1 Introduction
As mentioned in previous papers, humicolous scorpions are globally rare [1,2]. The first instance to be precisely reported was that of Akentrobuthus leleupi, a buthid found in forests of the Kivu Province in Congo and described by Lamoral [3]. Just before the publication of Lamoral (1976), Vachon [4] described a new genus and species, Lychasioides amieti from the forest of Otomoto in the Cameroon. According to the collector of this species, J. L. Amiet, it was also to be found in organic soil. It was therefore considered as humicolous (Vachon, in litt.). More recently, a new genus of true humicolous buthid scorpions, Microcharmus, was described from Madagascar [5]. With the discovery of several new species and a second new genus, this group of micro-scorpions has been accommodated in its own family Microcharmidae [5–10].
Tropical American species of humicolous scorpions appear to be even rarer than those found in both Africa and Madagascar. Only two species belonging to the ‘Ananteris group’, Microananteris minor Lourenço and Ananteris cryptozoicus Lourenço have recently been described from French Guiana and Brazilian Amazon [1,2]. In the present article, a new species of humicolous buthid scorpion is described from rainforest in French Guiana. Although certain morphological characters could associate it with the genus Microananteris, others suggest that the new species can be also associated to the genus Ananteris. The definition of Microananteris in relation to Ananteris was largely based on important differences in the structure of the peg-shaped sensillae of the pectines. Since, for technical reasons, this character was not investigated at present, the new species is for the moment accommodated in the genus Ananteris. It can be suggested that some of the morphological features of these humicolous scorpion species may be an adaptation to the soil dwelling life. The new species described here represents the third humicolous scorpion to be reported in the Neotropical Region.
2 The genera within the ‘Ananteris group’ and their possible evolution from endogeous to epygean environments
The subfamily Ananterinae was first proposed by Pocock [11] to accommodate the genus Ananteris Thorell. Pocock wrote as follows: “I propose to eliminate from this subfamily (Buthinae) the isolated Neotropical genus Ananteris, which differs strikingly from the rest of the family in the structure of the pectines. The subfamily Ananterinae may be created for its reception.” Subsequently, the matter of the subfamily Ananterinae was discussed by several authors, in particular by Kraepelin [12] who accepted the proposal and by Birula [13] who rejected it. The last author to discuss the issue in detail was Mello-Leitão [14], but in every case Ananterinae was considered only in relation to the genus Ananteris.
The position of subfamilies within the family Buthidae remained, however, a point of controversy. Even if in recent years, the subfamily Ananterinae has been the subject of new discussion, attempts to propose a diagnosis to this subfamily have not been successful [2], since the used characters were generally unreliable. Consequently, it is preferable not to retain the subfamily Ananterinae until further studies on the totality of the buthoid elements may be available. Instead, one may refer to the “Ananteris phylogenetic group” as defined by Fet et al. [15]. The group is used as an informal rank, whereas the rank of subfamily has to be used as a formal taxonomic category regulated by the International Code of Zoological Nomenclature.
The biogeographic patterns presented by extant and fossil elements of the ‘Ananteris group’, with a worldwide geographical distribution, confirm not only a typical model of panbiogeography but also correspond to old Pangaean patterns. Several elements of the genus Lychas C. L. Koch, for example, suggest possible links between elements of the most basal ‘Ananteris group’ [16] and other buthids. In fact, as already pointed out by Lourenço [9], the large number of genera currently accepted as being valid within the Buthoidea cannot be classified at a single evolutionary level. At least four or five different evolutionary gradients (groups) need to be defined. This was already attempted by Fet et al. [15] in their definition of groups within the Buthoidea.
In a recent paper dealing with the genera of the ‘Ananteris group’, both Ananteris and Ananteroides Borelli from Africa, have been revalidated by Lourenço [16]. In other recent publications, Lourenço [5,6] reopened the study of the Malagasy genus Tityobuthus Pocock, and suggested clear affinities with the genus Ananteris. This was followed by the description of a new genus Himalayotityobuthus Lourenço [17] from the Himalayas, which was also associated with the genus Tityobuthus from Madagascar. Finally, in another publication, Lourenço [18] suggested affinities between the African genus Lychasiodes Vachon, with both Ananteris and Tityobuthus.
