1 Introduction
In ants, colony-mate recognition is based on chemical cues, mainly a mixture of low-volatile cuticular hydrocarbons of genetic origin, but other compounds can be acquired from the environment. During self- and allo-grooming and trophallaxy, the workers continually gather their own compounds and those of their colony-mates in the postpharyngeal gland where they homogenize them into a mixture, or “colony odour”, which is then spread onto their cuticle. This colony odour is learned by the colony members, becoming a neural template that they compare to the cuticular compounds (CCs) of encountered individuals; a mismatch generally results in aggressiveness [1].
Eciton burchellii is an epigaeic-foraging, Neotropical army ant characterized by large colonies, nomadism and obligate group predation during raids consisting of a main column whose front widens to up to 20 m. Daily raids last more than 10 hours with workers continuously leaving the bivouac to join the front and then returning, some of them carrying prey, so that the flow of workers along the trails is bi-directional. This species preys mostly on wasp and ant brood, with ant brood representing more than 50% of its diet. When raiding a prey-ant colony, E. burchellii workers collect only the brood and callow workers, so that almost all older workers and queens are neither killed nor injured and later re-establish the colony [2,3]. Workers from many prey-ant species run away (likely due to the perception of a propaganda-like substance, as is known in slave-making ant raids; see [4]) and carry a part of their brood away from the nest, so that they are seldom in contact with the raiding Eciton. The workers of some other species are aggressive toward army ants; among them, Pheidole megacephala, an invasive species of African origin, counterattacks Eciton raids [3]. When returning to their bivouac, these Eciton workers are killed by outgoing colony-mates. Indeed, whereas the P. megacephala workers’ defence is primarily based on the fact that their huge colonies permit them to mount efficacious counterattacks, it has also been shown that their CCs are transferred from their cuticle onto that of the Eciton raiders through worker-to-worker contact, so that outgoing Eciton workers mistake their returning colony-mates for prey and kill them [3,5].
One may ask if this transfer of CCs and possibly other chemicals triggering intra-colonial aggressiveness in Eciton exists only for P. megacephala (which is an introduced, invasive species), or if such transfer also exists during raids on Neotropical ants. Indeed, in French Guiana, we noted that some E. burchellii workers were attacked by their colony-mates after raiding mounds of the native ant species, Solenopsis saevissima. So, we hypothesized that E. burchellii workers may be sensitive to alterations in the cuticular cues due to contact with workers from certain prey species. These alterations lead to mismatches in colony-mate recognition and may exert a selective pressure on army ants.
2 Materials and methods
This study was conducted at the field station at Petit Saut, Sinnamary, French Guiana (5°03′39″ N; 53°02′36″ W) in July 2013.
Prior to the experiments, we gathered parts or entire colonies of four ground-nesting ant species: Atta sexdens, Pheidole fallax, S. saevissima and Camponotus blandus. They were selected because they are commonly found along forest edges where E. burchellii raids are frequent and because the size of their workers permits easy manipulation if compared with the much smaller workers of Crematogaster tenuicula and Wasmannia auropuncta, two other species frequent in this habitat [6,7]. With colonies of 5–8 million workers, A. sexdens is a leaf-cutting, fungus-growing ant, while the three other species are generalist foragers [6–8]. S. saevissima, whose workers forage mostly nocturnally, forms supercolonies extending over up to 54 km in its native range in French Guiana [6–9]. In C. blandus colonies, consisting of ca. 6000 individuals, the workers forage during the hottest hours of the day, whereas P. fallax colonies, containing fewer than 1000 workers, are active all around the clock [6–8].
We placed these ants in large plastic boxes with earth from their nests and transported everything to the laboratory. There, we opened the boxes (whose sides were covered with Fluon® to prevent the workers from escaping), furnished these ants with pieces of cotton imbibed with water and with diluted honey and allow them to calm down during one night (they rearranged the earth in the boxes, grouped the brood and proceeded with numerous acts of self- and allo-grooming). The next day, we delicately placed the boxes in a freezer where the workers progressively became numb. We took these precautions to limit the possibility that defensive compounds remained on the workers’ cuticle (some may have been released while we were gathering them). We then looked for an E. burchellii bivouac from which we gathered several hundred workers plus leaf-litter. We placed these ants and the leaf-litter in a large, smooth-walled plastic box, transported the box to the laboratory, and waited until the workers had calmed down.
