1 Introduction
Agro-silvo-pastoral systems are recognized as crucial for maintaining the viability of rural areas in Europe and have particular significance for resource and nature conservation [1]. However, they are endangered by the dramatic changes towards intensification and abandonment that are occurring in the agricultural and agroforestry systems of many areas in the world, which in the long run can result in a general change of biodiversity and consequently of functionality [2–6]. In the last decades, the European agricultural and environmental policies have started to recognize productive, environmental and societal services of these systems, but the formulation of effective management proposals to balance human activities and biodiversity conservation would greatly benefit from more detailed information on the patterns of diversity [7,8]. The growing concern for the conservation of agro-silvo-pastoral systems makes it relevant to fill the gaps in knowledge, particularly in the Mediterranean region, where the effects of land use changes on the above- and below-ground biota have been traditionally investigated separately.
Mediterranean agro-silvo-pastoral systems are complex mosaics shaped over the long term by human activities based on multiple-use-oriented management [9] characterized by different levels of management practice intensity [10]. Each element of the mosaic is related to a cosmos of biota, potentially inter-linked, exhibiting different levels of diversity and providing different types and levels of ecosystem services [11–14].
The objective of this paper was to compare the diversity patterns of vascular plants with those of below-ground microorganisms under a gradient of land-use disturbances. We focused on five different land uses in the context of a long-term observatory representative of Mediterranean agro-silvo-pastoral systems under uniform environmental conditions [15,16]. In this long-term observatory, a number of multidisciplinary teams are analysing the relationships between land use, biodiversity and ecosystem services in the framework of different research projects (e.g. SOILSINK: http://soilsink.entecra.it/; ECOFINDERS: www.ecofinders.eu). Several pieces of information concerning the patterns of below-ground microorganisms of the long-term observatory have been already published by specialists of different taxonomic groups [17–21], but never compared with each other. Moreover, vascular plant patterns have not been assessed at all.
Starting from the general assumption that under the same ecological conditions, different land uses are relevant in shaping vascular plant biodiversity [22–25], we hypothesized that: (1) the land uses affected vascular plant diversity patterns, (2) the composition, richness and Shannon index patterns of vascular plants mirrored those of below-ground microorganisms, and (3) the relative importance of the components of γ-diversity, namely relativized species replacement, similarity and richness difference, presented similar patterns for plant and below-ground microorganisms under contrasting land uses.
To test the hypothesis, (1) we assessed and compared vascular plant composition, richness and Shannon index in the same sites that had been characterized for below-ground microorganisms; to test the hypothesis (2), we assessed the co-variation between plants and below-ground microorganism patterns; to test the hypothesis (3), we partitioned the γ-diversity of plants and below-ground microorganisms into the three additive components and compared their relative importance in shaping the biodiversity patterns of vascular plants and below-ground microorganisms.
2 Materials and methods
2.1 Study area and sampling sites
The field experiment was conducted at the Berchidda-Monti long term observatory located in Sardinia (Italy) (40°48’N; 9°17’E). The observatory includes private farm fields for which no specific permissions were required, as these fields are not located in protected areas and do not involve endangered or protected species. The observatory is representative of the Mediterranean agro-forestry systems of Gallura that are shaped by human activities practiced since centuries [26]. The area is included in the Meso-Mediterranean phytoclimatic belt (http://www.globalbioclimatics.org), characterized by an aridity index of 0.53, average annual precipitation of 632 mm with at least 70% of annual rain falling from October to May and average annual temperature of 14.2 °C. All the sampling sites were located on same soil type, Typic Dystroxerept [27], with the same morphology, on a granitic substratum [28]. The current Natural Potential Vegetation [29,30] of the area is represented by a Quercus suber forest, referable to the association Violo dehnhardtii–Quercetum suberis [31]. Nowadays, the forest is widely thinned and replaced by field crops or plant community successions [32].