The association of all these different genera within the ‘Ananteris group’, suggests a Gondwanan pattern of distribution for this undoubtedly ancient lineage of buthid scorpions. The recent discovery of fossil forms in Baltic amber, namely Palaeoananteris and Palaeotityobuthus Lourenço and Weitschat, which are related to Ananteris and Tityobuthus added further confirmation of both the Gondwanan pattern of distribution and antiquity of the ‘Ananteris group's lineage [19,20].
The morphological traits of these genera of scorpions demonstrate their relationships. These are: small size, the persistence of neotenic structures in the adults (e.g. absence of fulcra on pectines) and, in most instances, cryptozoic behaviour – as well as the existence of some humicolous species. Their ecology and biology are poorly known, but detailed inventories carried out during the last 30 years, have shown that the number of species is significantly greater than was initially expected. During this period, the number of known species increased from three to more than 70 in Ananteris and from one to almost 20 in Tityobuthus. These species are, however, invariably extremely rare and present very limited and patchy ranges of distribution.
Another specific feature observed for most species of the ‘Ananteris group’ is the frequent absence of juvenile forms from collections in general. These have been based mainly on overturning rocks and the use of ultraviolet light and pitfall traps. Only the use of extraction methods, such as those of Berlese, Winkler and Kempson, has resulted in more frequent collection of juvenile forms. Extraction methods also led to the discovery and description of humicolous species. These methods have been more often used in Madagascar than in the Neotropics, and have resulted in the collection of juvenile forms of Tityobuthus. One question can therefore be addressed: why are the juvenile forms of these cryptozoic but epygean species obtained almost exclusively by extraction methods?
Scorpions became adapted to terrestrial environments between the Carboniferous and Triassic periods [21,22]. It is quite possible that transitional forms may have existed then, although these are difficult to identify [21]. In every case, the early terrestrial forms, would have been unable to survive in extreme environments such as savannas and deserts which are today colonized by numerous species. According to their degree of adaptation to life on land, different types of soil would have been utilized by different stages of the evolution and adaptation of early scorpions. The evaporating power of the air is the most important physical factor of the environment affecting the distribution of cryptozoic animals. This is because small creatures have a very large surface in proportion to their mass; consequently, the conservation of water is the prime physiological problem of their existence [23–25]. The majority of cryptozoic animals are restricted to moist conditions, although these must not be so wet that they engender waterlogging. It is probable that the evolutionary transition of many invertebrates from aquatic to terrestrial life may have taken place via the soil where aerial respiration is not associated with desiccation [23–25]. The present eco-physiological situation of the scorpions belonging to the ‘Ananteris group’, could suggest that this lineage was originally, exclusively composed of soil dwellers. During evolutionary time, adult forms learned to explore the epygean environment, but juveniles of most species remained endogean. This kind of situation is frequently observed in insects but unknown among scorpions in general [26,27]. This could be a possible explanation for the frequent absence of juveniles outside the soil environment.
3 Taxonomic treatment
Family Buthidae C.L. Koch, 1837
Subfamily Ananterinae Pocock, 1900
Genus Ananteris Thorell, 1891
Ananteris intermedia sp. n. (Figs. 1–4)

Ananteris intermedia sp. n. Male holotype. Habitus, dorsal aspect.
