1 The field lizard Podarcis siculus
The loss of pristine terrestrial habitats due to the rapid and continuous urbanization is the primary threat to wildlife survival. Among animals, arthropods are particularly endangered, but also many vertebrate species are threatened [1]. It is estimated that human activities such as unsustainable agriculture and industrialization, causing habitat destruction and water and soil pollution, are the main causes of the decline of Amphibians [2,3]. In such a scenario, the survival of more adaptive species is less compromised. An example is given by the field lizard Podarcis siculus (Fig. 1), the most abundant reptile in Italy, widespread in all the country, with the exception of the Alps (Fig. 2).

Podarcis siculus specimens during a mating ritual.
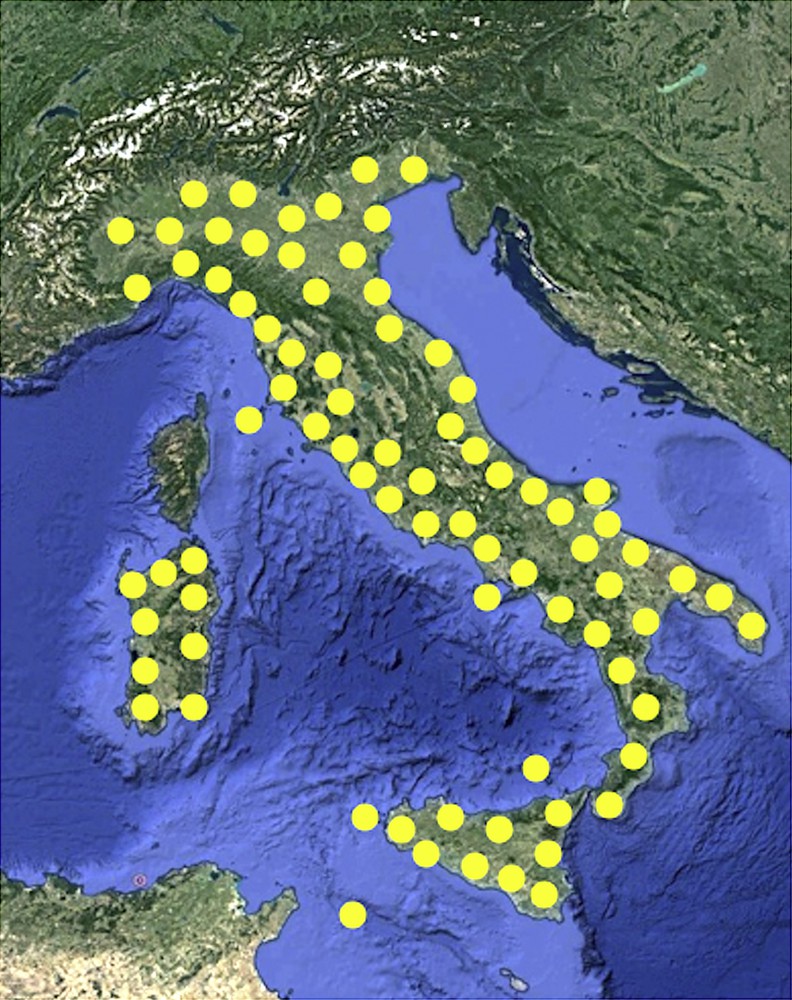
Geographical distribution of Podarcis siculus lizard in Italy.
This species has been also introduced into a number of locations elsewhere in the Mediterranean region (Portugal, Spain, France, Montenegro, Croatia, Turkey, Libya, Tunisia) [4].
P. siculus specimens are quite small (20–25 cm total length, 8–10 g body weight), ubiquitously inhabiting pristine areas, cultivated fields and city parks. They feed mainly on insect larvae and adults and worms, occasionally on fruits and vegetables. It is an oviparous species characterized by an annual reproductive cycle. After a period of semi-hibernation, in early spring the animals leave their winter shelters and start eating. Gonads and reproductive tracts become active and start growing. In the female, the massive hepatic synthesis of vitellogenin (VTG) begins and egg deposition takes place from May to June; 4–8 eggs are spawned in clutches and 2 or 3 clutches may occur during spawning [5].
