1 Introduction
Laccases (benzenediol: oxygen oxidoreductases; EC1.10.3.2.) are produced by many different species of white rot fungi (WRF) [1]. WRF laccase is one of the enzymatic components required to degrade the major components of lignocellulosic biomass into low-molecular-weight compounds that can be assimilated for fungi nutrition [2,3]. Laccase production by WRF strongly depends on the cultivation conditions. In recent years, two types of cultivation of WRF has been developed: solid state fermentation (SSF) and submerged fermentation (SmF).
Laccase production depends on the nature of the carbon source, which in some cases may come from different agro-industrial lignocellulosic residues. Moreover, lack of kinetic and design data associated with various fermentation processes may present additional limitations [4]. Submerged cultivation (SC) is a strategy to improve laccase production yield in a relatively short time as compared to SSF [1]. Physiological regulation of laccase production using SmF is comparatively simpler than in SSF [5]. Substitution of a synthetic carbon source with different agro-industrial lignocellulosic residues in SmF was carried out successfully for laccase production by WRF [6,7]. A goal of this approach was to reduce the cost of production of the enzyme to allow large-scale industrial applications [8].
Galhaup and Haltrich [9] showed that extracellular laccase formation could be greatly stimulated by the addition of Cu2+ to the growth medium. Many other authors have also shown that different phenolic compounds induce laccase production by SC of WRF [10–12].
In the present investigation, laccase was produced in a 120-L volume by SmF of Cerrena unicolor C-139 growing on wheat bran in the presence of 1 mM Cu2+ as an inducer. In a previous small-scale laboratory study, it was shown that, as compared to literature data on other WRF, laccase was produced at the very high level of 416.4 U·mL−1 by C. unicolor C-139 growing on wheat bran in SmF [8]. This previous contribution appeared as a good platform for the present scaling-up study. Recently, it was shown that Pycnoporus sp. SYBC-L3 laccase can be produced in a 65-L airlift reactor with a conventional medium containing glucose mineral salts at the level of 72 units enzyme mL−1 [13]. To our knowledge, the present report is the first one concerning a SmF production of laccase at the 120-L scale with a stirred bioreactor and using a cheap lignocellulosic (wheat bran)-based medium.
2 Materials and methods
2.1 Organisms and inoculum preparation
C. unicolor C-139 was obtained from the culture collection of the University of Regensburg, Germany. The fungus was maintained on 2% (w/v) malt agar plate at 4 °C. The inoculum was prepared by growing the strain on a rotary shaker at 120 rpm and 30 °C in 3 × 2-L flasks containing each 400 mL of the following defined medium (g·L−1): glucose 10; KH2PO4 0.8; Na2HPO4 0.25; MgSO4·7H2O 0.5; yeast extract 2.0; peptone 2.0 (Duchefa). Each flask was inoculated with 4 malt agar-plugs coming from Petri dishes (6-mm diameter). After four days of cultivation, preculture (1200 mL) was used to inoculate a 42-L reactor (Techfors-S: Infors) with a 30-L working volume, containing the same medium.
The cultivation was carried out at 26 °C and at pH 5.0 using NaOH (10%) for pH control. The minimum level of dissolved oxygen was hold at 25% of saturation of air oxygen in water (100% ≈ 7 mg O2/L at 30 °C) by controlling the impeller speed between 200 to 360 min−1 (six-blade Rushton impeller) at a constant aeration rate of about 5 L·min−1. After four days of cultivation, 10 L of the medium were used as an inoculum to start the production fermenter.
2.2 Production of laccase at the 120-L scale
Large-scale production of laccase was performed in an Infors ISF-300 stirred bioreactor with a 120-L working volume, using wheat bran (40 g·L−1) as the main organic substrate. The medium described in Table 1 was sterilized for 30 min at 121 °C. The addition of 5.5 g/L of glucose was used to support the initial growth after inoculation. Temperature was controlled at 30 °C and pH at 6.0 by the use of ∼1.4 kg H3PO4 (20%) and ∼0.3 kg NaOH (20 kg). The addition of an antifoam reagent was not necessary, because foam formation was sufficiently controlled by a mechanical foam reducer.
Cerrena unicolor C-139 cultivation batch-process in a 150-L bioreactor (firm: Infors).
| Component | Concentration |
| Peptone | 2 g·L−1 |
| Yeast extract | 2 g·L−1 |
| Glucose·1H2O | 5.5 g·L−1 |
| Wheat bran (Wb) | 40 g·L−1 |
| KH2PO4 | 0.8 g·L−1 |
| Na2HPO4·2H2O | 0.25 g·L−1 |
| MgSO4·7H2O | 0.45 g·L−1 |
| CuSO4·5H2O | 0.25 g·L−1 |
| Working volume (Start) | – |
| Inoculum | 8% |
| Tap water | 100% |
| Number of stirrers | 3 |
Dissolved oxygen was fixed at 25% of saturation by variation of the aeration rate (1–40 m3/h) and agitation rate (130–420 min−1, 3 × six-blade Rushton impeller). A single addition of copper sulphate (1 mM) was realized via a syringe on day 4 and lid-septum. The process parameters of the cultivation of C. unicolor C-139 in the 150-L bioreactor is shown in Table 2.
