1 Introduction
The position of subfamilies within the family Buthidae has always been a controversial matter. Kraepelin [1] recognized three subfamilies in the family Androctonidae (Androctonini, Isometrini and Centrurini). Later, using the family name Buthidae, he listed only the subfamilies Buthinae and Centrurinae, distinguishing them by the presence or absence of tibial spurs on the legs [2].
The subfamily Ananterinae was proposed by Pocock [3] to accommodate the genus Ananteris Thorell. Pocock wrote as follows: “I propose to eliminate from this subfamily (Buthinae) the isolated Neotropical genus Ananteris, which differs strikingly from the rest of the family in the structure of the pectines. The subfamily Ananterinae may be created for its reception.”
Subsequently to his previous publications [1,2], Kraepelin [4] added Ananterinae, previously described by Pocock [3] and the Tityinae, within the Buthidae. The Ananterinae was diagnosed by the absence of fulcra on the pectines, but the other subfamilies were diagnosed by differences in the dentition of the pedipalp chela fingers. Birula [5] distinguished three subfamilies (Buthinae, Isometrinae and Orthochirinae) using a completely different set of diagnostic characters. However, the majority of subsequent authors working with the Old World Buthidae [6–8] accepted Kraepelin's [4] scheme. Mello-Leitão [9], the last author discussing this issue in detail, rejected the scheme of Kraepelin [4], and explicitly accepted Birula's [5], listing all Neotropical buthids under the Isometrinae. The characters defining the Ananterinae (or the ‘Ananteris group’—as preferred by some authors), was already discussed by Lourenço [10].
In this paper, I provide new comments on the subfamily Ananterinae (sensu Pocock). One remarkable new species is described in the genus Ananteris Thorell, representing the first record of this genus for Peru.
2 The composition of the Ananterinae and their geographical distribution
A number of genera may be accommodated in the subfamily Ananterinae, namely: Ananteris Thorell, 1891, Tityobuthus Pocock, 1893, Ananteroides Borelli, 1911, Lychasiodes Vachon, 1974, Himalayotityobuthus Lourenço, 1997, †Palaeoananteris Lourenço and Weitschat, 2001, and Microananteris Lourenço, 2003.
Another Caenozoic fossil scorpion, Palaeotityobuthus longiaculeus Lourenço and Weitschat, 2000 was associated with the extant genus Tityobuthus. This fossil, however, is extremely incomplete [11]. Consequently, this genus was not included in the previous list.
Lourenço [12] proposed the synonymy of the monotypic genus Ananteroides with Ananteris. More recently, the study and redescription of another African genus, Lychasioides Vachon [13] suggested that the latter was closer to Ananteris than to Ananteroides. Consequently, the genus Ananteroides was revalidated.
In recent publications [14,15], the Malagasy genus Tityobuthus Pocock was exhaustively studied. These suggest clear affinities with the genus Ananteris. Other new genera were also thoroughly described, such as Himalayotityobuthus Lourenço from the Himalayas, which was clearly associated with the genus Tityobuthus from Madagascar, and Microananteris Lourenço, from French Guiana [16,17]. Clear affinities were also suggested among Lychasiodes, Ananteris, and Tityobuthus [13].
The association of all these different genera within the subfamily Ananterinae clearly indicates a Gondwanian pattern of distribution for this undoubtedly ancient lineage of buthid scorpions. The recent discovery of fossil forms in Baltic amber, namely Palaeoananteris Lourenço and Weitschat, which appears to be closely related to Ananteris, added further confirmation of both the Gondwanian pattern of distribution and antiquity of the Ananterinae lineage [11,18].
3 Methods
Illustrations and measurements were produced using a Wild M5 stereomicroscope with a drawing tube and an ocular micrometer. Measurements follow Stahnke [19] and are given in millimetres. The trichobothrial notations follow Vachon [20] and the morphological terminology mostly follows Vachon [7] and Hjelle [21].