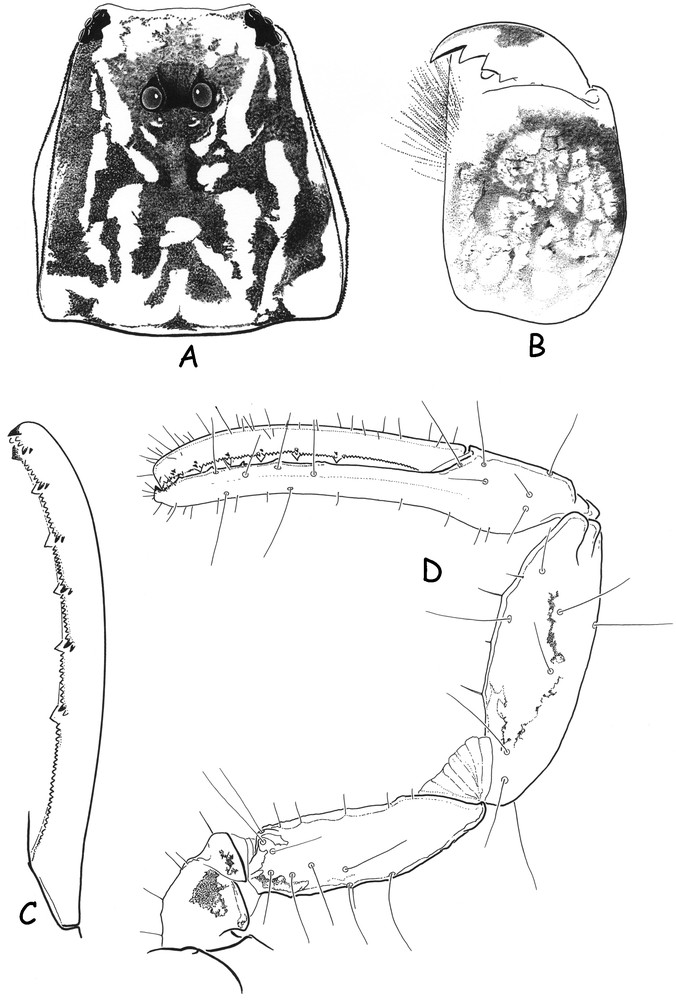
Ananteris intermedia sp. n. Male holotype. A and B. Carapace and chelicera, dorsal aspect, showing pigmentation pattern. C. Movable finger of pedipalp chela with rows of granules. D. Right pedipalp, dorsal aspect, showing trichobothrial pattern.
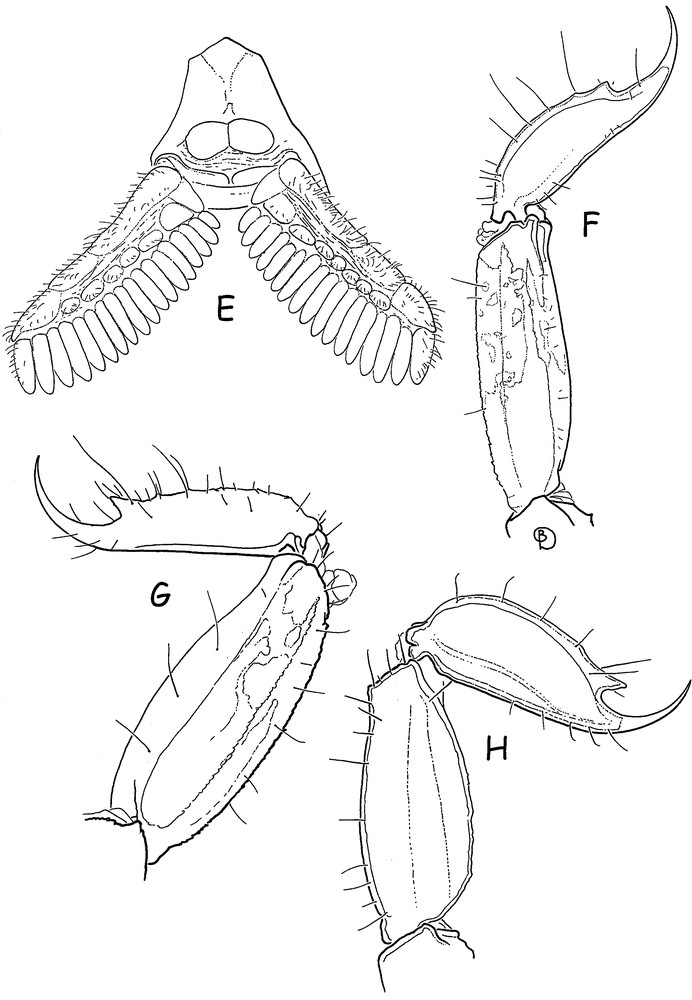
Ananteris intermedia sp. n. Male holotype. E. Sternum, genital operculum and pectines. F. Metasomal segment V and telson, lateral aspect. G and H. Idem for Ananteris bonito Lourenço, male holotype and Microananteris minor Lourenço, female holotype.
