1 Introduction
Bumblebees occur universally and are characterized by their large body size. They have economic and ecological importance as many native plants and agricultural plantations are pollinated almost exclusively by them [1–4].
The adaptive success of insects is partly a result of the variety of structural and physiological properties of the digestive tract, which enables the use of a great diversity of resources vital for their survival [5].
In insects, the digestive tract is distinguished into the foregut, midgut, and hindgut [6]. The midgut is the chief region where actual digestion and absorption occur [7,8]. In bees, the midgut is a tubular structure lined by single-layered epithelium, comprising three cell types, viz., digestive or principal cells, regenerative cells and endocrine cells [9–11].
In bees, the digestive cells are the most numerous of the cell types occurring in the midgut. They are responsible for the synthesis of digestive enzymes and nutrients [12–15].
The regenerative cells are found clustered in nests and distributed among the digestive cell bases. They help in the renewal of the midgut epithelium [14,16–18]. However, the differentiation mechanisms of the regenerative cells has been discussed, with the occurrence of cell proliferation in the adult Lepidoptera [8] and Diptera [19], although evidence of mitosis in the regenerative cells has not been observed in Orthoptera [20] and Hymenoptera [10,21].
The midgut endocrine cells, on the other hand, produce several bioactive peptides that regulate the functions of the digestive epithelium, visceral muscle contraction, cell differentiation and development [11,13,22–25].
The midgut is a dynamic structure incorporating several morphological variations along its entire length, based on the physiological status of the insect or the different functions associated with each one of its regions [14,17], as reported in Dendroctonus jeffreyi and Diatraea saccharalis (Lepidoptera) [26,27] and bees [10].
This paper describes the morphology of the different cell types occurring in the anterior and posterior midgut regions of Bombus morio and aims at contributing to a better understanding of the digestive process in this bee.
2 Materials and methods
2.1 Bees
A total of 25 B. morio forager workers were collected from the flowers growing in the Viçosa region, Minas Gerais, Brazil (20°46′S 42°51′W).
2.2 Histology
First, five bees were dissected in saline solution for insects (0.1 M NaCl, 20 mM KH2PO4 and 20 mM Na2HPO4), and the midguts were removed and placed in Zamboni's fixative solution for 4 h at room temperature. Following the dehydration of the midguts in a graded ethanol series (70–95%), they were embedded in historesin JB-4. After sectioning, the resulting 3-μm-thick slices were stained with hematoxylin and eosin and examined under the light microscope.
2.3 Whole midgut
As described above, five bees were dissected and following the transfer of the midguts to Zamboni's fixative solution for 2 h at room temperature, the samples were washed in distilled water. The midguts were separated into the anterior and posterior regions and stained with diamidino-2-phenylindole (DAPI) for 30 min, mounted on slides with 50% sucrose and analyzed using a fluorescence microscope.
2.4 Immunofluorescence
After fixation in Zamboni's solution, all five midguts were washed thrice, for 30 min each, in a sodium phosphate buffer 0.1 M at pH 7.2 containing 1% of Triton X-100 (PBST) and incubated for 24 h at 4 °C with anti-FMRFamide antibody (Peninsula Lab.) 1:400 in PBST. The samples were once again washed thrice with PBS (for 5 min each), and incubated with anti-IgG fluorescein isothiocyanate (FITC) conjugated (Sigma) 1:500 in PBST for 24 h at 4 °C. After three washes in PBS, the samples were stained with DAPI for 30 min, mounted in 50% sucrose and analyzed under a fluorescence microscope.
Negative control was conducted in the B. morio midguts following the procedure described above, omitting the step involving the treatment with anti-FMRFamide antibody.
Five midguts of the stingless bee, Melipona quadrifasciata, were used as positive controls as the occurrence of these cells in this organ was described earlier [11].