The male genital apparatus shows a seasonal rhythm in reproductive function, divisible into different phases: spring–half summer (April–June) reproductive phase, summer stasis (July–August), autumnal resumption (September–November), winter stasis (December–February), spring resumption (March–April). Stasis is the period of refractory or blocked testicular activity; resumption is the period in which spermatogenesis renewal occurs. During summer and winter, stasis seminiferous tubules are composed of spermatogonia and Sertoli cells only; in the autumnal resumption, tubules are composed of spermatogonia, spermatocytes, spermatids and a few spermatozoa not released into the lumen. The testis of the reproductive period shows tubules consisting of germ cells in all the differentiating stages with numerous spermatozoa, ready to be ejaculated [6–8].
Habitat and diet adaptability make it a competitive and successful lizard, not currently considered in the Italian red list of threatened species (IUNC) [9]. However, evidence exists on adverse effects on the health status and reproductive fitness of these animals as a result of food, water, and soil pollution [10–18]. It has been also demonstrated that lizard embryos, albeit developing in cleidoic eggs, i.e. enclosed eggs that protect and nourish embryos, are affected by the presence in the incubation soil of pollutants, such as heavy metals and pesticides [19–24]. Also, physical stress such as a sharp change in temperature can alter the correct lizard development [25,26].
2 Environmental pollutants and P. siculus health status
In the last past years, our researches aimed to evaluate the adverse effects of food and soil pollution on P. siculus health status and reproductive fitness. We demonstrated that cadmium (Cd) exerts toxic effects both in adults and in embryos: in the former, the morphology of the liver and gonads is altered, leading to the impairment of reproduction in females [11,12]; in the latter, the in ovo Cd-exposure, obtained incubating eggs in a Cd-contaminated soil, induces malformations in encephalic vesicles [22–24] and dysregulation of embryonic gene expression [21].
More recently, we investigated the potential toxicity of nonylphenol (NP) on P. siculus specimens. This chemical product is an endocrine disruptor widely distributed in the environment. NP is used in many industries as a surfactant; it is present as co-formulant of pesticides and biocidal products used in conventional farming. It accumulates in the lipids of living organisms entering the food chain and acts as an estrogenic compound being able to mimic the action of oestradiol 17β (E2) by binding to the oestrogen receptors (ERs) [27,28].
Collected data demonstrate that an experimentally NP-polluted diet is able to affect liver and gonad conditions of sexually mature P. siculus specimens. First of all, we demonstrated the xeno-estrogenic activity of NP also in these animals; indeed, NP induces the VTG synthesis in male liver [27]. The protein, undetectable in untreated males, reaches the value of about 4 μg/μl in the plasma of the experimental ones. The NP-polluted diet also elicits the expression in the liver of ERα, the ER isoform known to be related to VTG synthesis in Podarcis females [29].
Always in male lizards, NP-polluted food and water administered during the mating period cause a slowdown of spermatogenesis and affects the testicular and epididymal structure, bringing it back to the typical morphology of the non-reproductive period [14]. In these animals, NP treatment also changes the distribution in the testis and epididymis of mRNA for the oestrogen receptors α (ERα) and β (ERβ) and the androgen receptor (AR): the expression of the ERα, ERβ, and AR genes is down-regulated in spermatogonia and primary spermatocytes, the ERα gene is up-regulated in epididymis, thus causing a switch-off of the secretory activity of the epididymal corpus [14].
In Podarcis females, the oestrogenic activity of NP seems to be negligible, at least under the conditions experimented in these studies (unpublished data).
Also, environmental exposure to other chemicals used in intensive crop production, such as the fungicide methyl thiophanate, affects P. siculus welfare. Cells from exposed lizard show clear signs of genomic damage; changes in corticosterone and catecholamines plasma levels, alterations in adrenal gland morphology, such as hypertrophy of the steroidogenic tissue, enlargement of blood capillaries, lymphocyte and macrophage infiltrations are also evident [13]. More recently, a study performed with the insecticides imidacloprid, a neonicotinoid belonging to a new class of highly effective and widely used insecticides for crop protection, demonstrated that P. siculus lizards environmentally exposed to this substance showed dose-dependent changes in testicular architecture, increased apoptotic processes and decreased levels of sex hormones and steroid receptor mRNAs [30].