The landscape is characterized by a variety of land uses related to different types of agro-silvo-pastoral activities, which are mainly represented by grape-growing (mostly the DOC vine cultivar “Vermentino di Gallura”), livestock farming, and cork extraction. Vineyards are conventionally managed applying annually one or rarely two inversion tillage treatments in spring to reduce the competition of the grass cover (tilled vineyards [TV]) or allowing the development of a permanent inter-row grass cover (grass covered vineyards [GV]).
Livestock farming is practiced in the area since centuries and hence it is frequent to observe fields that have been managed in the same way since over 50–60 years [15]. The main land uses related with livestock farming are secondary grasslands (GR) and hay crops (HC). GR in fact represent the grass covered fallow between two subsequent HC. In the study site, GR followed a less intensive rotation scheme than HC (e.g., five years of fallow grassland and one year of annual forage crop). HC has a complementary scheme of more intensive forage crop rotation (e.g., up to five years of cultivation and one year of fallow grassland). Cork extraction is operated in Q. suber forests (cork oak forests, CO) approximately every 10–11 years. Further details about the land use managements in the study area were reported by Bagella et al. [32].
2.2 Experimental design
The experimental sites were represented by fields of 1–4 ha, characterized by uniform management since at least 50 years, except for vineyards, which were planted 20 years before the survey was made. They were selected according to a gradient of increasing land-use intensity: CO → GR → HC → GV → TV [32]. A mono-factorial design considering land uses as fixed factors was applied. Four replicated sampling areas were randomly chosen within each field. Vascular plants and below-ground microorganisms were sampled in the same areas in May 2007.
2.3 Vascular plant sampling
Herbaceous communities (i.e. GR, HC, GV and TV) were sampled in spring 2007 using the linear transect methodology. Along each transect the presence/absence of each plant species over 100 vertical point quadrates at 1-m intervals was detected [33]. Woody plant communities (i.e. CO) were sampled inside a 100 × 100 m2 area by visual census [34]. The percent cover of each species in each land use was assessed by averaging the data from the four replicates. Plant species richness was calculated as the average of four replicates for each land use [35]. Diversity was assessed using the Shannon index [36].
2.4 Below-ground microorganism data sets
Below-ground microorganisms were not specifically sampled, but datasets available for the five land uses in the long-term observatory were used for the analysis. The data were obtained from soil core samples collected at a depth of 20 cm in the same plots and in the same year as for plant communities. The details concerning materials and methods and sampling season applied for each taxonomic group are reported in the papers utilized as sources (Table 1).
Selected below-ground microbial assemblage parameters.
| Biota | Land use | Source | ||||
| TV | GV | HC | GR | CO | ||
| Richness (no. taxa/OTUs) | ||||||
| Basidiomycota | 9 | 9 | 12 | 29 | 35 | Table 2 in [19] |
| Ascomycota | 58 | 36 | 55 | 52 | 42 | Table 2 in [19] |
| Glomerales | 41 | 32 | 21 | 52 | 22 | Derived from Table S1 in [18] |
| Diversisporales | 12 | 9 | 8 | 15 | 4 | Derived from Table S1 in [18] |
| Denitrifying bacteria nirK | 3 | 1 | 4 | 6 | 6 | Derived from Fig. 1 in [20] |
| Active eubacterial microflora | 26 | 28 | 26 | 28 | 25 | Derived from Fig. 3 in [21] |
| Shannon index | ||||||
| Microorganisms | 2.6 | 2.7 | 2.8 | 2.8 | 2.8 | Derived from Fig. 2 in [17] |
| Basidiomycota | 1.5 | 0.9 | 1.0 | 2.5 | 2.7 | Derived from Table S1 in [19] |
| Ascomycota | 2.6 | 2.7 | 2.4 | 1.8 | 2.7 | Derived from Table S1 in [19] |
| Glomerales | 2.4 | 2.8 | 1.5 | 2.5 | 1.3 | Derived from Table S1 in [18] |
| Diversisporales | 2.3 | 2.1 | 1.9 | 2.3 | 1.5 | Derived from Table S1 in [18] |
| Denitrifying bacteria nirK | 0.9 | 0.3 | 0.9 | 1.4 | 1.2 | Derived from Fig. 1 in [20] |
| Active eubacterial microflora | 3.1 | 3.2 | 3.0 | 3.1 | 3.0 | Derived from Fig. 3 in [21] |
| Biomass (μg C g−1) | ||||||
| Microorganisms | 136 | 138 | 242 | 487 | 694 | Table 1 in [17] |
As a synthetic proxy of all microorganism biota, Lagomarsino et al. [17] provided the microbial C biomass. The same authors reported the related Shannon index as an indicator of soil functional diversity.