2.5 Transmission electron microscopy
Five worker bees were dissected in the presence of a sodium cacodylate buffer 0.1 M at pH 7.2 and their midguts were transferred into 2.5% glutaraldehyde in the sodium cacodylate buffer, and kept for 4 h at room temperature. Then, the pieces of the anterior and posterior midgut regions were washed in the same buffer and post fixed in 1% osmium tetroxide for 2 h in the dark. The samples were then washed in sodium cacodylate buffer, dehydrated in 70% ethanol and embedded in LR White resin. After sectioning, the ultrathin slices were stained with aqueous uranyl acetate and lead citrate [28] and analyzed using a transmission electron microscope, Zeiss EM 109, at the Nucleus of Microscopy and Microanalysis, Federal University of Viçosa, MG, Brazil.
2.6 Morphometry
Six fields of each one of the midgut regions (anterior and posterior) were randomly photographed using a fluorescence microscope with a 40× objective lens (total area = 0.414 mm2) to analyze the digestive and regenerative cells; a 10× objective lens (total area = 0.6 mm2) was utilized for the endocrine cells. From the images of the entire midgut, the number and nuclear areas of the digestive, regenerative and endocrine cells were recorded. Twenty nuclei from the digestive cells and 10 from the regenerative cells per field were used to measure the nuclear areas. All data were obtained using the software Image-Pro Plus 4.5 (Media Cybernetics) to study the midguts of five bees.
2.7 Statistics
The morphometric data and the number of cells were tested for normality with the Kolmogorov–Smirnov test and subjected to analysis of variance (ANOVA test) for normally distributed data (number of regenerative and endocrine cells, area of regenerative cell nuclei, length of the epithelium and striated border). The nuclear areas of the digestive and endocrine cells and the number of digestive cells were subjected to Kruskal–Wallis test. Both tests were performed at a significance level of 5%.
3 Results
In B. morio, the midgut was lined with a single-layered epithelium composed of columnar digestive cells with a well-developed apical striated border. The midgut lumen revealed a peritrophic membrane enclosing the food. Externally, two muscle layers were present (Figs. 1 and 3c).
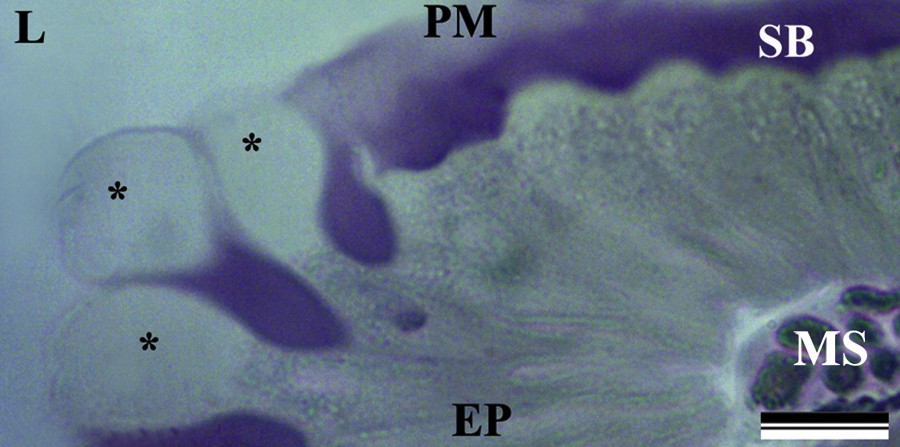
(Color online.) Light micrograph of the midgut epithelium of Bombus morio. The epithelium (EP) showed “bubbles” (asterisk) in the apical region, peritrophic membrane (PM), striated border (SB) and muscle fibers (MS). Note the lumen (L) of the midgut. Scale bars: 20 μm.