3 Organic farming and P. siculus health status
The growing concerns on the effects of chemical fertilizers and pesticides to wildlife, human health, and environmental impact led in recent years to a sharp increase in the agricultural practices of organic crops, that allow the use of many natural derivates as fertilizers and plant protection products. Among fertilizers, manure, consisting of a mixture of cow and chicken dung, straw and plant litter, is the soil amendment most used in organic farming.
However, manure may contain residues of hormonal substances and/or their metabolites [31,32], thus displaying oestrogen-like activity and ability to activate steroid receptors in animals [32,33]. Indeed, vertebrates excrete conjugated or free steroid hormones that may interfere with the endocrine system, affecting the reproduction and development in wildlife [34]. It has been demonstrated that metabolites of steroid hormones persist in manure for several months [35]; conjugated and biologically inactive forms can be converted into free steroids by soil microorganisms [36]. In addition, manure may contain residues of drugs (antibiotics, cortisone) used in animal husbandry and/or pollutants bioaccumulated in plants, such as heavy metals [37].
The amount of studies regarding the potential adverse effects of manure on terrestrial vertebrates is very scarce. So, considering the increasing shift towards organic farming and manure use as soil amending, we decided to investigate if manure application could adversely affect lizard populations. We captured P. siculus adult specimens in two organic farms (certified by the Italian Inspectorate for the Protection of Quality and Fraud Prevention of Agri-Food Products) located in the neighbourhood of Sorrento (southern Italy); both farms were of wide extent, well isolated from non-organic crops, and used as fertilizer the manure of animals (cows, sheep, horses, pigs and chicken) bred in the same farms. In these animals, we examined the morphological condition of liver, the main detoxifying organ in vertebrates, and, to ascertain a possible xeno-estrogenic effect of manured soil, we determined in male livers the presence of VTG and ERα, typical biomarkers of estrogenic contamination. In addition, on the same tissue, the concentration of Cd and lead (Pb) ions was evaluated, together with the expression of the metallothionein (MT), the principal biomarker of heavy metal contamination, also known to be a good scavenger of reactive oxygen species [10,38,39].
To detect possible impacts on reproductive fitness, we also paid attention to gonad conditions in both males and females.
All data were compared with those obtained from lizards treated with nonylphenol, as a positive control of oestrogenic contamination, and with those retrieved from lizards caught in pristine, uncultivated sites, representing the wild specimens on which many studies on P. siculus were performed and that never showed signs of oestrogenic contamination (referred to as controls).
4 Liver condition
4.1 Morphology
The liver of P. siculus shows the typical hepatic architecture consisting of cords of hepatocytes radiating from the central vein toward the periphery; liver sinusoids occupy the spaces between cords.
In control lizards living in uncultivated habitats, the morphological characteristics of liver parenchyma were mostly normal; hepatocytes showed a round, well-defined nucleus containing a single nucleolus and a dense cytoplasm, marginally vacuolated. Livers of lizards inhabiting manured soils showed essentially the same histological conditions, whereas livers of lizards fed with NP-polluted water and food appeared more suffering, hepatocytes showed a more vacuolated cytoplasm, many pyknotic nuclei were also present (Fig. 3).