Cerrena unicolor C-139 cultivation process parameters in a 150-L bioreactor.
| Temperature | 30 °C |
| pH, start | 5.4 |
| pH, set point | 6.0 |
| pO2, set point | 25% |
| Aeration | 1–40 m3·h−1 |
| Agitation | 130–420 min−1 |
2.3 Sugar and protein determination
Sugars were determined as the total amount of reducing saccharides using 3,5-dinitrosalicylic acid (DNS method of [14]).
The amount of total protein in the culture liquid was determined by [15] protein assay using the Folin–Ciocalteu reagent.
2.4 Laccase activity assay
Laccase activity was determined by monitoring the A420 change related to the rate of oxidation of 1 μmol of 2,2-azino-bis-[3-ethylthiazoline-6-sulfonate] (ABTS) in 20 mM of a sodium acetate buffer (pH 4.5). Assays were performed in a 1-mL spectrophotometric cuvette at 30 ± 1 °C with an adequately diluted culture liquid. One unit of laccase activity was defined as the amount of enzyme catalysing the oxidation of 1 μmol of ABTS per minute (Songulashvili et al. 2007) [1].
2.5 Micro- and ultrafiltration
The culture liquid was first separated from solids by centrifugation with a Sigma 8K10 apparatus operated at 11,300 g for 10 min at 4 °C. Microfiltration was performed as crossflow filtration using a Sartoflow-β unit (Sartorius, technical scale). The filter cassettes were made of 0.2 μm hydrosart (HA) material.
Crossflow ultrafiltration was realized with two S-slice units (Sartorius, laboratory scale) equipped with three filter cassettes made of 10 kDa or 5 kDa hydrosart and two cassettes made of a 10-kDa polyethersulfone (PESU) material. The process parameters are described in Table 3.
Cross flow filtration of Cerrena unicolor C-139 culture fugate, processing parameters.
| Parameter | Microfiltration | Ultrafiltration | ||
| Module | Sartoflow beta, Sartorius | 2 × Sartocon slice, Sartorius | ||
| Filter material | Hydrosart | Hydrosart/PESU | ||
| Cut-off | 0.2 | μm | 10.000 | Da |
| Number of filter cassettes | 4 | – | 5 | – |
| Area filter cassette | 0.6 | m2 | 0.1 | m2 |
| Total filtration area | 2.4 | m2 | 0.5 | m2 |
| Filtration temperature | 13.5 | °C | 6.5 | °C |
| Medial pressure feed–input | 0.35 | bar | 0.4–0.7 | bar |
| Medial pressure retentate–output | 0.3 | bar | 0.0 | bar |
| Medial pressure filtrate–output | 0.0 | bar | 0.0 | bar |
| Volume feed | 91.3 | L | 81.5 | L |
| Duration | 2.8 | h | 14.6 | h |
| Filtrate flow per unit of area | 12.3 | L/m2·h | 11.5 | L/m2·h |
3 Results and discussion
3.1 Scaling-up of laccase production by C. unicolor C-139
C. unicolor C-139 was previously characterized at the laboratory scale as a high laccase producer in SmF [8]. Scaling-up of C. unicolor C-139 cultivation was done at a working volume of 120 L. The strain was cultivated during 14 days. The growth medium described in Materials and methods was chosen for cultivation, with a wheat bran concentration of 40 g·L−1 in combination with 5.5 g·L−1 of glucose. Glucose was used as a starter carbon source for the good development of mycelia at the initial stage of cultivation. Wheat bran was used as a cheap lignocellulosic growth substrate. The total amount of soluble saccharides was approximately 8 g·L−1 in the initial medium (Fig. 1). At the beginning of the cultivation of the WRF, the concentration of saccharides increased moderately and peaked at 10 g·L−1 at the end of day 1 (Fig. 1). The level of saccharides remained stable at approximately 1.8 g·L−1 after 5 days of cultivation until the end of the 14-day experiment. It should be noted that during cultivation, the total protein concentration varied slightly (Fig. 1), starting at 3.2 g·L−1, further decreasing at 1.8 g·L−1 at day 4 to finally stabilizing at 2 g·L−1 from day 7 until day 14. At the same time, laccase monitoring showed that enzyme production started after 2 days of cultivation, attaining a maximum activity of 416.4 U·mL−1 at day 12 of fermentation (Fig. 1). One millimole of copper sulphate was added to the growth medium as an inducer after 4 days. At the end of cultivation, laccase activity slightly declined to attain a level of 365.5 U·mL−1 on day 14 (Fig. 1). This result was in agreement with a previous result, showing that a similar profile of laccase production was obtained with C. unicolor C-139 cultivated in 100-mL flasks containing 50 mL of the wheat bran medium [8]. These results clearly indicated that the strategy of the upscaling process was planned correctly. The amount of laccase produced by C. unicolor C-139 growing on a cheap medium gave us the opportunity to study at a post-laboratory scale