In keeping with the idea that simple, practical research approaches are needed to study the basic biology of social insects [10], we used the simplest possible technique to transfer cuticular compounds onto live ants, which consisted of rubbing a given Eciton individual with the thorax from a different worker ant [11,12]. After trying this method during a preliminary study conducted in the field on three E. burchellii raids, we adapted it for use in laboratory conditions. To do so, we picked up an E. burchellii worker using a pair of supple forceps without perturbing its colony-mates. Then, using a second pair of forceps, we picked up a heterospecific worker (which we had allowed to thaw) and rubbed its thorax against the E. burchellii worker during 2 min (for small Pheidole and Solenopsis workers, two successive individuals were used). The E. burchellii workers were then released among their colony-mates and we noted if they were admitted, antennated (i.e., successive touching with antennae as a sensory probe [8]), pursued or bitten. The same process was undertaken for each sympatric ant species tested and for a control lot, for which we used an E. burchellii colony-mate (n = 30 in all cases).
Statistics were conducted using Fisher's exact-tests (GraphPad Prism software) and the ‘false discovery rate’ adjustment (FDR) (BY correction; [13]) for simultaneous comparisons.
3 Results and discussion
When we reintroduced ‘manipulated’ workers among their colony-mates, those from the control lot were never attacked. The difference with the control lot was not significant for E. burchellii workers rubbed with A. sexdens or P. fallax individuals, while we noted a significantly greater level of aggressiveness toward those rubbed with S. saevissima and C. blandus individuals (Fig. 1).
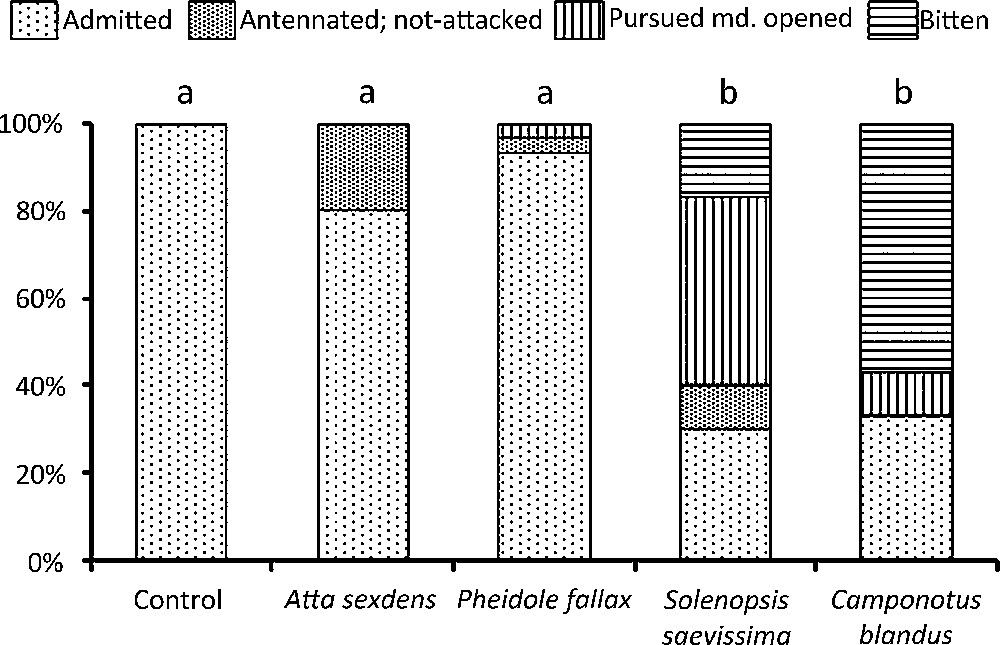
The fate of Eciton burchellii workers from the control (rubbed with the thoraxes of colony-mates for ∼ 2 min) and the experimental lots (rubbed with the thoraxes of heterospecific workers during ∼ 2 min) when introduced into the bivouac of colony-mates (n = 30 in all cases). Statistical comparison: Fisher's exact-tests and false discovery rate’ adjustment (BY correction); different letters indicate significant differences at P < 0.001. Pursued md. opened: the experimented E. burchellii worker, which had received cuticular compounds from alien ant species, is itself perceived as an alien by its colony-mates which pursued it their mandibles wide open, ready to bite (this is a level of aggressiveness lower than biting [6,8]).