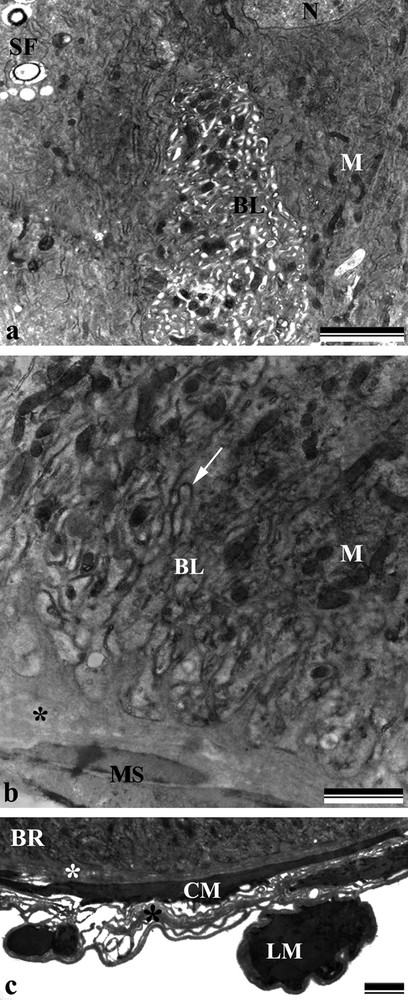
Electron micrographs of the anterior and posterior region of the midgut of Bombus morio. a: anterior portion of the midgut with the basal region of the digestive cell showing dilated and sinuous basolateral labyrinth (BL), nucleus (N), spherocrystals (SF), and mitochondria (M); b: posterior portion of the midgut with the basal region of the digestive cell showing mitochondria (M) and without dilatation of the basolateral labyrinth (BL) and several openings into the hemocoel organized by the folds of the basal plasma membrane (arrow). Note the basal lamina (asterisk) and the muscle lining (MS); c: outer covering along the basal region (BR) of the midgut showing a thin basal lamina (asterisk) and circular (CM) and longitudinal (LM) muscular fibers. Scale bars: (a) 2 μm, (b) 1 μm and (c) 2 μm. Masquer
Electron micrographs of the anterior and posterior region of the midgut of Bombus morio. a: anterior portion of the midgut with the basal region of the digestive cell showing dilated and sinuous basolateral labyrinth (BL), nucleus (N), spherocrystals (SF), ... Lire la suite
Both the anterior and posterior midgut regions showed the presence of some fragments of digestive cells that had been cast into the midgut lumen as “bubbles” in the cell apex (Fig. 1).
Long microvilli and mitochondria-rich apical cytoplasm were observed in the digestive cells (Fig. 2a). The spherical nucleus revealed decondensed chromatin and a well-developed nucleolus, as well as a perinuclear cytoplasm with an abundance of rough endoplasmic reticulum (Fig. 2b). The basal plasma membrane was highly invaginated, forming a labyrinth associated with the mitochondria (Fig. 3a and b) and vesicles containing spherocrystals and electron-lucent vesicles (Figs. 2a,b and 3a). The cell itself was based on a thin basal lamina anchored by circular (internal) and longitudinal muscle fibers (Fig. 3c).