the processes involving this enzyme in environmental, food and pharmaceutical applications [8,13,16,17]. It should be also noted that the production of laccase by C. unicolor C-139 is very much higher than a typically reported range of 4–100 U·mL−1 produced by several fungal strains [9,13,18,19]. At the same time, the cost analysis of laccase production was an important feature for industrial exploitation. The calculation of laccase production cost should include equipment, medium, and operating costs. As shown by Osma [20], most part of laccase production cost depended on the medium cost, accounting for 81–89% of the total cost of post-laboratory production of laccase. The operational cost of laccase production does not differ significantly according to the laccase production medium. For example, laccase production cost in the absence of inducers in SmF of Trametes pubescens, using glucose as a carbon source, was around 17 cents of euros per unit of enzyme produced, while glucose substitution with wheat bran decreased the final price of laccase to 1.59 cents of euro per unit, however in SSF at the flask scale [20]. It should be noted that the maximal laccase production by T. pubescens never attained more than 41.1 U·mL–1 (SSF tray bioreactor scale) as compared to 416.4 U·mL−1 attained here by the C. unicolor strain in SmF. In our study, the use of C. unicolor strain as a high enzyme producer and that of a cheap wheat bran-based growth medium were the main assets to reduce laccase production cost. When using Osma's [20] approach, it can be estimated that the final price of C. unicolor laccase obtained by SmF in the presence of Cu2+ is lower than 0.01 € cent/unit enzyme as compared to the lowest price of 0.04 € cent/unit obtained with T. pubescens in a SSF tray bioreactor in the presence of Cu2+ and tannic acid as inducers [20]. The price of C. unicolor laccase is apparently in the range of the price calculated for the Pycnoporus sp. SYBC-L3 laccase produced in a 65-L airlift reactor [13]. However, one point that should not be forgotten is that a given preparation of laccase from a particular strain of WRF is not necessarily appropriate for all possible applications. For example, C. unicolor laccase was found to be particularly suitable for immobilization with the aim of eliminating endocrine disruptor micropollutants in wastewaters [8]. Among the different laccases produced by WRF, the Trichoderma harzianum enzyme appears as one of the more suitable for pulp bleaching and treatment of paper industry effluents [21,22]. Yet, the enzyme from Trichoderma appeared as not suitable for treating optimally endocrine disruptors (Songulashvili et al., unpublished). Accordingly, laccases are not necessarily exchangeable for different applications and it is possible that we have to use in some cases more expensive preparations.

(Color online.) Profile of laccase production by Cerrena unicolor C-139 in the 150-L bioreactor.
It is therefore essential to always look for the method allowing the highest and cheapest production of an enzyme suitable for a particular application. Many industries are currently pursuing enzymatic approaches for developing green chemistry technologies. This is mainly due to the shortcomings of physicochemical methods, to growing environmental concerns, to legal restrictions, and to increasing scientific knowledge. Laccase-assisted reactions, in particular, are currently intensively investigated as they are generally eco-friendly and have a wide application potential [23]. However, the price of laccase contribution in industrial processes can depend strongly on the nature of the planned application [21–24]. In environmental applications using non-purified enzymes, the cost of laccase utilisation is not far from that of enzyme production. In food, and especially in pharmaceutical industries, the price of laccase utilisation may increase substantially if a purified catalyst is needed. The yield and cost of a downstream treatment to concentrate enzyme from the culture fluid for further application should be also taken into account.
3.2 Treatment of culture liquid from C. unicolor C-139 for laccase concentration
In a first step, 116 L of total culture liquid were centrifuged for 10 min at 11,300 g for separation of the cells and of other insoluble materials. The supernatant after centrifugation contained a total of 3,3370,150 units of laccase activity. This value was assumed as the 100% absolute activity in order to evaluate the yields of further crossflow filtration processes.