Taxonomic treatment
Family BUTHIDAE C.L. Koch, 1837
Subfamily ANANTERINAE Pocock, 1900
Genus Ananteris Thorell, 1891
Ananteris cisandinus sp. n. (Figs. 1–3)
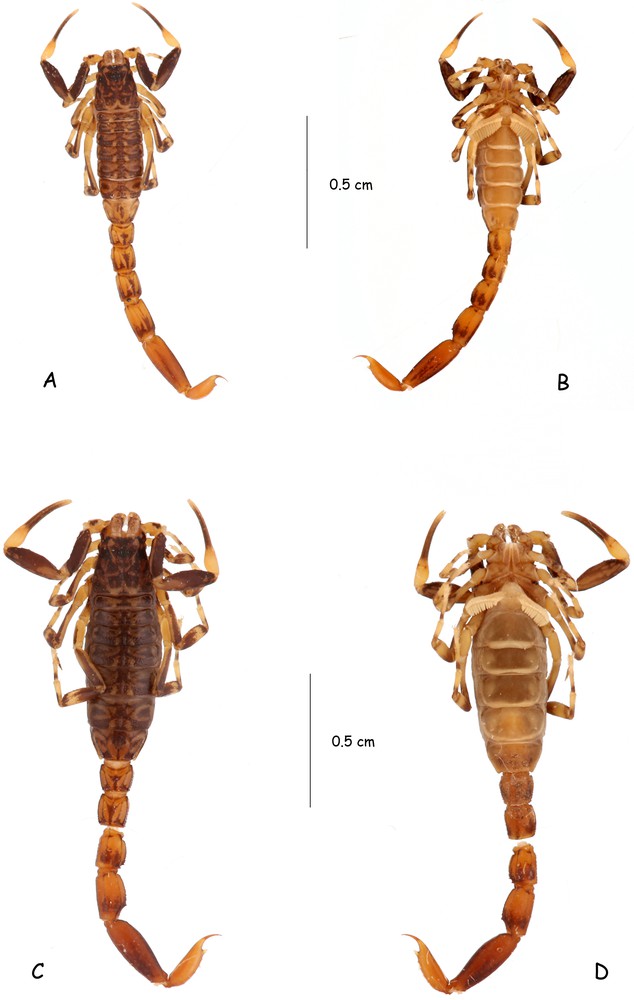
(Colour online.) Ananteris cisandinus sp. n. Habitus. A–B. Male holotype. C–D. Female paratype, dorsal and ventral aspects.
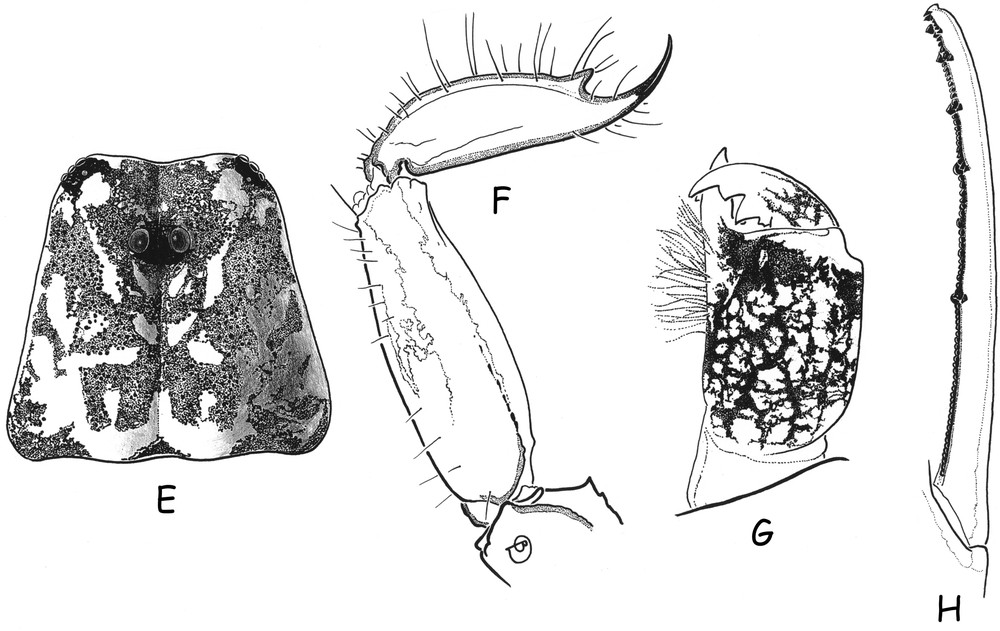
Ananteris cisandinus sp. n. E, G–H (male holotype). F (female paratype). E. Carapace. F. Metasomal segment V and telson, lateral aspect. G. Chelicera, dorsal aspect. H. Cutting edge of a movable finger, showing rows of granulations.