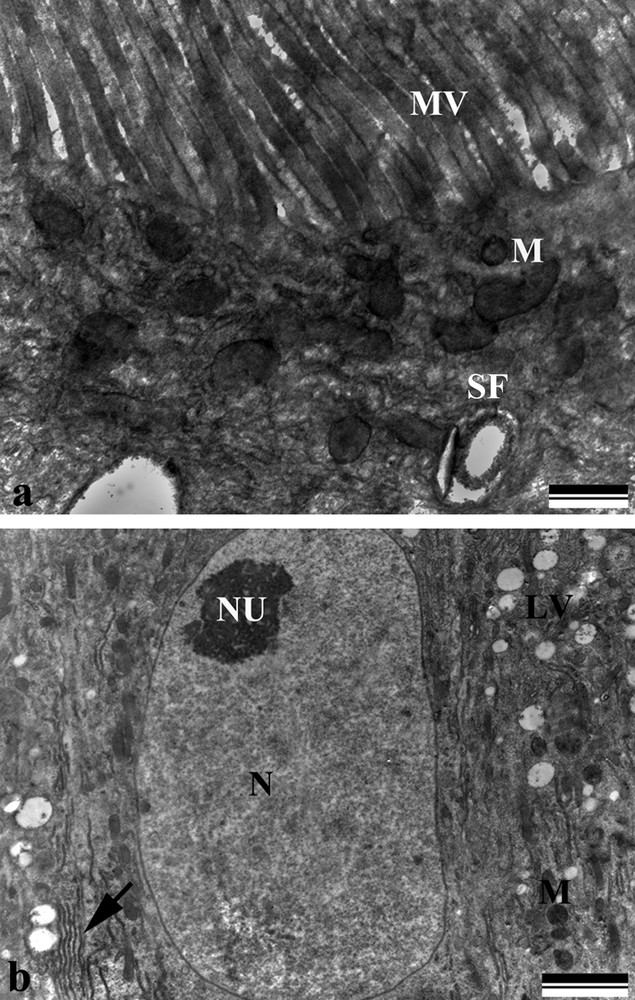
Electron micrographs of the midgut digestive cells of Bombus morio. a: apical region showing microvilli (MV), mitochondria (M), and spherocrystals (SF); b: middle region showing nuclei with decondensed chromatin (N), nucleolus (NU), rough endoplasmic reticulum (arrow), mitochondria (M), and electron-lucent vesicles (LV). Scale bars: (a) 500 nm and (b) 2 μm.
The anterior midgut region showed digestive cells with a sinuous and dilated basolateral labyrinth (Fig. 3a) having a few openings into the hemocoel, whereas in the posterior midgut region, the plasma membrane invaginations showed several openings into the hemocoel (Figs. 3b and c). The apical surface showed electron-dense vesicles scattered among the microvilli (Fig. 4a).
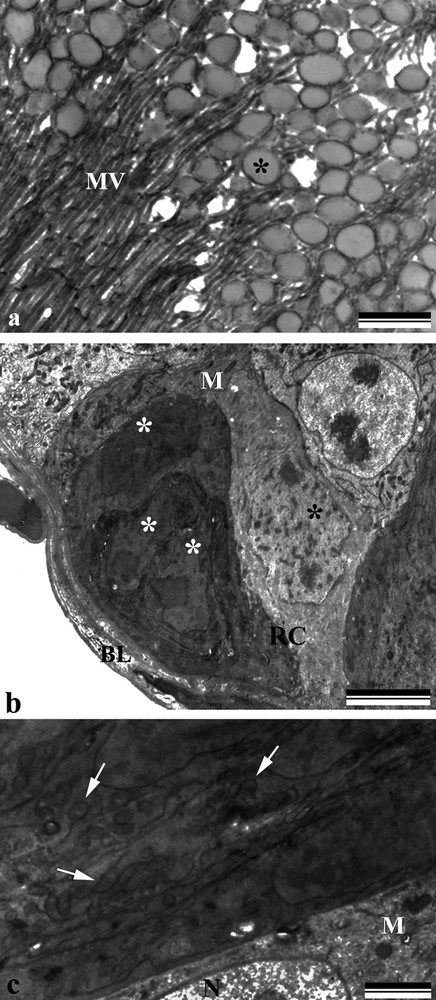
Electron micrographs of the midgut Bombus morio. a: digestive cell of the posterior region of the midgut showing electron-dense vesicles (asterisk) scattered among the microvilli (MV); b: nests of regenerative cells (RC) showing nuclei with decondensed chromatin (asterisk) and mitochondria (M). Note the basal lamina (BL); c: regenerative cells showing mitochondria (M), nucleus (N) and cisternae of the endoplasmic reticulum (arrows). Scale bars: (a) 1 μm and (b) 5 μm (c) 1 μm.