Basidiomycota and Ascomycota Operational Taxonomical Units (OTUs) represented the dominant components of the soil fungal assemblages amplified by the ITS1F-ITS2 primer pair (ITS1 region) [19].
Similarly, Glomerales and Diversisporales were the two most represented taxa of arbuscular mycorrhizal fungi (Glomeromycota) amplified with primers AMV4.5NF/AMDGR, Archaesporales and Paraglomerales being retrieved exclusively from GR [18]. The number of reads included in a given OTU was taken as a proxy for the abundance of the associated molecular species.
As for the denitrifying bacterial assemblages, only the expression of Nitrite Reductase Genes (nirK) based on the amplification of cDNA (DGGE bands) was considered in the comparisons because it was the only one presenting differences among land uses in terms of richness (number of bands) and of Shannon index, as reported by Pastorelli et al. [20].
The active eubacterial microflora based on rRNA DGGE profiles, as assessed by Pastorelli et al. [21], was also considered for the comparisons.
2.5 Data analysis
In order to test hypothesis (1), plant species cover values were compared by permutational multivariate analysis of variance (PERMANOVA) [37]. Non-parametric multidimensional scaling (nMDS) ordination analysis was performed to visually show the results. Bray–Curtis measures of dissimilarities on square root-transformed data were used for calculating a matrix of distances between sample pairs. The taxa responsible for differences among assemblages, as indicated by an a posteriori test following PERMANOVA, were identified by SIMPER analysis [38]. The taxa contributing at least to 5% of dissimilarity for any comparison were considered to be important differential factors. Plant assemblage richness and Shannon index were compared by a one-way analysis of variance (ANOVA). Prior to the analysis, the homogeneity of variance was tested with the Cochran's test [39], and data were transformed whenever necessary. When ANOVA identified a significant difference, the post hoc Student–Newman–Keuls (SNK) test was applied to determine the specific differences between each pair of data [40].
In order to test hypothesis (2), the co-variation between vascular plant and below-ground microorganism composition was evaluated comparing the similarity matrices based on Sørenson's similarity indices using the Pearson correlation coefficient, while richness and Shannon index, whenever available, were compared using the Spearman correlation coefficient.
In order to test hypothesis (3), the SDR simplex approach [41] was used, limited to the biota for which presence/absence data for each species were available. This method is based on the evaluation of the relative importance of three complementary indices of γ-diversity: similarity (i.e. the number of shared species between two sites), relative species replacement (i.e. the maximum fraction of species turnover which is equally shared by the two sites), and relative richness differences (i.e. the component of species turnover that is attributable only to richness changes, and is given by the absolute difference between the species numbers of two sites).
The PRIMER (v6.0) statistical package [42] with the PERMANOVA add-on [43] was used to conduct PERMANOVA, nMDS and SIMPER analysis.
ANOVA was performed using the GMAV 5 software (University of Sydney, Australia).
The γ-diversity partitioning was defined with the SDR Simplex application (http://ramet.elte.hu/∼podani).
3 Results
3.1 Vascular plant composition, richness and Shannon index patterns
Overall, 177 vascular plant taxa were detected. Fifty percent of them were unique to one land use, whereas just one, Trifolium subterraneaum, was shared by all of them.
The PERMANOVA analysis indicated a significant effect of land use on vascular plant assemblage composition. In particular, all the a posteriori pairwise comparisons were significant (Pseudo-F4,15 = 27.9; P < 0.001). This result was also visually confirmed by the nMDS plot (Fig. 1).