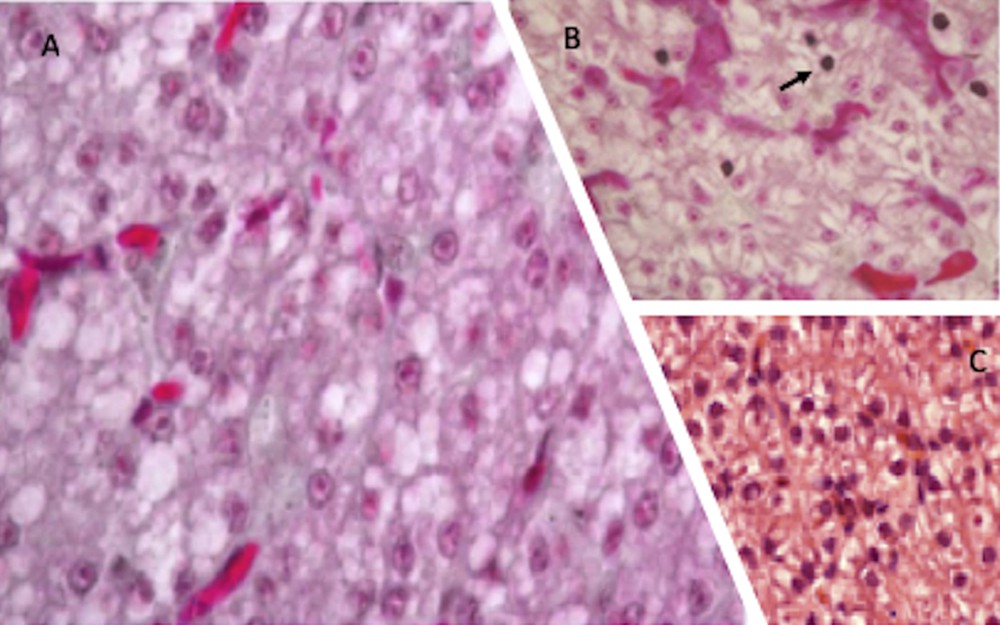
Haematoxylin-eosin stained P. siculus liver sections. Livers from control lizards (C) and from individuals dwelling in organic farming soils (A) show a similar morphology. In NP-contaminated lizards (B) hepatocytes cytoplasm is vacuolated and some pyknotic nuclei, typical of cells undergoing apoptosis, are evident (arrow). Magnification: 20 × .
4.2 Cadmium, lead and metallothionein content
Heavy metal contamination has been increased due to the anthropogenic activities; atmospheric fall-out, pesticides, fertilizers and irrigation with water of poor quality are some of the causes of soil contamination with metals [40]. Lead contamination comes from mining and smelting, burning of gasoline, municipal sewage and paints, while cadmium comes from metal smelting and refining, fossil fuel burning or application of phosphate fertilizers [41,42]. These metals cannot be destroyed biologically but are only transformed from one oxidation state or organic complex to another.
Cadmium and lead accumulation in liver is typical of terrestrial vertebrates inhabiting soils contaminated by anthropogenic activities. Biomonitoring of heavy metal pollution can be performed measuring the metal concentrations in animal body fluids and organs [43]; more often, it is preferred to determine the metallothionein (MT) contents in the liver and the kidney, as specific biomarkers of heavy metal pollution [44]. Indeed, MTs are proteins rich in the amino acid cysteine and consequently exhibit a high affinity for soft, polarisable metal cations. Metal-induced expression and synthesis of MT causes this protein to play a predominant role in the regulation of essential metals (Cu, Zn) and in the sequestration and detoxification of non-essential metals (Cd, Pb) in a wide variety of animals. MTs have become of great interest for assessing pollution in both aquatic and terrestrial environments and today they are the most powerful biomarkers of metal exposure in animals [45–47]. In the latter, the absorbed metals are accumulated mostly in the liver and excreted primarily in urine and in faeces [48]; therefore, this could lead to an unwanted accumulation of metals in the manure used in organic farms.
The soils of the two organic farms from which the lizards came from showed a very low content of heavy metals (Cd < 0.4 mg/kg; Pb < 55 mg/kg); they also showed a low level of phenols (<0.010 mg/kg), a value that certifies that chemical fertilizers and pesticides are not used in the two farms.
As a consequence of this weak metal content in the soils, we determined a low accumulation of the same metals in the livers of animals inhabiting these areas. The values reported in Table 1 show that the Cd and Pb levels were higher in livers from specimens referred to as control group, living in uncultivated areas, probably more exposed to vehicular traffic and illegal waste dumping.
Real-time PCR analysis performed to measure the amount of MT transcripts in livers confirmed the data obtained from metal determination: MT expression was two-fold higher in livers of control lizards (Fig. 4). It is known that MT genes can be induced by stress factors other than metals, such as inflammation and oxidative stress [50]. Hence, this analysis reinforces the morphological observations, and it can be stated that livers of animals inhabiting manured fields seem to be in good health condition.