Regenerative cells were found clustered in nests, dispersed throughout the midgut. Their cytoplasm showed regions of different electron densities, with the more electron-dense cells found closer to the centre of the nests having mitochondria, nuclei with decondensed chromatin and cisternae of the endoplasmic reticulum (Fig. 4b, c).
The nuclei of the digestive and regenerative cells showed different shapes from those of the regenerative cells; the nuclei of the regenerative cells were 4 to 20 in number per nest and were smaller than those of the digestive cells (Figs. 5a,b and 6a).
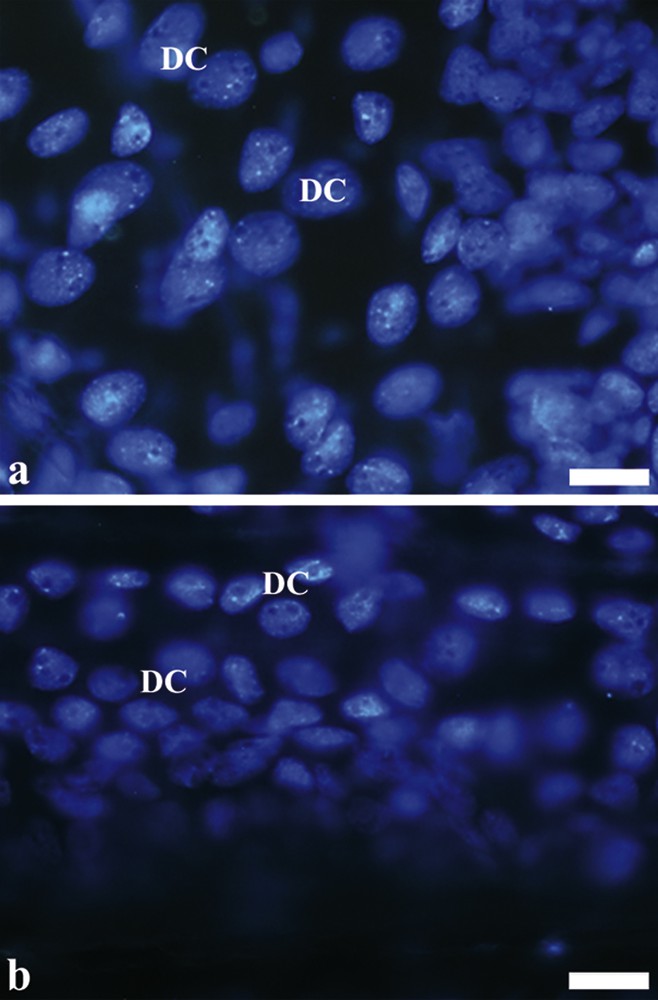
(Color online.) Total assembly from nuclei of digestive cells of the midgut of Bombus morio. a: nuclei of digestive cells (DC) of the anterior region of the midgut; b: nuclei of digestive cells (DC) of the posterior region of the midgut. Scale bars: (a) 10 μm and (b) 10 μm.