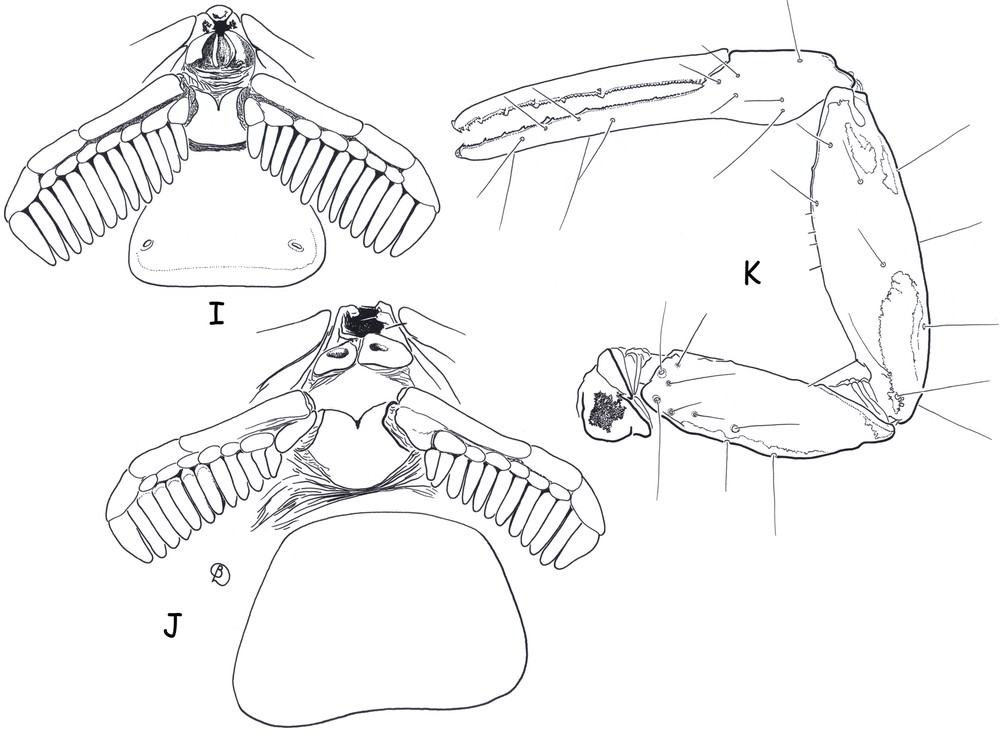
Ananteris cisandinus sp. n. I–J (male holotype and female paratype). Ventral aspect showing sternum, genital operculum, pectines and sternite III. K. (female paratype). Pedipalp, dorsal aspect, showing trichobothrial pattern.
Type material. Peru, Depto. Loreto, Cerros de Campanquiz, N of Borja/Maranon, X/1994 (collected by local Indians for Missionaries); under bark, deep buried in the soil. One male holotype (RS-8969) and one female paratype (RS-8970), deposited in the Muséum national d’histoire naturelle, Paris.