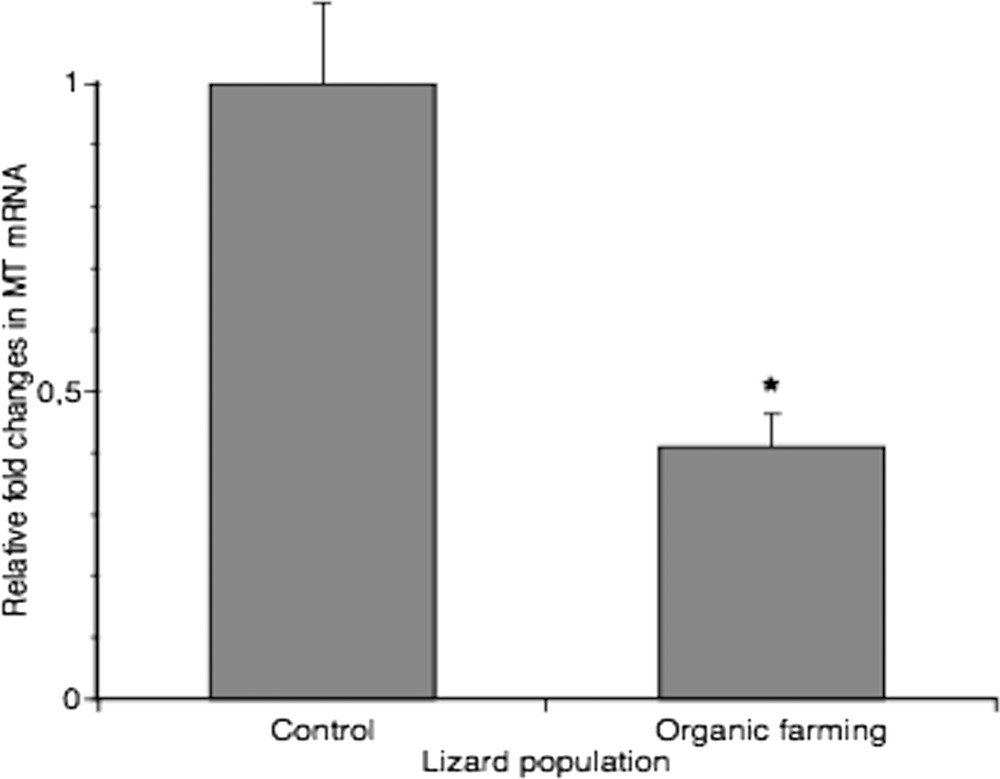
Real-time PCR analysis of MT expression in liver from two different lizard populations. MT mRNA level was normalized to that of β-actin mRNA and converted in fold change, compared with the control lizards. Data represent the mean ± s.e.m. * = p < 0.05.
4.3 Estrogenic effect
To ascertain a possible estrogenic effect of manure, we searched for the typical hepatic biosynthetic alteration indicative of this hormonal signalling, i.e. the presence in male hepatocytes of detectable amounts of both VTG and ERα transcripts. In situ hybridization analysis on liver sections from P. siculus males captured in the two organic farms showed the presence of transcripts for both VTG and ERα in the cytoplasm of hepatocytes, as previously detected in the livers of NP-treated males (Fig. 5). Sections of livers from control males remained unlabelled (Fig. 5).
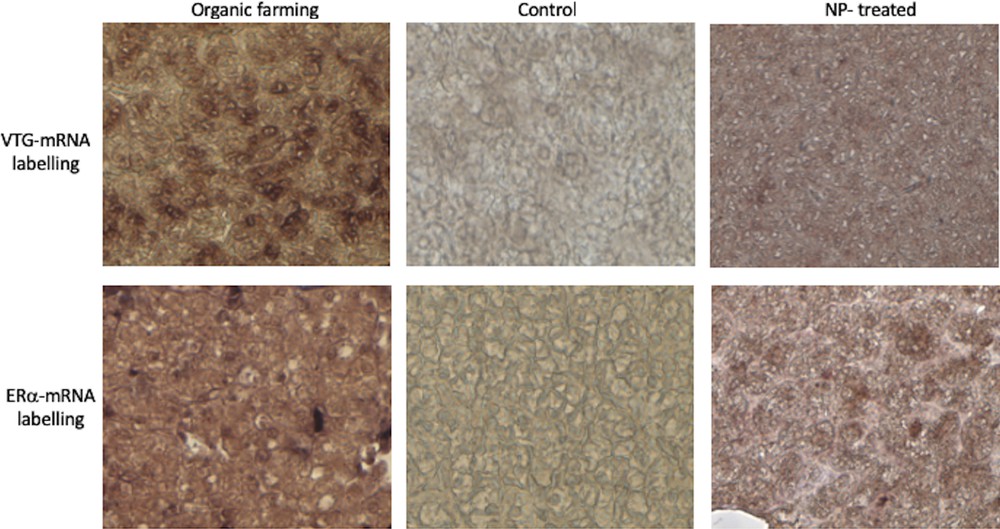
VTG and ERα expression in the liver of P. siculus. Sections were incubated with DIG-probes to detect VTG- and ERα-mRNA. The brown hybridization signal was always absent in the liver of males collected on uncultivated areas (control). The signal was evident for both probes on liver sections of males treated with NP-polluted food or dwelling in organic farming soils. Magnification: 20 × .