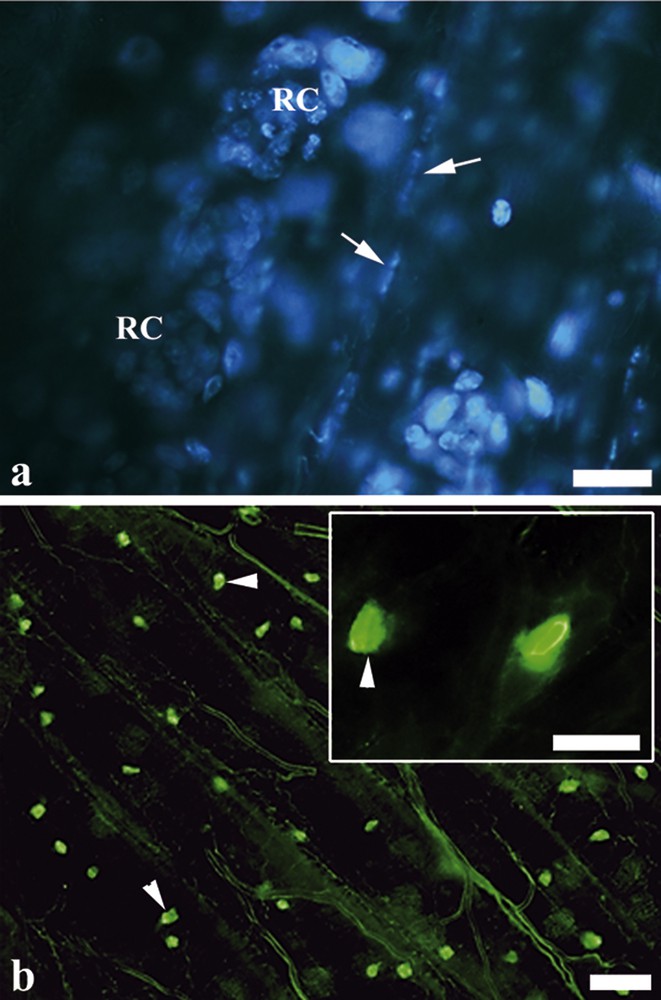
(Color online.) Marked nuclei of regenerative cells and endocrine cells. a: nests of regenerative cells (RC) evidenced by an agglomeration of small nuclei. Note the flattened nuclei of muscle fibers (arrow); b: FMRFamide-positive endocrine cells (arrowheads) found scattered only in the posterior midgut region. Insert: FMRFamide-positive endocrine cells (arrowheads). Scale bars: (a) 10 μm, (b) 30 μm and insert 10 μm.
The FMRFamide-positive endocrine cells were found scattered only in the posterior midgut region (Fig. 6b).
The morphometric data from B. morio midgut revealed significant differences between the regions. The posterior midgut region had a longer striated border (8 ± 2.1 μm) (P < 0.05, ANOVA test), although the epithelial height (38 ± 1.1 μm) was similar to those of the anterior region (P = 0.951, ANOVA test) (Fig. 7a). On analyzing the entire midgut, the nuclei of the digestive cells of the anterior region were found to be located higher (238 ± 7.1 μm2) than nuclei of the posterior midgut region (157 ± 9.3 μm2) (P < 0.05, Kruskal–Wallis test) (Fig. 7b). However, the number of digestive cells was higher in the posterior midgut, with 35.5 ± 1.2 cells/area, than in the anterior region with 25.6 ± 2.4 cells/area (P < 0.05, Kruskal–Wallis test) (Fig. 7c).
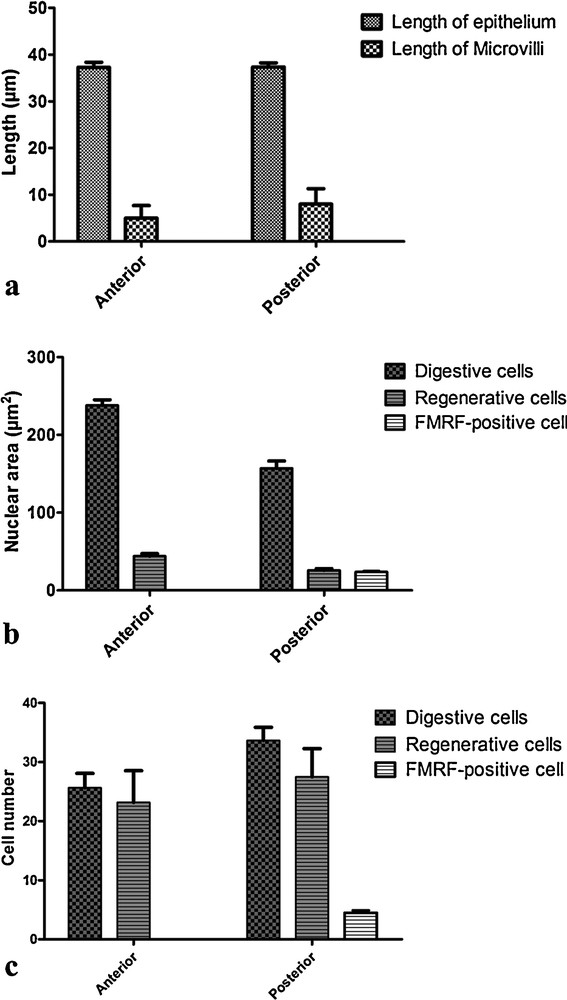
Morphometric analysis of midgut regions Bombus morio. a: epithelial height and length of the striated border in histological section; b: area of nuclei of digestive cells, regenerative cells and FMRFamide-positive endocrine cells; c: number of digestive cells, regenerative cells and FMRFamide-positive endocrine cells.