Etymology. The specific name refers to the geography of the region where the new species was found, between the Amazon River and the Oriental Andes in Peru.
Diagnosis. Species of very small size compared to the average size of the other species within the genus (14.9 mm in total length for the male and 19.3 for the female; see morphometric values after the description). General coloration yellow to reddish–yellow, intensely marked with brownish variegated spots. Pedipalps rather short; fingers with six rows of granules; male and female pectines small with 13–13 and 12–12 teeth respectively. Carinae and granulation moderately to strongly marked. Trichobothriotaxy, type A-β-beta [20,22]; trichobothria db and esb of chelal fixed finger situated almost at the same level.
Relationships. Mainly by its small size, aspects of the pigmentation pattern, and other morphological characters, the new species shows affinities with Ananteris festae Borelli, 1899. These two species are respectively known from the northwestern region of Ecuador and the Amazonian/cis-Andean region of Peru. However, the new species shows a combination of distinct characters:
- • overall darker coloration with chelicerae totally covered by an intense variegated dark pigmentation;
- • better marked granulations on carapace, tergites, and metasomal segments;
- • trichobothria db and esb of chelal fixed finger situated almost at the same level.
Moreover, both species are possible micro-endemic elements distributed respectively in the occidental and oriental Andean regions (Fig. 4).
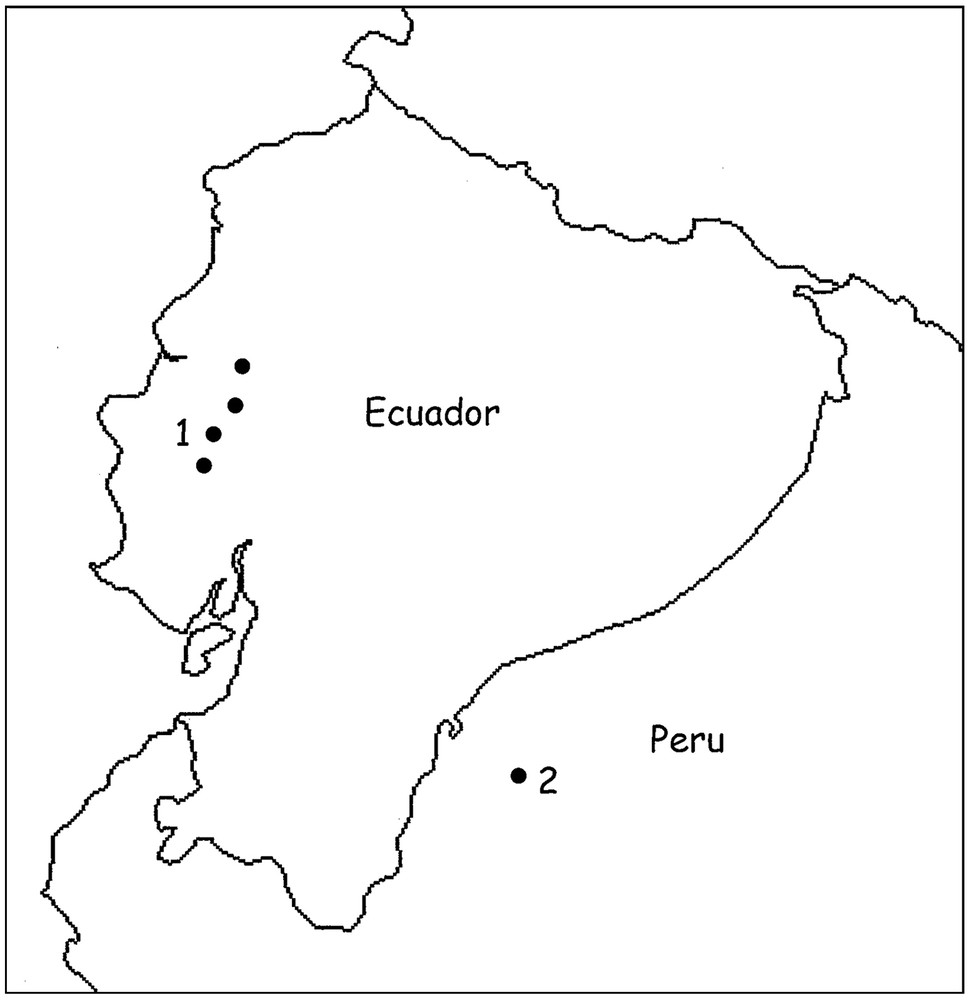
Map showing Ecuador and Northwestern Peru with the zones of distribution of Ananteris festae (1) and Ananteris cisandinus sp. n. (2).