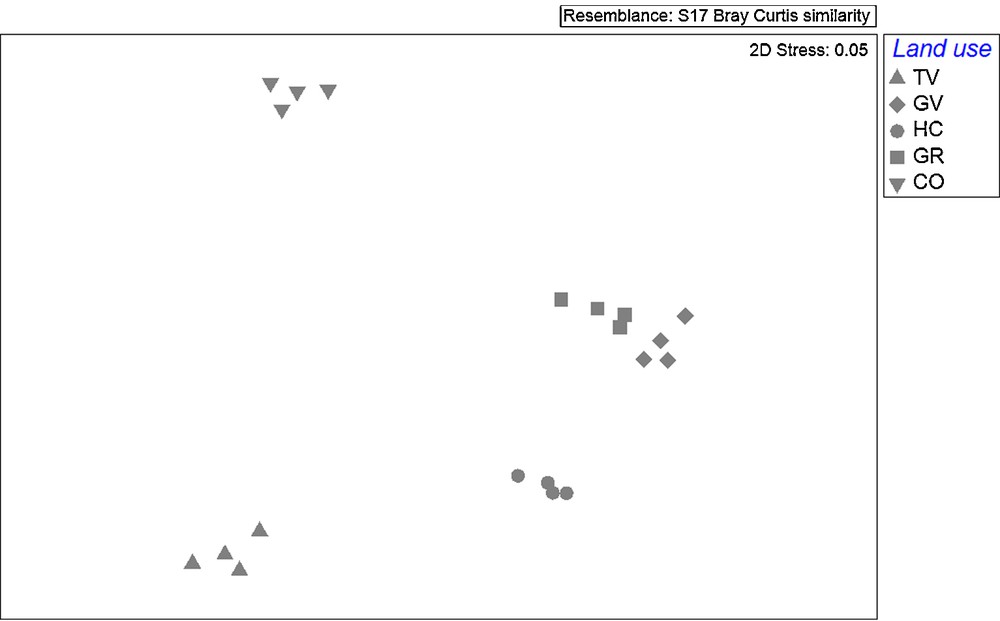
nMDS plot relative to vascular plant assemblage composition pattern under the five land uses. TV: tilled vineyards; GV: grass-covered vineyards; HC: hay crops; GR: secondary grasslands; CO: cork-oak forests.
The results of the SIMPER analysis showed that overall 59 species contributed to differences among vascular plant assemblages under the five land uses, which accounted for more than 90% of the total dissimilarity. Twelve top species contributed more than 5% each to the dissimilarity (Table 2). Among the latter, only four species (Cytisus villosus, Geranium molle, Quercus suber and Spergula arvensis) presented a percentage cover higher than 20% at least under one land use (Table 2).
Results of the SIMPER pairwise comparisons for vascular plants.
