1 Introduction
Army ants are characterized by large colonies, nomadism and obligate group predation. The colony cycle of Eciton burchellii and E. hamatum, both epigaeic foraging Neotropical Ecitoninae, is characterized by two alternating phases. During the “statary” phase, the colonies reside in a fixed bivouac from which successive raids radiate away; the queen lays numerous eggs that hatch into larvae at the end of this phase. To feed these larvae, the colonies enter their “nomadic” phase, emigrating and forming new raids daily. As the larvae pupate, a new statary phase begins. Swarm-raiding E. burchellii workers form a main column whose front widens, creating a “carpet” of workers that fans out to a width of up to 20 m; the swarm front proceeds at a speed of ca. 15 m/h. The raids last more than 10 hours each day with workers continuously leaving the bivouac to join the front and then returning, sometimes carrying a reward, so that the flow of workers along the trails is bi-directional. The same is true for E. hamatum, but it is a column raider whose workers separate into small foraging groups and produce trails that branch out into a tree-like pattern [1–3].
Both species prey particularly on wasps and/or ants whose brood represents more than 50% of the E. burchellii prey and most of the E. hamatum diet. When they successfully raid an ant colony, they generally collect only the brood and callow workers, so that only a relatively minor proportion of older workers are injured or killed; surviving workers and queens, which are not generally injured, later re-establish the colony [1,2]. Workers from species with small or vulnerable colonies panic and carry a part of their brood away from the nest; the workers of some other species are aggressive toward army ants [4]. Among the latter, the African ant, Pheidole megacephala, is an invasive species that, after being introduced into the Neotropics, forms huge colonies depleting the prey available for colonies of native ant species and even raiding them [5–9]. Also, P. megacephala counterattacks raiding Eciton workers; when returning to their bivouac, these Eciton workers are spread-eagled and killed by outgoing colony mates [4].
In ants, nestmate recognition is based on chemical cues that are mainly a mixture of low-volatile cuticular hydrocarbons of genetic origin, but other compounds can be acquired from the environment [10]. During self- and allogrooming, trophallaxis and inter-individual contact, the workers continually gather their own chemicals and those of their colony mates in the postpharyngeal gland where they homogenize them into a mixture that is then redistributed. The resulting “colony odour”, learned by colony members, represents a neural template, which is compared to the cuticular chemicals of encountered individuals, a mismatch generally resulting in aggressiveness [10].
In this study, we hypothesized that some compounds from P. megacephala are passed onto the cuticle of the raiding Eciton workers during the combats, so that they are not recognized by their outgoing colony mates. In keeping with the idea that simple, practical research approaches are needed to study the basic biology of social insects [11], we used a water-based technique permitting different compounds, including cuticular hydrocarbons, to be extracted from and transferred onto live ants (Fig. 1). The success of this process was previously demonstrated using gas chromatography-mass spectrometry analysis and bioassays [12].
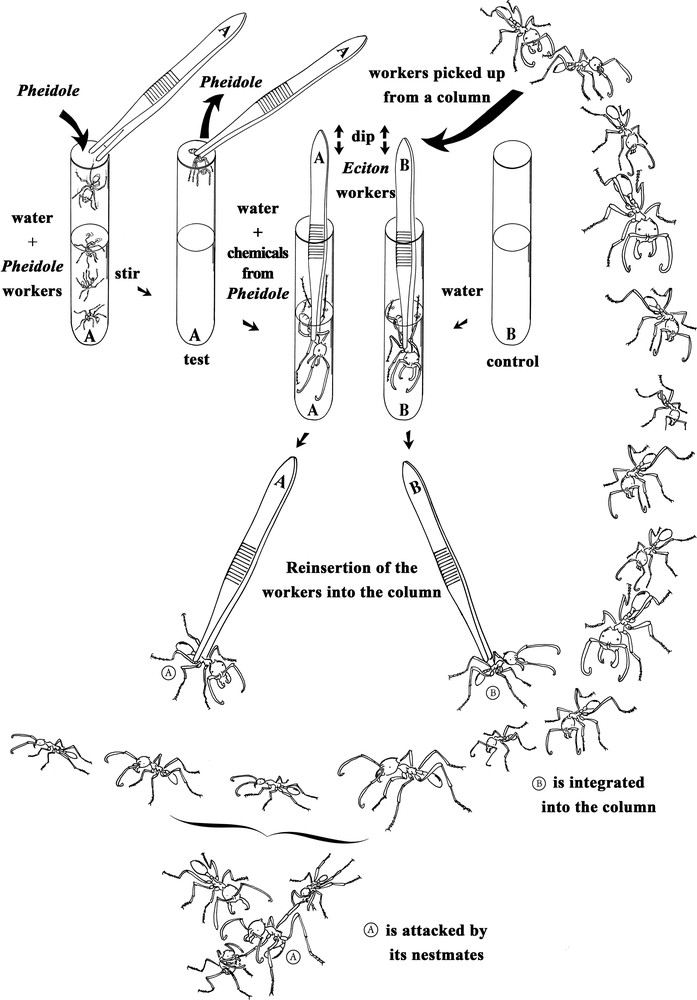
Representation of the experimental set-up. At top left, the preparation of experimental Eciton burchellii workers, which are soaked in a water-based emulsion of the cuticular compounds of Pheidole megacephala workers. At top right, the preparation of E. burchellii from the control lot which were soaked in ultrapure water. At the bottom of the figure, representation of the release of the manipulated E. burchellii workers into their own column; those from the experimental lot were attacked, spread-eagled and killed by their colony mates, while those from the control lot entered the column without being attacked.