Description. The description is based on male holotype and female paratype. (Morphometric measurements after the description).
Coloration. Generally yellow to reddish–yellow with brown to dark brown variegated pigmented zones on the body and its appendages. Prosoma: carapace yellow with dark brown spots on anterior, lateral, and posterior edges; eyes surrounded by black pigment. Mesosoma: yellow with confluent brownish zones on posterior and lateral edges of tergites. Metasoma: segments I to IV reddish–yellow; V reddish; all segments marked with dark brown spots. Vesicle yellow without spots; base of aculeus yellow, tip reddish. Venter yellow to pale yellow; coxapophysis and sternite VII infuscate. Chelicerae yellow with variegated blackish spots over the entire surface; fingers with blackish spots; teeth yellow. Pedipalps: yellow; femur and patella strongly marked with dark brown spots; chela hand yellow; fingers brown. Legs yellow, with several dark brown spots.
Morphology. Carapace with moderately to strongly marked granulation; anterior margin slightly emarginate. Anterior median superciliary and posterior median carinae weak or absent. All furrows moderate to weak. Median ocular tubercle distinctly anterior to the centre of the carapace; median eyes separated by a little more than one ocular diameter. Three pairs of lateral eyes. Sternum subpentagonal. Mesosoma: tergites with moderately marked granulation, less intense than those of carapace. Median carina moderately to weakly marked on all tergites. Tergite VII pentacarinate. Venter: genital operculum divided longitudinally, each plate more or less suboval in shape. Pectines small: pectinal tooth count 13–13 in male holotype (12–12 in female paratype); basal middle lamellae of pectines not dilated; fulcra absent. Sternites smooth; only VII slightly granular; spiracles rather short; setation moderate to weak; sternite VII with very weakly marked carinae and granulation. Metasomal segment I with 10 carinae, crenulate; segments II to IV with 8 carinae, crenulate; segment V slightly rounded and smooth, with vestigial carinae; intercarinal spaces strongly granular on segments I to III; weak on IV. Telson elongate and smooth; aculeus short and weakly curved; subaculear tooth moderately to strongly marked and spinoid. Cheliceral dentition characteristic of family Buthidae [23]; fixed finger with two strong basal teeth; movable finger with two vestigial basal teeth; ventral surface of both finger and manus with long, dense setae. Pedipalps: femur pentacarinate; patella and chela with weak to vestigial carinae; internal face of patella with only vestigial spinoid granules; all faces weakly granular, almost smooth. Fixed and movable fingers with six, almost linear, rows of granules; two small external and one internal accessory granule present at base of each row; three granules at the extremity of the fingers. Trichobothriotaxy; orthobothriotaxy A-β-beta [20,22]; trichobothria db and esb of fixed finger situated almost at same level. Legs: Tarsus with very numerous, fine, median setae ventrally. Tibial spurs strongly developed on leg IV, moderate on leg III.