The regenerative cell nuclei, however, were larger in the anterior midgut region (44 ± 3.1 μm2) than in the posterior midgut region (26 μm2 ± 0.7) (P < 0.05, ANOVA test) (Fig. 7b). However, the number of regenerative cells was similar in both the midgut regions (P = 0.175, ANOVA test) (Fig. 7c).
The digestive and regenerative cells were similarly distributed between the anterior and posterior midgut regions (P = 0.288, ANOVA test) with 27 ± 2.9 and 33 ± 4.7 cells/area, respectively (Fig. 7c).
In B. morio, the FMRFamide-positive endocrine cells were found exclusively in the posterior midgut region, with 4.3 ± 0.9 cells/area (Fig. 7c). Their nuclei were similar in size (24 ± 1.1 μm2) with those of the regenerative cells and were smaller than those of the digestive cells (P < 0.05, Kruskal–Wallis test) (Fig. 7b).
Although 25 bumblebees were studied in total, proliferating cells were completely absent in the midgut region.
4 Discussion
The midgut of B. morio was lined by simple columnar epithelium with a well-developed striated border, having scattered nests of regenerative cells and two layers of muscle, very similar in structure to the midgut of other hymenopterans [14,15,29–33].
The “bubbles” released into the digestive cell apex could be regarded as an apocrine secretion, involving the loss of apical cytoplasm and usually containing digestive enzymes and other cell compounds [10,34–37]. Although regional differences are often encountered in the insect midguts [10,26,27,38], the B. morio midgut shows a longer striated border in the posterior than in the anterior midgut region, whereas in the stingless bees, Trigona hypogea and Trigona spinipes, the microvilli length is similar in both the midgut regions [39]. The parasitoid wasp, Campoletis flavicincta, has a striated border, which is longer in the anterior midgut region [15]. The length of the striated border appears to imply a higher degree of absorption in the posterior midgut region in B. morio, similar to that reported for the hemipteran Brontocoris tabidus [36], the mosquito, Aedes aegypti [40] and bees A. mellifera and M. quadrifasciata [13]. Besides, in B. morio, the apical cytoplasm of the digestive cells is rich in mitochondria, which coupled with the microvilli features suggests some active transport mechanism [29,36,41].
The presence of mitochondria associated with basal plasma membrane infoldings observed in the digestive cells of B. morio suggests an active movement of compounds into this cell region, as reported for other insects [42,43]. However, in B. morio, the anterior midgut region shows sinuous and dilated basolateral labyrinths, similar to that reported in the solitary orchid bee, Euglossa townsendi, where these cells appear to play a vital role in water and ion transport [44]. Forager bees feeding on a liquid diet (nectar) [29], and the anterior midgut region of B. morio could be responsible for the rapid uptake of the water content of the nectar to prevent dilution of the digestive enzymes along the midgut. Rapid water absorption in the anterior midgut has also been reported in the midguts of insects fed on diluted fluids [36]. In B. morio, the posterior midgut region contains electron-dense vesicles that are released into the lumen. This may indicate the release of some digestive enzymes, as reported in this midgut region for A. mellifera [45,46].