So, while, on the one hand, organic farming avoids soil contamination by chemicals and heavy metals, on the other hand, the use of manure as fertilizer may lead to an accumulation in soils of natural steroids and/or EDC that may affect the reproductive processes.
It has been demonstrated that the activation of oestrogen signalling in male vertebrates can lead to detrimental effects on spermatogenesis and fertility parameters [14,51]. VTG expression in oviparous males has been also interpreted as a warning of reproductive adverse consequences [52].
5 Ovary and testis condition
5.1 Ovary morphology
The ovary of P. siculus has a cluster shape, with protruding follicles. In early spring, follicles resume their growth, and several oocytes become increasingly yolky. The ovarian follicle is composed of a central oocyte surrounded by a bi-layered, acellular membrane, the zona pellucida, which is bounded by the follicular epithelium. Follicular growth involves not only the growth and maturation of the oocyte, together with the storage of large quantities of yolk in the ooplasm, but also morphological and functional changes in the follicular epithelium [53]. In primary follicles (<150 μm in diameter), the epithelium is composed of a single layer of small stem cells; in mid-previtellogenesis (<1500 μm in follicle diameter), the epithelium becomes multi-layered, and small and pyriform cells are recognizable; during late previtellogenesis (>2000 μm in diameter), cells regress via apoptosis [54] and follicular epithelium gradually restores the monomorphic monolayered condition in which the small cells persist as unique components of epithelium until ovulation [53]. Surrounding the ovarian follicle there is the theca, composed of connective tissue, blood vessels, and secretory cells.
As shown in Fig. 6, ovaries of all the three different lizard populations (from uncultivated areas, from manured soils and NP-polluted) showed the expected morphology, consistent with the phase of the reproductive cycle examined. Once again, the ovary proves to be a structure less sensitive to xeno-estrogenic substances, which, at least under the conditions tested in this study, fail to disrupt the natural hormonal cycle in this organ. Noteworthy, remarkable oestrogen amounts are present in the blood during all the oocyte growth period [55]; the incidence of this additional oestrogenic stimulation might be very poor.