| Twelve top vascular plant species | Percentage contribution to the dissimilarity | Percentage cover | |||||||||||||
| TV vs GV | TV vs HC | TV vs GR | TV vs CO | GV vs HC | GV vs GR | GV vs CO | HC vs GR | HC vs CO | CO vs GR | TV | GV | HC | GR | CO | |
| Anthemis arvensis L. | 0.0 | 8.4 | 0.0 | 0.0 | 8.8 | 0.0 | 0.0 | 7.5 | 8.3 | 0.0 | 0.0 | 0.0 | 16.4 | 4.2 | 0.0 |
| Cytisus villosus Pourret | 0.0 | 0.0 | 0.0 | 15.8 | 0.0 | 0.0 | 15.8 | 0.0 | 15.8 | 16.0 | 0.0 | 0.0 | 0.0 | 0.0 | 31.4 |
| Geranium molle L. | 14.5 | 0.0 | 0.0 | 0.0 | 15.4 | 20.8 | 14.5 | 0.0 | 0.0 | 0.0 | 0.0 | 28.9 | 0.0 | 0.4 | 0.1 |
| Hordeum leporinum Link | 8.2 | 0.0 | 7.9 | 0.0 | 8.7 | 5.1 | 8.2 | 9.4 | 0.0 | 7.9 | 0.0 | 16.4 | 0.0 | 15.6 | 0.0 |
| Lolium multiflorum Lam. | 0.0 | 7.0 | 0.0 | 0.0 | 7.2 | 0.0 | 0.0 | 8.0 | 6.8 | 0.0 | 0.0 | 0.0 | 13.6 | 0.3 | 0.0 |
| Medicago polymorpha L. | 4.6 | 0.0 | 0.0 | 0.0 | 4.6 | 0.0 | 4.6 | 0.0 | 0.0 | 0.0 | 0.0 | 9.1 | 0.4 | 3.3 | 0.0 |
| Poa annua L. | 5.1 | 5.2 | 5.1 | 5.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 10.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| Quercus suber L. | 0.0 | 0.0 | 0.0 | 18.8 | 0.0 | 0.0 | 18.9 | 0.0 | 18.9 | 19.1 | 0.0 | 0.0 | 0.0 | 0.0 | 37.4 |
| Sisymbrium officinale (L.) Scop. | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 4.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.2 | 6.6 | 0.0 |
| Spergula arvensis L. | 35.8 | 36.4 | 35.9 | 35.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 71.1 | 0.0 | 0.2 | 0.0 | 0.0 |
| Trifolium subterraneum L. | 0.0 | 0.0 | 4.6 | 0.0 | 5.1 | 6.0 | 0.0 | 4.9 | 0.0 | 4.6 | 0.1 | 1.0 | 1.2 | 9.2 | 0.2 |
| Vulpia bromoides (L.) S. F. Gray | 0.0 | 5.5 | 0.0 | 0.0 | 5.7 | 0.0 | 0.0 | 6.5 | 5.4 | 0.0 | 0.0 | 0.0 | 10.8 | 0.1 | 0.0 |
The ANOVA showed that land use significantly influenced vascular plant richness and Shannon index (Fig. 2). Plant richness increased along the gradient TV, GV, HC, GR, CO, while the Shannon index showed the highest values in the intermediate situations (GR and HC).

Patterns of vascular plant richness and Shannon index along the land-use gradient. Vertical bars indicate standard errors. Bars bearing the same letter indicate that means of each variable are not significantly different (P < 0.05 SNK test). TV: tilled vineyards; GV: grass-covered vineyards; HC: hay crops; GR: secondary grasslands; CO: cork-oak forests.
3.2 Co-variation between vascular plants and below-ground microorganism patterns
A significant correlation was observed between Sørenson's similarity indices of vascular plants and Basidiomycota or Ascomycota (Table 3). The richness in vascular plant was also significantly correlated with microbial biomass C, and the richness of Basidiomycota and denitryfing bacteria. No significant relationship was observed between the Shannon index of vascular plant and that of below-ground biota.
Pearson correlation coefficients (rp) and significance level (P) between Sørenson's similarity indices of vascular plants and microorganisms assemblage composition and Spearman correlation coefficients (rs) and significance level (P) between richness and Shannon index of vascular plants and microorganism assemblages.
| Vascular plants vs | Sørenson indices | Richness | Shannon index | P | ||
| r p | P | r s | P | r s | ||
| Microbial biomass (μg C g−1) | n.a. | – | 1.00 | 0.000 | 0.73 | n.s. |
| Basidiomycota | 0.73 | 0.017 | 0.97 | 0.004 | 0.36 | n.s. |
| Ascomycota | 0.74 | 0.014 | –0.40 | n.s. | –0.61 | n.s. |
| Glomerales | 0.19 | n.s. | 0.20 | n.s. | 0.24 | n.s. |
| Diversisporales | 0.50 | n.s. | 0.40 | n.s. | –0.03 | n.s. |
| Denitrifying bacteria nirK | n.a. | – | 0.90 | 0.043 | n.a. | – |
| Active eubacterial microflora | n.a. | – | –0.38 | n.s. | –0.05 | n.s. |
3.3 γ-Diversity partitioning
The relativized species replacement (R) of vascular plants was by far the most important component of γ-diversity along the land-use gradient, followed by richness differences (D) and similarity (S). R was the main component of γ-diversity also for Ascomycota, while D and S exhibited an opposite trend when compared to those of vascular plants. A clear trend of γ-diversity along the land use gradient was also observed for Basidiomycota, D being the main component, followed by R and S. By contrast, Glomerales and Diversisporales γ-diversity component partitioning was nearly random, with a substantial equilibrium among S, D and R (Table 4).