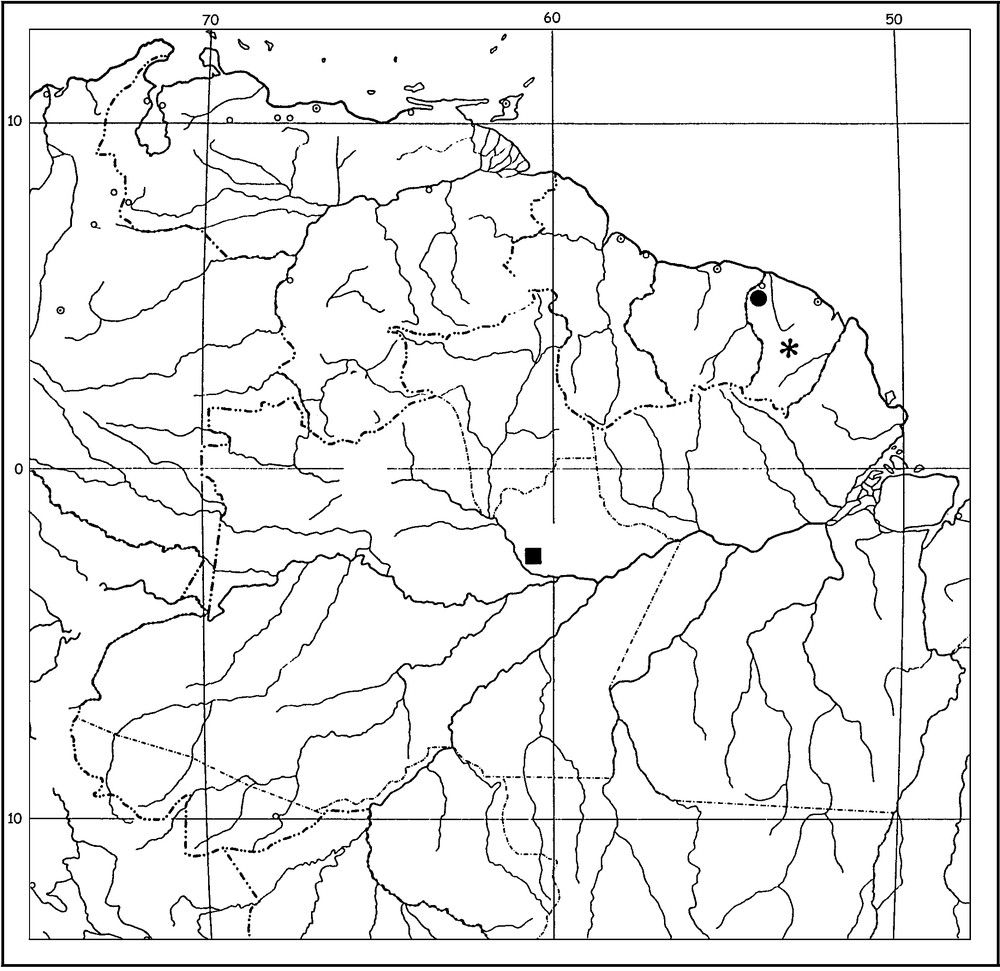
Map of the Guiano-Amazonian region showing the known distribution of humicolous ‘Ananteris group’ species. Microananteris minor (black flower), Ananteris cryptozoicus (black square), Ananteris intermedia sp. n (black circle).
Type material: male holotype: French Guiana, St. Jean, road to St. Laurent, 12/VI/1987 (W. Lourenço); primary rainforest, Winkler 06. Deposited in the Muséum national d’Histoire naturelle, Paris.
Diagnosis: Very small scorpions, when compared with the average size of most species of micro-buthid genera, and measuring only 9.3 mm in total length (see morphometric values). General coloration yellow to pale yellow with carapace and tergites intensely marbled with dark brown spots. Pedipalps rather short; fingers with 6 rows of granules; male pectines with 17–18 teeth. Telson with a fusiform shape and an extremely reduced subaculear tubercle. Carinae and granulation weakly marked or absent. Trichobothria db and est of fixed finger situated at distinct levels; external trichobothria of femur very close to each other.
