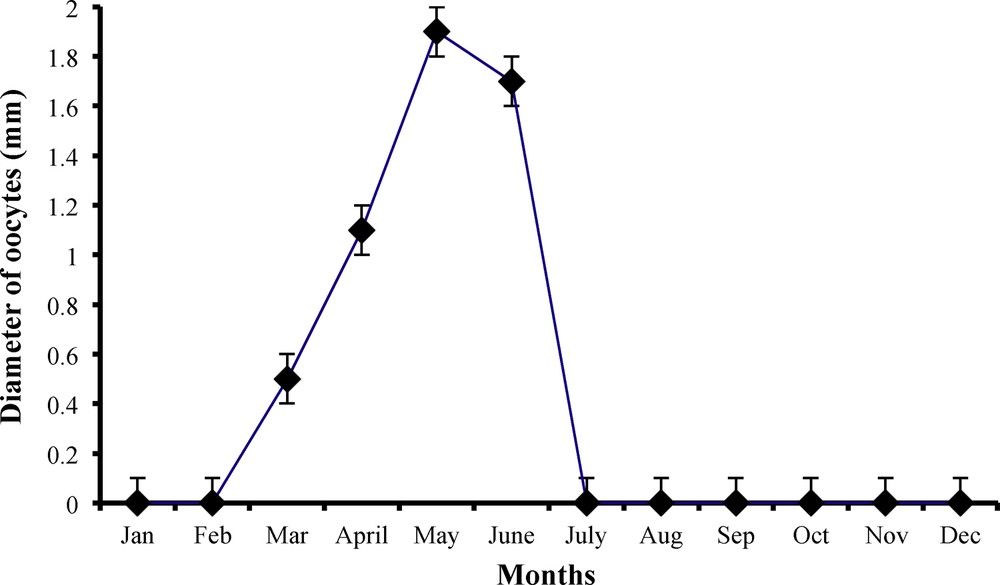
Monthly variations in oocyte diameter (mm) of female worm lizard Diplometopon zarudnyi (vertical lines represent
3.1.2.4 Regression phase
The ovarian size was minimal and atrophy started throughout July, August and September. No ovarian structure was observed on the ovary during this stage. Hatchability was expected to occur during the middle of September and recently hatched youngsters were seen in the middle of October (Table 2).
3.1.3 Fat body cycle
Fat bodies represent the stored adipose content, which is considered as energy storage. This fat content fluctuates according to food availability and organism activity. Following hibernation, fat bodies of D. zardunyi started to increase when it reached its maximum weight during May (
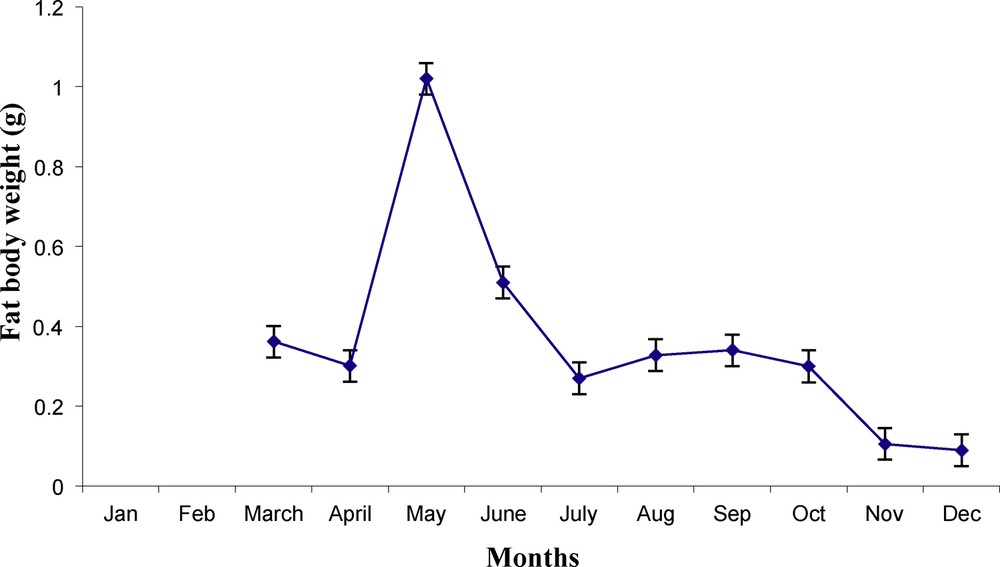
Monthly variations in fat body weight (g) of male worm lizard Diplometopon zarudnyi (vertical lines represent

Monthly variations in fat body weight (g) of female worm lizard Diplometopon zarudnyi (vertical lines represent
4 Discussion
The breeding season of D. zarudnyi occurs in just one period throughout the year in the Riyadh region. This was consistent with the findings of [4] on lizard B. tridactylus in Mexico, and with those of [11] on Blanus cinereus in Spain. Moreover, this result of the current study agreed with various reproductive studies carried out on reptiles in desert areas of Saudi Arabia [12] on Uromastyx aegyptius [3] and on Uromasyx ocellatus philbi [13]. It was also found that the duration of the breeding season of D. zarudnyi was a short period, lasting from 75 to 90 days (beginning of April till the end of June). The reproductive activity of females started a little earlier (end of March) than that of males, which might indicate a longer ovarian cyclicity and a greater hormonal response to improved environmental conditions in females. The number of eggs was almost equal in both ovaries, which is in agreement with the results of [7] on A. kingii. It was noted that D. zarudnyi was oviparous. Similar findings were obtained by [4] on species belonging to the genus Bipes. Continuity of ovarian atrophy was observed in D. zarudnyi till the time of hibernation; this was consistent with the results of various reproductive studies done in desert areas of Saudi Arabia using different species of reptiles [3,13,14]. These previous studies pointed out the gonadal atrophy of reptiles upon completion of hibernation, and then maximal gonadal size was attained throughout the breeding season. In the current study, the end of May represents the peak of sexual activity of D. zarudnyi, since copulation occurs during this period. This might be attributed to an improvement of the environmental conditions. The incubation period of D. zarudnyi eggs extends from the beginning of July till the end of August, which is in agreement with the findings of [4]. The juveniles of D. zarudnyi were seen during mid-October, when their ages ranged from 1–1.5 months and they resembled their parents in phenotype and behavior. These results agreed with those of [3] on U. aegyptius, with those of [12] on Scincus mitranus, and with those of [14] on C. gasperettii in desert areas of Saudi Arabia. On the other hand, fat bodies were increased upon the end of hibernation, preparing for a new phase of reproductive activity. Similar results were obtained by numerous investigators using different species of reptiles in desert areas of Saudi Arabia (e.g., [13] on U. philbi). These fat bodies were completely utilized as a source of energy throughout the duration of the breeding season. Fat bodies were then increased just prior to getting in hibernation, which then diminished to their smallest sizes during hibernation that occurred during February of most reptiles in desert lands of Saudi Arabia.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgement
The authors would like to express their sincere appreciation to the Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia for funding this Research Group Project No. RGP-VPP-289.