2 Materials and methods
This study was conducted along the Caribbean coast of Quintana Roo, Mexico, around Puerto Morelos (20° 50′ 52.2″ N, 86° 52′ 41.3″ W) where the E. burchellii and E. hamatum density (i.e., 0.71 colony per hectare) is relatively high [13].
For those cases where we were able to observe Eciton workers leaving the P. megacephala galleries being attacked by their outgoing colony mates, we noted the details of the attacks. Each time we arrived early enough to witness the beginning of the Eciton raids on the P. megacephala colony, we noted the duration of time separating this moment from the first attacks on returning Eciton workers by their colony mates, and then between those first attacks and the end of this intracolonial slaughter. In six cases for both E. burchellii and E. hamatum, we noted the number of returning individuals killed during 10 two-minute-long periods of observation of the zones where the ants encountered one another. We then estimated the total number of individuals killed by their colony mates by extrapolating these results to the duration of the raids on the P. megacephala colonies.
We also conducted bioassays where we confronted each time an E. burchellii or an E. hamatum worker with colony mates from a column after it was soaked in an emulsion made using 30–50 P. megacephala workers. These workers were placed in a test tube containing 20 mL of ultrapure water (at ambient temperature or 30–35 °C) and vigorously shaken by hand for 5 min with the aim of emulsifying the cuticular compounds (other chemicals can also be added; see details of this water-based method in [12]). These ants were removed and the Eciton workers from the experimental lots were placed directly into the emulsion, shaken for 10 s, and left for 5 min (see A in Fig. 1). The Eciton workers from the control lot were placed into test tubes containing 20 mL of ultrapure water, shaken for 10 s, and then left in the water for 5 min (see B in Fig. 1). In both cases, the Eciton were then removed and put into a glass container where they dried for 20 min. The behavioural confrontations that followed consisted in placing the ‘experimental’ Eciton workers by the side of one of their colony's trails and noting their reactions, those of their colony mates in the column, and if they were killed or not (see bottom of Fig. 1). We used E. burchellii minor, media and major workers (30 cases each; the worker caste is highly polymorphic). The experiments were conducted using workers from six different raids for E. burchellii and two raids for E. hamatum. Note that this water-based method, previously used for ants and termites [12,14,15], is possible because many tropical ground-nesting ant species are adapted to flooding and do not drown when they are immersed [16].
All statistics were conducted using Fisher's exact-tests (Past 2.14 software).
3 Results
3.1 Details of the attacks on the Pheidole megacephala colony and the consequences
Both E. burchellii and E. hamatum raided P. megacephala nests (24 and 11 cases observed, respectively) and, in all cases, they began by successfully plundering a part of the brood. Yet, both around their nest entrances and inside the nests (which we observed by lifting rocks and concrete slabs), the P. megacephala workers reacted by counterattacking and spread-eagling numerous Eciton raiders.
When we arrived before the Eciton began the raids, we noted that the first returning Eciton workers leaving the P. megacephala nests were attacked by outgoing colony mates that spread-eagled them, killing most of them (see Table 1). Attacked returning individuals did not defend themselves but rather crouched, their antennae folded backward or adopted a posture where their thorax was vertical and gaster bent forward (bottom of Fig. 1).