Components of vascular plant and below-ground microorganism γ-diversity.
| Relativized species replacement 100 Rrel (%) | Relativized richness difference 100 Drel (%) | Similarity 100 SJac (%) | |
| Vascular plants | 52.1 | 30.1 | 17.7 |
| Ascomycota | 62.7 | 13.8 | 23.5 |
| Basidiomycota | 36.9 | 46.6 | 16.4 |
| Glomerales | 38.7 | 30.6 | 30.7 |
| Diversisporales | 34.6 | 33.9 | 33.9 |
4 Discussion
The analysis of the patterns of vascular plants and below-ground microorganisms confirmed hypothesis (1), as vascular plant assemblage composition, richness and Shannon index were substantially different along the land use intensity gradient; partially confirmed hypotheses (2) and (3), as (i) vascular plant assemblage composition and richness patterns partially co-varied with the composition and richness of below-ground microorganism patterns, and (ii) the pattern of γ-diversity components of vascular plants weakly mirrored that of below-ground microorganisms.
4.1 Vascular plant patterns
The differences in plant assemblage composition along the land use gradient were mainly associated with the different abundance of plant species that were peculiar to each land use.
According to previous observations documented in the Iberian Peninsula [44], tillage was determinant in separating TV from GV plant assemblages. Spergula arvensis, i.e. the main differential species for TV, is typical of the segetal communities under tillage in early spring before the vineyard sprouting [45] and is very common as weed in vineyards [46]. The high contribution of Geranium molle in GV compared with HC and GR was attributed to the shading effect of the vine plants over the grass cover, as this species is typical of shaded areas [47]. Trifolium subterraneum was the only species detected under all land uses, although it was the main differential species between GR and all the other land uses. This result confirms the role of grazing in facilitating the reproduction of this species [48], which is one of the most relevant forage species in Mediterranean agro-silvo-pastoral systems [28].
Vascular plant richness and Shannon index presented a different trend along the land-use gradient. The highest richness was found in CO, although it was expected in GR, since grassland plant assemblages are generally richer than those of wooded habitats [49]. This finding was interpreted as a consequence of the positive effect of the anthropic disturbance on richness in the CO, as found in other Mediterranean woody vegetation [50]. Indeed CO woodlands are periodically disturbed by the shrub cutting that occurs to allow cork collection and by the light shrub grazing at the end of the summer, when grasslands are dry. On the contrary, the strong dominance of Quercus suber and Cytisus villosus in the tree and shrub layers, respectively [28], was the main reason for the lower Shannon index compared to GR. The lower Shannon index of the herbaceous cover detected for the vineyards reflected the strong dominance of Spergula arvensis in TV and Geranium molle in GR.
4.2 Co-variation between vascular plant and below-ground microorganism patterns
Vascular plant assemblages effectively mirrored specific below-ground microorganism patterns along the five contrasting land uses under comparison. Basidiomycota diversity patterns were those better mirrored by vascular plant patterns. The significant correlation between vascular plant and Basidiomycota richness indicates that along a gradient of land-use intensity, the richness trend of these two biotas is very similar, showing a decline with increasing the level of disturbance. The co-variation between vascular plants and Basidiomycota was also confirmed in terms of assemblage composition. Since most of the recovered Basidiomycota OTUs were ectomycorrhizal, the high proportion of Basidiomycota in CO fits well with the Q. suber cover, because of the symbiotic relationships between them [19,51,52].