Morphometric values (in mm) of male holotype/female paratype. Total length (including telson) 14.9/19.3. Carapace: length 1.7/2.2; anterior width 1.1/1.6; posterior width 1.6/2.3. Mesosoma length 4.1/5.8. Metasomal segment I: length 0.8/1.1, width 1.0/1.3; II: length 0.9/1.2, width 0.9/1.2; III: length 1.2/1.4, width 0.8/1.1; IV: length 1.4/1.8, width 0.8/1.2; V: length 2.4/3.0, width 0.7/1.1, depth 0.8/1.0. Telson length 2.4/2.8. Vesicle: width 0.6/0.8, depth 0.5/0.8. Pedipalp: femur length 1.6/2.0, width 0.4/0.5; patella length 2.0/2.7, width 0.5/0.8; chela length 2.5/3.2, width 0.4/0.6, depth 0.3/0.5; movable finger length 1.9/2.3.
4 Ecology and population densities
The ecology and biology of Ananterinae are poorly known, but detailed inventories carried out during the last 30 years have shown that the number of species is significantly greater than was initially expected. During this period, the number of known species increased from 3 to more than 70 in Ananteris and from 1 to almost 20 in Tityobuthus. However, in most cases, the species are extremely rare and present very limited disjunct distributions. According to Giupponi et al. [24], Lourenço and Duhem [25] and Lourenço et al. [26], the totality of the known material of Ananteris preserved in official collections may be represented by less than 600 specimens.
Only a few species of Ananteris proved to be ‘common’. These were reported by González-Sponga [27] and by Brüehmüller Ramos [28]. In fact, recent ecological studies using pitfall and Winkler traps, showed remarkable results and clearly suggested that at least some species are much more common than was originally suspected [28].
Another particularity of Ananteris and Tityobuthus is the almost total absence of juvenile forms from museum collections. Collections have been based mainly on overturning rocks and the use of ultra-violet light and pitfall traps. Only the use of extraction methods, such as Berlese, Winkler and Kempson, has permitted the collection of juvenile forms [29]. Extraction methods also led to the discovery and description of a new humicolous genus and species of Ananterinae Microananteris minor [17]. These methods have been used more in Madagascar than in the Neotropics, and have resulted in the collection of juvenile forms of Tityobuthus [15]. One question can therefore be addressed: why are the juvenile forms of these cryptozoic but epigean species only found by extraction methods?
Scorpions became adapted to terrestrial environments between the Carboniferous and Triassic periods [30,31]. It is quite possible that transitional forms may have existed then, although these are difficult to identify [30]. In every case, the early terrestrial forms would have been unable to survive in extreme environments such as savannahs and deserts, which are today colonized by numerous species. According to their degree of adaptation to life on land, different types of soil would have been utilized during different stages of the evolution and adaptation of early scorpions. The evaporating power of the air is the most important physical factor of the environment affecting the distribution of cryptozoic animals. This is because small creatures have a very large surface in proportion to their mass; consequently, the conservation of water is the prime physiological problem of their existence [32–34]. The majority of cryptozoic animals are restricted to moist conditions, although these must not be so wet that they engender waterlogging. It is probable that the evolutionary transition of many invertebrates from aquatic to terrestrial life may have taken place via the soil, where aerial respiration is not associated with desiccation [32–34]. The present eco-physiological situation of the scorpions belonging to the Ananteridae could suggest that this lineage was originally exclusively composed of soil dwellers. During evolutionary time, adult forms learned to explore the epigean environment, but juveniles remained endogean. This kind of situation is frequently observed in insects, but generally unknown among scorpions [35,36]. This could be a possible explanation for the almost total absence of juveniles outside the soil environment.
Disclosure of interest
The author declares that he has no conflicts of interest concerning this article.
Acknowledgements
I am most grateful to Bernard Duhem and Élise-Anne Leguin, Muséum, Paris, for respectively the preparation of the drawings and photographs, to Michael M. Webber, University of Nevada, Las Vegas, USA, for her review of an earlier version of the manuscript. Finally to three reviewers for their useful remarks to the text.