Microfiltration of the centrifuged culture liquid was done as described in Material and methods. In this step, colloidal particles were removed. After microfiltration, the filtrate volume resulted into 81.5 L containing 29,274,800 units of laccase. According to these data, the loss of laccase was 12.3%, between the first and the second steps. Microfiltration was a preliminary step for further ultrafiltration in order to prevent fouling of the ultrafiltration membrane and to avoid microbial contamination of the culture liquid.
The micro-filtrate was further ultra-filtrated with 10 kDa hydrosart and polyethersulfone filter cassettes. After this step, 600 mL of a laccase liquid concentrate containing 9,081,600 units were obtained (Table 4). Only 27% of the initial enzyme activity was recovered after ultrafiltration. A loss of activity of more than 70% during both micro- and ultrafiltration constituted an absolutely critical result (Fig. 2). Ultrafiltration was apparently the “bottle neck” factor during downstream treatment and had to be optimized thoroughly. A laccase activity higher than 30% was found in the ultrafiltrate. One explanation could be that the processing pressure applied here for a long time on 10 kDa filters increased permeability of the membrane resulting in enzyme leakage. Another explanation could be an enzymatic degradation that led to partial membrane damage. Hydrosart material is based on cellulose and could possibly be partially degraded by cellulases present in the cultivation liquid. Furthermore it is possible that laccases agglomerated in the concentrate, so that an underestimation of laccase activity caused by blocked functional groups cannot be excluded. In view of obtaining a higher final laccase yield, the 10-kDa cut-off filters were exchanged for a 5-kDa cut-off material. Apparently, the change of size of porosity of hydrosart and polyethersulfone filter cassettes appeared as the crucial factor in the ultrafiltration process. The recovery rate of the enzyme was 66.5% of the initial amount, with 22,203,176 U per total laccase activity (Table 4.) Moreover, it should be noted that the use of 5-kDa cut-off membranes in combination with a decreased inlet pressure and temperature should also be taken into account for explaining a lower laccase loss during the ultrafiltration step.
Output of laccase yield on different stage of the downstream treatment by crossflow filtration of a Cerrena unicolor C-139 culture liquid.
| Downstream step | Volume (L) | Laccase specific activity (U mL−1) | Laccase absolute activity (U) | Laccase distribution (%) |
| Supernatant centrifugation | 91.3 | 365.5 | 33,370,150 | 100.0 |
| MF–filtrate | 81.5 | 359.2 | 29,274,800 | 87.7 |
| UF–10-kDa retentate | 0.6 | 15136 | 9,081,600 | 27.2 |
| UF–5-kDa retentate | 0.6 | 37005 | 22,203,176 | 66.5 |
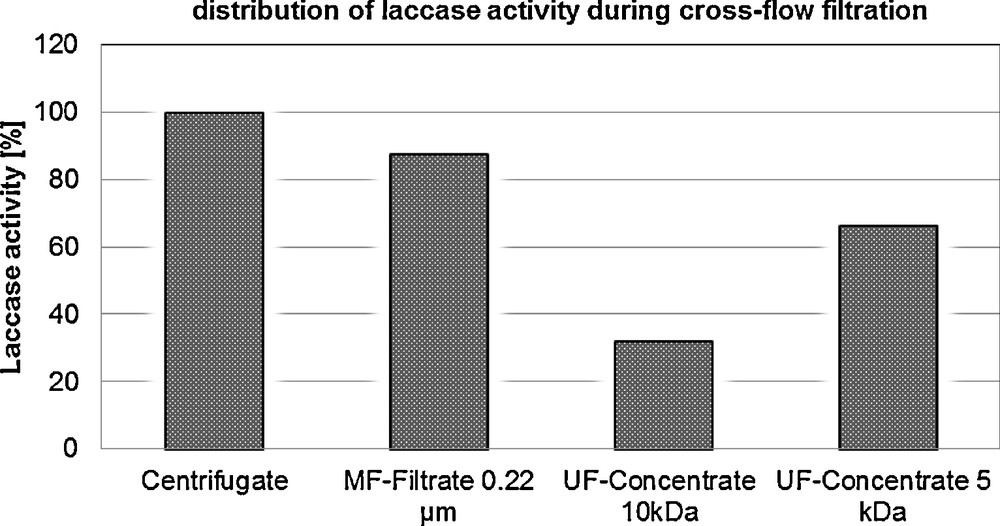
Profile of laccase activity in the different steps of downstream processing by crossflow filtration of a culture liquid from Cerrena unicolor C-139.
In general conclusion, the present study underlines the need to develop a correct and consistent scaling-up process for a high production of cheap laccase by WRF in order to obtain enough material for studying the contribution of the enzyme in different agronomical, environmental, or pharmaceutical applications.
Acknowledgments
This work was done in the frame of OXEROM project. The OXEROM project is supported by Brussels-Capital Region, Belgium (Convention IP-ENVI 10).