Relationships: By a number of characters, the new species shows affinities with both the genera Ananteris and Microananteris. It appears as an intermediate form between those already known for the two groups. However, since the analysis of the microstructure of its pectine peg-shaped sensilla was not possible at this stage, I decided to include it on the genus Ananteris. The new species can be easily distinguished from all the other in the genus by a combination of characters:
- • small global size;
- • carapace narrowed posteriorly and with the anterior margin straight;
- • carapace and tergites weakly granular to smooth;
- • pectines elongated with 17–18 teeth in male;
- • telson with the subaculear tubercle extremely reduced;
- • external trichobothria of femur situated very close to each other.
The new species may be a possible endemic element of the rain forests of the northwest region of French Guiana.
Description: Coloration. Basically yellow to pale yellow with carapace and tergites marbled with dark brown, producing an overall spotted appearance. Prosoma: carapace yellow, almost totally covered with brown spots; eyes surrounded by black pigment. Mesosoma: yellowish-brown with three longitudinal stripes. Metasomal segments I to V yellow to pale yellow, with several brown annular spots distally; segment V with better marked spots. Telson: vesicle yellow without spots; aculeus yellow at the base and reddish at the tip. Venter pale yellow with infuscations only on sternite VII. Chelicerae pale yellow with diffused variegated spots over their entire surface; better marked anteriorly; fingers pale yellow with reddish teeth. Pedipalps pale yellow, only slightly infuscate on the femur and patella; chela paler than patella; fingers pale yellow with the rows of granules slightly reddish. Legs yellow, densely marked with brownish spots.
Morphology. Carapace weakly granular to smooth; anterior margin almost straight. Anterior median superciliary and posterior median carinae weak to vestigial. All furrows weak. Median ocular tubercle distinctly anterior to the centre of carapace; median eyes separated by more than one ocular diameter. Three pairs of lateral eyes. Sternum subpentagonal to pentagonal. Mesosoma: tergites weakly granular to smooth. Median carina weak in all tergites. Tergite VII pentacarinate. Venter: genital operculum divided longitudinally, each plate having a more or less oval shape. Pectines rather long: pectinal tooth count 17–18; basal middle lamellae of the pectines not dilated; fulcra absent. Sternites smooth with short semi-oval to round spiracles; VII with a few granulations and vestigial carinae. Metasomal segments I and II with 10 carinae, weakly crenulate. Segments III and IV with 8 carinae, weakly crenulate. Intercarinal spaces weakly granular to smooth. Segment V with 5 carinae. Telson with a ‘fusiform-like’ shape, smooth with one vestigial ventral carina; aculeus moderately long and weakly curved; subaculear tubercle extremely reduced to vestigial. Cheliceral dentition characteristic of the family Buthidae [28]; fixed finger with two moderate basal teeth; movable finger with two very weak basal teeth; ventral aspect of both finger and manus with dense, long setae. Pedipalps: femur pentacarinate, with carinae weakly marked; patella with a few vestigial carinae; chela smooth; internal face of patella with some vestigial granules; all faces weakly granular, almost smooth. Movable fingers with 6 almost linear rows of granules; two accessory granules present at the base of each row; extremity of movable fingers with three accessory granules. Trichobothriotaxy; orthobothriotaxy A-β [4,29]; external trichobothria of femur situated very close to each other. Legs: tarsus with very numerous fine median setae ventrally. Tibial spurs weakly developed on legs III and IV.
Morphometric values (in mm) of the male holotype. Total length, 9.3. Carapace: length, 1.2; anterior width, 0.8; posterior width, 1.1. Mesosoma length 2.7. Metasomal segment I: length, 0.5; width, 0.7; II: length, 0.5; width, 0.5; III: length, 0.7; width, 0.5; IV: length, 1.0; width, 0.5; V: length, 1.4; width, 0.5; depth, 0.5. Telson length 1.3.Vesicle: width, 0.4; depth, 0.4. Pedipalp: femur length, 0.9, width, 0.3; patella length, 1.3, width, 0.4; chela length, 1.6, width, 0.3, depth, 0.3; movable finger length, 1.1.
Disclosure of interest
The author declares that he has no conflict of interest concerning this article.
Acknowledgements
I am most grateful to Bernard Duhem and Elise-Anne Leguin, Service des Collections, MNHN, Paris for their help in the preparation of the illustrations and to Victor Fet, Marshall University, USA for his comments and review of the manuscript.