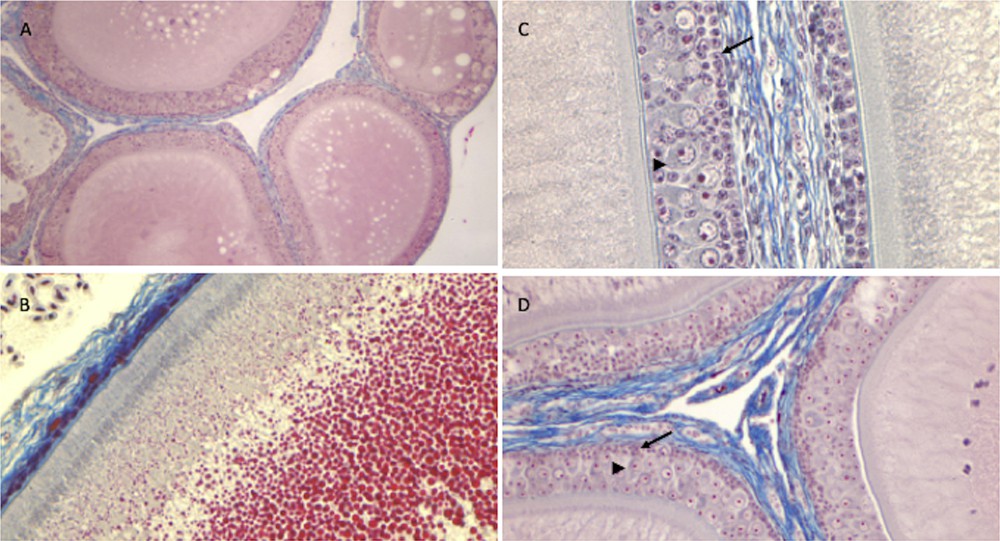
Histology of the ovary of P. siculus. Previtellogenic (A) and vitellogenic (B) follicles of control lizards. Note in B the yolk droplets (in red) filling the oocyte cytoplasm. (C) Mid-previtellogenic follicles of lizards from agricultural manured soils. (D) Mid-previtellogenic follicles of NP-polluted lizards. Note in C and D the small (arrow) and pyriform cells (arrowhead) surrounding growing oocytes. Staining: Mallory's trichrome; magnification: (A) 4 × (B, D) 10 × (C) 20 × .
5.2 Testis morphology
Testes of control males stained with Mallory's trichrome staining showed the seminiferous epithelium filled with all germ cells from spermatogonia to spermatozoa, as expected in the spring resumption period (Fig. 7). In the lizard population inhabiting soils devoted to organic farming, seminiferous tubules showed the same morphology, with ordinated layers of germ cells from spermatogonia (at the basement membrane) to spermatids and spermatozoa protruding toward the narrow lumen (Fig. 7). In the animals exposed to the NP-polluted diet, testes showed an altered morphology: the lumen of the tubules was wide, the seminiferous epithelium was reduced in thickness and several empty spaces were evident (Fig. 7), likely due to a decrease in the amount of the germ cells, albeit all stages of their differentiation were detectable.
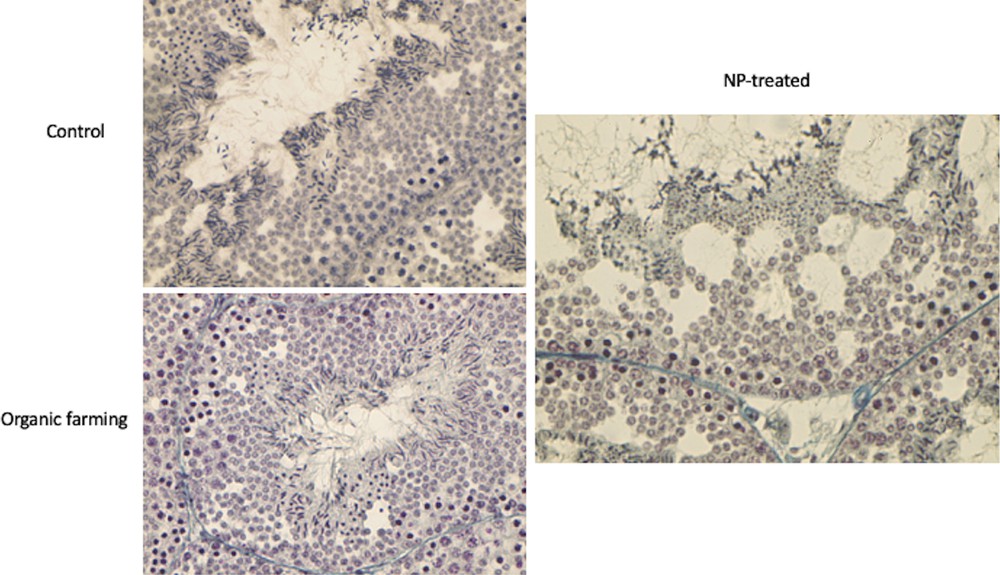
Histology of the testis of P. siculus in the spring resumption period. Control: all stages of spermatogenesis are evident in the seminiferous epithelium. Organic farming: testis condition is similar to control, with all the differentiating stages of germ cells organized from the basal membrane (spermatogonia) to the lumen (spermatids and spermatozoa); note the narrow lumen of the tubule. NP-treated: in the thin seminiferous epithelium, empty spaces are evident; the lumen and interstitial spaces of the tubule are enlarged. Magnification: 20 × .