Duration of Eciton burchellii and E. hamatum raids on Pheidole megacephala nests, and estimation of the number of returning Eciton individuals killed by their outgoing colony mates after they left the Pheidole nests.
| Eciton burchellii: 300,000 to 650,000 workers per colony [4] | ||
| Total number of cases observed | 24 | |
| No. of cases analyzed for evaluation | 17 | |
| Duration of the raidsc | ||
| First phasea | 34.1 ± 2.7 min | ca. 0 h 34′ |
| Second phaseb | 46.7 ± 4.5 min | ca. 0 h 46′ |
| Total | 80.8 ± 3.9 min | ca. 1 h 21′ |
| Mean no. of workers killed per series of observationd | 76.77 ± 2.33 | |
| Number of workers killed during the second phasee | From 1571 to 2025 | Mean: 1792 |
| % Workers killed based on 300,000 workers | From 0.52% to 0.67% | Mean: 0.60% |
| % Workers killed based on 650,000 workers | From 0.24% to 0.31% | Mean: 0.27% |
| Eciton hamatum: up to 250,000 workers per colony [4] | ||
| Total number cases observed | 11 | |
| Number of cases analyzed for evaluations | 6 | |
| Duration of the raidsc | ||
| First phasea | 36.5 ± 4.3 min | ca. 0 h 36′ |
| Second phaseb | 40.7 ± 7.1 min | ca. 0 h 41′ |
| Total | 77.2 ± 6.5 min | ca. 1 h 17′ |
| Mean number of workers killed per series of observationd | 64.7 ± 2.0 | |
| Number of workers killed during the second phasee | From 1052 to 1594 | Mean: 1315 |
| Percentage of workers killed based on 250,000 workers | From 0.42% to 0.64% | Mean: 0.52% |
a First phase: from entering the Pheidole megacephala nest to the first attack on returning colony mates.
b Second phase: from the first attack on returning colony mates to the end of the raid.
c Means ± SE calculated from 17 cases for Eciton burchellii raids and from 6 cases for 11 E. hamatum raids.
d Means ± SE calculated from six cases; 10 series of 2-min observations each time.
e Evaluation of the number of returning workers killed by their colony mates calculated from the duration of the second phase.
The counterattacks by the P. megacephala workers kept the Eciton raiders inside their nest during a relatively long time as more than 30 min passed before the first individuals left the Pheidole nests; then, other Eciton individuals continued to leave the Pheidole nests during 40–50 min (details in Table 1). Meanwhile, the line of encounter between outgoing and returning Eciton workers moved away little by little from the Pheidole nest entrances. When the flow of returning Eciton workers stopped, the line of encounter with outgoing individuals was more than 10 m further from the Pheidole nests, and the column had already begun to move in a new direction as other prey were discovered. The P. megacephala nests were finally abandoned because little by little the recruitment of new E. burchellii or E. hamatum workers ceased. So, the P. megacephala lost a part of their brood, but the core of the colony was spared.
During such raids, E. burchellii colonies lost ca. 1800 workers each time and E. hamatum ca. 1300 workers, representing between 0.24% and 0.67% of the worker force of the colonies (see details in Table 1).
3.2 Experiment using a water-based transfer of compounds
The E. burchellii workers from the control lot, previously soaked in water, easily returned to their columns when released (only one major was attacked but not killed), while all of those from the experimental lot, previously soaked in an emulsion made using P. megacephala workers, were spread-eagled. Only two of these workers survived whereas the corpses of the others were retrieved to the bivouac as were the prey; the differences between the control and experimental lots were significant for each sub-caste of workers (P < 0.0001; Fig. 2).
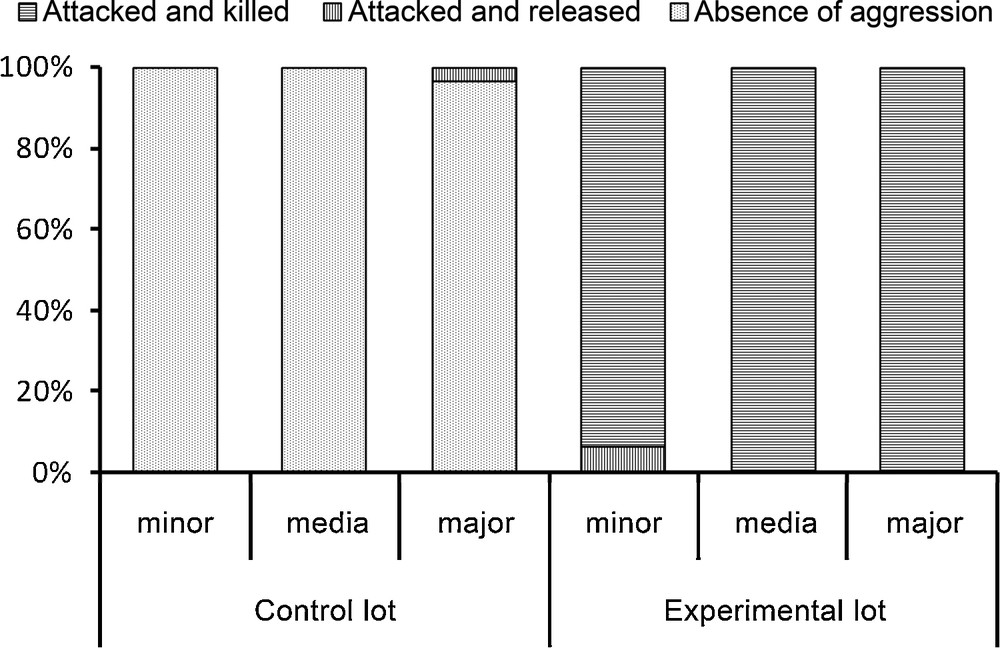
Results of the fate of Eciton burchellii workers from the control (soaked in ultrapure water) and the experimental (soaked in an emulsion of cuticular compounds from Pheidole megacephala workers) lots. Each time minor, media and major workers were tested (30 cases each). Statistical comparisons: Fisher's exact-test, P < 0.0001 for each sub-caste of workers.