The clear differences in the reactions toward experimented colony-mates show that our very simple technique (having rubbed the ants with the thoraxes of colony-mates or of workers from different species) is sufficient for such studies (on the condition that basic precautions are taken). This indeed suggests the transfer of some chemicals (likely, mostly CCs) from potential prey-ants to E. burchellii individuals. The effects of such a transfer have been discussed in the literature. For instance, adding a dimethylated alkane to the Camponotus herculeanus cuticle elicits aggressiveness from nestmates, but not a mono-methylated nor a linear alkane [14]; in the Argentine ant, Linepithema humile, adding a methylated alkane to an individual's cuticle elicits its nestmates’ aggressiveness if this compound has a different branch position, but not if it has the same branch position, even if the chain length is different [15].
When E. burchellii raid ant species with small colonies (the case for P. fallax and C. blandus [6–8]), the only effective response for the prey-ant is to escape: they lose most of their brood and callow workers, but the society is safe and able to recover. A counterattack at the colony level would be “suicidal” and therefore such a strategy could not have exerted selective pressure. Therefore, even when transferred CCs trigger intra-colonial aggressiveness in E. burchellii (as experimentally shown in the case of C. blandus), such an event is rare in nature (observed in Mexico during raids on Camponotus spp. colonies; AD, pers. obs.). Indeed, in natural conditions, the raided workers evacuate their nests and E. burchellii workers gather only the brood and callow workers that have not yet acquired the CCs responsible for the colony odour [16]; contact with “old” raided workers is extremely rare. Moreover, for species with small colonies, the number of raiding army ant workers is so great that the CC transfer is “diluted” and thus cannot become a defence mechanism.
When army ants raid ant species with large colonies, the two species being evenly matched, a counterattack by the raided ants may be an effective strategy as observed for the invasive ant, P. megacephala, whose workers react aggressively to the presence of E. burchellii. This promotes physical contact between workers that facilitates the transfer of CCs from the raided workers to E. burchellii raiders and consequently triggers intra-colonial aggressiveness among the raiding army ants. Those previously in contact with P. megacephala workers are mistakenly attacked by their outgoing colony-mates when they return to their bivouac; they are then spread-eagled and killed [5]. This corresponds to a kind of by-product benefit for P. megacephala because this is not the result of a coevolutive process. Among Neotropical ants with large colonies, some instances of intra-colonial aggressiveness among E. burchellii colony-mates have been observed after contact with S. saevissima individuals, but the core of the individuals from the attacked mound move (polydomous colonies; AD, pers. obs.; this study). This corroborates the fact that S. saevissima workers react aggressively to E. burchellii CCs [6]. Yet, because most individuals evacuate their nest during E. burchellii raids, the situation is relatively similar to that of most ant species having small colonies. Similarly, when raided by Neivamyrmex compressinodis, the huge W. auropunctata colonies evacuate their nests due to a propaganda effect [17].
When the transfer of CCs does not trigger intra-colonial aggressiveness among raiding E. burchellii, as for A. sexdens, there is no selection pressure to avoid contact with these prey-ants. Yet, these ants are not preyed upon by Eciton spp., all generalist predators of ant brood, due to an unknown reason (likely related to CCs). Nevertheless, the army ant, Nomamyrmex esenbeckii, is specialized in Atta spp. predation although the colonies of the latter contain several million workers, many of them counterattacking and so generating numerous physical contacts with the raiders. It is likely that the transfer of Atta chemicals does not trigger any intra-colonial aggressiveness between N. esenbeckii individuals (as in the present study) because this fact has never been reported by those having witnessed these raids [18–22].
In conclusion, we have shown differences between potential prey-ants of E. burchellii relative to the transfer of chemicals. Because both a transfer of chemicals from prey-ants and the aggressiveness between colony-mates may occur in nature during army ant raids, chemicals from prey-ants likely had a role in the evolution of army ant predatory behaviour. Further research is needed to confirm this hypothesis and to verify what compounds are transferred.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We are grateful to Andrea Yockey-Dejean for proofreading the manuscript, Sardadebie Loza and Lucie Toussaint for technical assistance, and the Laboratoire Environnement de Petit Saut for furnishing logistical assistance. Financial support for this study was provided by the Programme Convergence 2007–2013, Région Guyane from the European Community (project Bi-Appli, 115/SGAR-DE/2011/052274).