The absence of significant co-variation between vascular plants and Ascomycota patterns reflects the variety of lifestyles of the obtained OTUs, which mostly encompassed saprotrophic taxa [18,19].
The significant co-variations found between vascular plant richness vs. microbial biomass C and denitrifying bacteria richness were possibly related to the increase of substrate availability on top-soil with decreasing land-use intensity [17]. On the contrary, the richness of arbuscular mycorrhizal fungi (Glomeromycota and Diversisporales) was not the highest under CO because of the reduced number of their host plants as compared to the other land uses [18].
The active bacterial microflora as well as vascular plants exhibited the lowest Shannon index in CO [21]. This pattern was interpreted as the consequence of competitive exclusion, as it often occurs for some bacterial taxa in natural soils [53].
4.3 γ-diversity partitioning
In spite of the congruence of co-variation in terms of richness and composition between vascular plants and Basidiomycota, the two biotas presented a different model of γ-diversity partitioning. The species replacement component was prevalent for vascular plants, while the relativized richness difference component was prevalent in the case of Basidiomycota. This indicated that the two biotas achieved the same pattern of richness and composition through different pathways. Indeed the variation in richness of Basidiomycota was not associated with the appearance/disappearance of species at the same rate observed for vascular plants and the core of the shared species among the contrasting land uses was greater for Basidiomycota than for the plants. This trend indicates that in these agro-silvo-pastoral systems, the plant species susceptible to changes in land uses are more numerous than Basidiomycota species.
The species replacement was also the main component of γ-diversity for Ascomycota. Actually, each land use encompassed unique Ascomycota OTUs, mostly represented by saprotrophic and ectomycorrhizal species, plus a few coprophilous species [19]. The change in terms of presence/absence of species of plants and Ascomycota along the land use gradient was indeed very similar, even if no significant co-variation between the two biotas was found in terms of richness, Shannon index, or composition.
The γ-diversity partitioning of Glomerales and Diversisporales confirmed what had already emerged from the comparative analysis of these two biota with vascular plants in terms of richness, Shannon index or composition co-variation. In fact, vascular plant diversity patterns showed a poor predictability for these two microbial communities.
5 Conclusions
The contrasting land uses associated with the Mediterranean agro-silvo-pastoral systems generated patchy diversity patterns of diversity of vascular plant and below-ground microorganism communities. The ability of vascular plant diversity patterns to mirror those of below-ground organisms cannot be generalized, as it was related to the microbial biota and diversity indicators used.
As already observed in other contexts [54–56], vascular plants are not always effective surrogates of biodiversity. The response of vascular plant richness to contrasting land uses mirrored that of Basidiomycota and of the total microbial biomass, but not the richness of the other below-ground microbial communities. The vascular plant Shannon index was independent of those of all below-ground microbial communities. The prevalence of relativized species replacement in the γ-diversity partitioning of vascular plants and Ascomycota suggests that, for these two biotas, the coexistence of the different land uses can be relevant to ensure the highest γ-diversity levels.
Our findings on diversity patterns of vascular plants and below-ground microorganisms are the first available for Mediterranean agro-silvo-pastoral systems and may represent a basis for careful management decisions aiming at biodiversity conservation.
The integrated assessment revealed that the impact of the most intensive land uses on vascular plant diversity was counterbalanced by the contribution of specific below-ground microorganisms to biodiversity. The patchy diversity patterns suggest that the maintenance of contrasting land uses associated with different productions typical of agro-silvo-pastoral-systems can guarantee the conservation of high levels of biodiversity for vascular plants and below-ground microorganisms.
Acknowledgements
This research was supported by the Italian National Project SOILSINK (Climatic Changes And Agricultural And Forestry Systems: impact on C Reservoirs and on Soil Microbial Diversity) and is ongoing in the context of the FP7 Ecofinders (www.ecofinders.eu) and Pascuum (Sardinia Region Grant) projects.