When re-introduced into their column, workers from the experimental lot crouched, their antennae folded backward or adopted a posture where their thorax is vertical and gaster bent forward (see again bottom of Fig. 1). Minor workers always crouched, while media and major workers crouched in 63.3% and 40% of the cases, respectively, or adopted a vertical position in the other cases (n = 30 for each sub-caste of workers). Then, these workers let themselves be attacked to death by their colony mates.
Very similar results were obtained using E. hamatum workers subjected to the same treatment as all 30 workers from the control lot returned safely to their columns, while all 30 from the experiment lot were spread-eagled (Fisher's exact-test: P < 0.0001).
4 Discussion
Here, we show that the aggressive reactions of the workers indirectly protect the P. megacephala colonies from raids by E. burchellii and E. hamatum, two of the main ant predators in the Neotropics. This aptitude stems from two of the most important characteristics known for invasive ant species in their introduced range: the ability to form huge colonies and a high level of aggressiveness towards native ants that are displaced or eliminated [5]. Indeed, due to its ability to develop huge colonies in areas where it has been introduced and its high predatory aptitude, P. megacephala can even raid native ant species [6,8]. Subsequently, when raiding P. megacephala nests, Eciton workers were kept inside these nests during a long time due to the combativeness of the counterattacking P. megacephala workers. This likely permits compounds to be transferred onto the cuticle of the raiding Eciton workers. So, it is because returning Eciton workers were attacked and killed by outgoing colony mates that the P. megacephala colony was spared. This corresponds to a kind of by-product benefit for P. megacephala due to the different characteristics of the colonies in their introduced range as no coevolutive processes are involved in this case (see also [17]). Yet, one can note that, in their native African range, P. megacephala workers react very aggressively to Dorylus driver ant raids (the Old World equivalent of army ants), but, here, the reaction is related to predatory behaviour. Indeed, the Dorylus workers are raided, spread-eagled and retrieved to be eaten (A.D. pers. obs.; [8]), the same has been noted for other African ant species [18,19].
Our simple, water-based experiment permits us to argue that some P. megacephala chemicals are indeed transferred onto the Eciton workers’ cuticle during the counterattacks occurring during raids. These transferred compounds disturb the recognition system of the Eciton workers to the point that they mistakenly attack and kill colony mates having experimentally acquired P. megacephala compounds. This transfer of chemicals is reminiscent of the case of slave-making Polyergus breviceps queens, which kill the queens of their Formica host colony. Then, they are adopted by the host workers because the chemical signatures of the Formica host queens are transferred onto the parasite queens during their aggressive interaction [20]. Note that colony mate recognition is sensitive to only certain modifications of the cuticular signature. For instance, in the Argentine ant, adding one synthetic methylated alkane to an individual confronted with colony mates elicits their aggressiveness if this compound has a different branch position, but not if it has the same branch position, even if the chain length is different [21].
One can note that, in addition to cuticular compounds, defensive chemicals might be transferred onto the Eciton cuticle when raiding P. megacephala nests. For instance, traces of Solenopsis saevissima venom in Camponotus blandus cuticular extracts were noted during a water-based experiment [12]. Because they have atrophied stingers, it has been suggested that P. megacephala workers release offensive secretions other than venom [6–8]. The pygidial gland is a good candidate for producing such compounds because in P. biconstricta it produces repellent and irritant components [22,23].
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We are grateful to Andrea Yockey-Dejean for proofreading the manuscript, and the Laboratoire Environnement de Petit Saut for furnishing logistical assistance. Financial support for this study was partially provided by the Programme Convergence 2007–2013, Region Guyane from the European Community (project Bi-Appli).