5.3 Oestrogenic effect
As described above, VTG synthesis occurs naturally in the liver of oviparous females; following oestrogenic contamination, VTG transcript and protein appear also in males, typically in the liver. However, evidence exists on the oestrogen-dependent, extrahepatic synthesis of VTG in organs other than liver [56]. In particular, it has been demonstrated that, in males of P. siculus, oestrogen contamination induces VTG expression and synthesis in testis and epididymis [56]. In situ hybridization analysis on testis sections from the three different lizard populations considered in this research reinforces the result obtained on the liver, indeed both manure fertilized soil and nonylphenol show a strong oestrogenic effect also on the testis, determining the transcription of the VTG gene in somatic cells (Sertoli and Leydig cells) and in all the differentiating stages of germ cells (Fig. 8). Noteworthy, the testis of the animals collected on organic farming soils appears morphologically in good conditions; therefore, the presence of VTG, demonstrated also at the protein level [56], does not seem to impair the correct maturation of male germ cells. However, the function, if any, of VTG in the testis is still a matter of debate. The failure to detect transcripts for the VTG receptors in testis under natural or oestrogenic conditions [56] suggests that the testicular VTG detected in these animals derives entirely from the biosynthetic locally oestrogen-activated process and that, most likely, VTG does not represent a functional protein in this organ.

In situ hybridization on sections of testes of P. siculus, incubated with the DIG-probe to detect VTG-mRNA. The brown hybridization signal was absent from the testes of wildlife males collected in uncultivated, rural areas (control). In the samples treated with NP-polluted food or dwelling in organic farming soils, the signal was evident in the cytoplasm of all the germinal cells of the seminiferous epithelium, from spermatogonia to spermatozoa, as well as in somatic cells. Magnification: 10 × .
6 Concluding remarks
The data summarized herein highlight that the P. siculus population inhabiting organic farm areas shows typical hepatic biosynthetic alterations indicative of an estrogenic contamination, easily demonstrable in male specimens. Male hepatocytes, in fact, contain detectable amounts of both VTG and ERα transcripts and proteins; in addition, in the same specimens, a testicular synthesis of VTG was also detected, in both somatic and germ cells. These oestrogen-related changes in gene expression were also observed in lizards receiving a nonylphenol-polluted diet; however, the morphological condition of liver and testis in the two lizard populations was very different: samples from NP-treated males resulted seriously impaired, whereas the conditions of the samples from organic farms areas resembled those of natural wildlife populations. Considering also the low level of Cd and Pb ions and of the metallothionein, a protein induced by metals, inflammation and oxidative stress, we can assume that the animals living on the soil fertilized with manure are healthy.
On the other hand, having ascertained the oestrogenic effect of manure in lizards, it cannot be ruled out that the prolonged use of manure as a fertilizer may affect reproductive processes. The accumulation in soils of natural steroids and/or EDC could lead to changes in the spermatogenic cycle of these animals, endangering their reproduction and survival. In addition, a continuous increased amount of oestrogen plasma levels could have adverse effects also in female specimens. In this regard, it has been demonstrated that exogenous oestradiol increases shell thickness in laying hens [57]. Yolk steroids of maternal origin may also influence sex determination and gonad differentiation during embryo development [58,59]. So, it is conceivable that a prolonged intake of exogenous oestrogens could affect the embryonic development, the offspring, and the reproductive cycle of P. siculus.
In conclusion, although less harmful than intensive farming, organic farming should focus more on crop rotation and encourage the biological cycles and the biological activity of soils rather than the massive use of manure as a fertilizer. It is also important to monitor the amount of oestrogen derivatives in manure to avoid possible undesirable effects on wildlife and human health. In the recent years, it has been estimated that organic farming has grown by 8.9% per year [60]; soil contamination by steroids and EDC could rapidly be a major risk, like pesticides and heavy metals.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
This work was supported by the University of Naples Federico II, Italy. All the experiments described here were carried out following the ethical provisions established by the 2010/63/EU directive for animal experiment and under the permit of the Italian Department of Health (78/2013).