The regenerative cells of B. morio are packaged in nests, similar to the pattern observed in the bees M. quadrifasciata [18] and A. mellifera [47]. However, the regenerative cells of B. morio possess different electron densities in the regenerative cell nest, which may be due to the difference in the differentiation stage that each regenerative cell is at, as reported for other insects [10,48–51].
In the whole mounted midgut of B. morio, the nuclei of the digestive cells are equidistant and greater than those of the regenerative cells, which are found at the base of the epithelium, forming nests in sets of 4 to 20 cells, as in the stingless bee, M. quadrifasciata anthidioides [14]. However, they are different from those of the parasitoid wasp, C. flavicincta, which has isolated regenerative cells surrounding the digestive cells [15].
Both the digestive as well as the regenerative cells vary in the size of their nuclei along the midgut of B. morio. This can be related to endopolyploidy, commonly observed in the digestive cells of insects associated with their intense protein synthesis [52–54], where the digestive cells synthesize digestive enzymes and regenerative cell proteins for differentiation into digestive or endocrine cells, as the case may be.
Although there are a greater number of digestive cells found in the posterior midgut region of B. morio, the nuclear size should be considered, as the digestive cells in the anterior region have larger nuclei, which might result in fewer cells per observation field. However, in M. quadrifasciata anthidioides, differences between the midgut regions are evident, although they have similar sized nuclei among them [14]. However, the physiological significance of larger nuclei occurring in the midgut region continues to remains unknown.
The FMRF-positive endocrine cells found only in the posterior midgut region of B. morio are similar to those described for other insects [11,13,22–25]. Despite the rather insufficient understanding of the functions of the midgut endocrine cells, it is thought that they may be related to gut peristalsis [23,55,56] and/or to the control of enzyme synthesis by the digestive cells [11,57,58]. Further, these endocrine cells are derived from the regenerative cells [20], which explains the similarity in the size of their nuclei as reported in this study.
In the three cell types present in the B. morio midgut, no evidence of mitosis is found, supporting the hypothesis that in adult Hymenoptera proliferation of cells is absent [10,11,14]. Further, the number of regenerative cells is similar along the entire midgut of B. morio, suggesting that the sheer number of these cells is enough to renew the digestive epithelium during the entire adult lifespan of the insect, without requiring cell proliferation at all. In bees with a long life cycle, such as the queen of the stingless bee, M. quadrifasciata, which lives for almost two years, mitosis was reportedly observed in the midgut cells [59]; however, mitosis did not occur in bumblebees with seasonal nests [60].
In bees, the occurrence of midgut endo-ectoperitrophic circulation has been suggested [7] for digestion and absorption, with enzyme release in the posterior region and absorption in the anterior midgut region [12,16,45,46]. Although more detailed physiological experiments are warranted, the morphological data from this study support the occurrence of an endo-ectoperitrophic circulation in the B. morio midgut. This is because the cells of the anterior midgut region show infoldings of the basal plasma membrane forming wide canals with openings for the hemocoel with the cells in this region concentrating the solutes, resulting in an osmotic gradient between the midgut lumen and the hemocoel, enhancing water absorption. A similar scenario is observed in the rectum of insects [61,62]. On the other hand, the digestive cells of the posterior midgut region of B. morio reveal invaginations of the basal plasma membrane with several openings for the hemocoel, resulting in water flow into the gut lumen, as observed in the malpighian tubules [37,61]. As the B. morio individuals studied were forager bees, which feed mainly on diluted food, nectar and honey [29], rapid water absorption from the lumen in the anterior midgut region might prevent dilution of the digestive enzymes along the entire length of the organ.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
Authors are grateful to Nucleus of Microscopy and Microanalysis of Federal University of Viçosa for technical support. This research was supported by Brazilian research agencies CAPES, CNPq, FAPEMIG and FINEP.


