1 Introduction
The white-rot basidiomycete fungus Pleurotus ostreatus is one of the most active microorganisms degrading lignin, a complex aromatic biopolymer that is extremely recalcitrant to degradation [1]. This fungus produces different oxidative enzymes, with broad substrate specificity, which can also be used to degrade a vast range of toxic aromatic pollutants [2,3]. Among these enzymes, the production of several laccase (E.C. 1.10.3.2) isoenzymes is prominent [4]. The variety of laccase isoenzymes is related to the diversity of their roles: lignin synthesis/degradation [1,5], fruiting bodies development [6], pigment production [7], cell detoxification [8], etc. [9]. Moreover, laccases result in biotechnologically relevant products because of their ability to oxidize both phenolic and non-phenolic lignin related compounds as well as highly recalcitrant environmental pollutants. These features are suitable for several different applications in industrial effluents disposal, medical diagnostics, bioremediation to degrading pesticides and explosives in soils, delignification processes in paper industries and in cosmetics formulation as additive [10]. Owing to the successful use of laccases in the above-mentioned biotechnological applications, the ever-increasing demand requires the production of large quantities of enzyme at low cost. As a fact, several production strategies have been adopted along with process optimization to achieve better process economics. Concurrently, studies on laccase producing organisms have been intensified in the recent years. The overexpression in suitable hosts and optimization of laccase production from different microorganisms would provide means to achieve high titers. On the other hand, several methods have been used for strain improvement in Pleurotus spp. including selection, hybridization and gene transformation [11–14]. Based on current legislation (European Directive 2001/18/CE), genetic transformation and mutagenic treatments produce strains not suitable for “natural or safe processes”. Therefore, the construction of genetically modified organisms cannot be chosen to improve the addressed quality of the fungus, and breeding should be based on classical genetic approaches. This last technique is based on the mating of two monokaryotic compatible strains of interest, whose hyphae are able to fuse and give rise to a dikaryotic mycelium in which the two parental nuclei remain independent [13,14]. Production of the monokaryotic strain, germinating from uninucleate basidiospores, is achieved when the fungus enters into a reproductive phase triggering basidiocarp formation: during basidia formation, karyogamy takes place immediately before the onset of the meiosis giving rise to four uninucleate basidiospores. Chaudhary et al. [15] developed single spore isolates from the white-rot fungi Pleurotus djamor, P. ostreatus var. florida, Pleurotus citrinopileatus and Hypsizygus ulmarius. The hybrids showed improved mycelial growth rate compared to parental strains. In another work, Sawashe and Sawant [16] developed hybrid cultures which required a significantly shorter period for spawn run as compared to the parent species.
Selecting new hybrid strains for enzyme production could be viewed as a solution to make the entire process cost effective, and further enhancement using inducers may be added to the benefit.
Use of inducers to enhance laccase production has been widely practiced in fungi especially in white-rots, where induction of laccase production by aromatic compounds is well established [17]. Increasing in laccase production has been also achieved in presence of other compounds like aminoacids [18], plant extracts [19] and copper [20,21] in the growth medium. However, use of above-mentioned inducers enhance production cost, because of their price and their likely toxicity which could negatively affect cost of wastewater disposable. In order to avoid these problems, studies on fungal autoinduction mechanisms [22] are being carried out by several groups. Autoregulations, and the signal molecules involved in, have been clearly elucidated in dimorphic fungi like Candida albicans and Saccharomyces cerevisiae [23]. However, several compounds have been correlated to the regulation of different aspects of fungal physiology in diverse classes of the kingdom mycota [22]. Schimmel et al. [24] reported a specific autoinduction effect on lovastatin synthesis in Aspergillus terreus, when a spent medium solution extracted from the fungal submerged culture is used to condition a new fresh growth of the same strain. Conversely, in the literature, no information about laccase autoinducers is available.
The aim of the present work has been to exploit both approaches: conditioning P. ostreatus growth by spent medium solution extracted from liquid culture and breeding the fungal strains by classical crossing. Finally, the potential exploitable effect of spent medium solutions on laccase production was combined with improved capabilities of new hybrids derived from the breeding of two P. ostreatus varieties.
2 Materials and methods
2.1 Organisms
All P. ostreatus monokaryotic and dikaryotic strains were maintained through periodic serial transfers and kept at 4 °C on agar plates in the presence of 2.4% potato dextrose and 0.5% yeast extract (PDY) (Difco).
Monokaryotic progeny were identified by a progressive number followed by the (lower case) letter of the parent strains (strains no.“f” and no.“o”, respectively). New dikaryotic varieties were classified by progressive numbers followed by the two lower case letters and separated by the “X” (no.f X no.o).
2.2 Fructification and basidiospores isolation
Mushrooms of two commercial dikaryotic strain of P. ostreatus, P. ostreatus variant Florida (strain F) and P. ostreatus variant ostreatus (strain O), were cultivated in 500 mL jars containing 400 g of wheat-straw (65% water content), which were sterilized by steam heated (121 °C) in autoclave for 1 h at 121 °C. Sterilization procedure was repeated a second time after an incubation time of 24 h at room temperature. Each jar was inoculated with four agar plug (13 mm diameter), and left to grow at 28 °C for 30 days in the dark. Fructification was promoted by opening the jars, and placing them in presence of daylight in a chamber at 15 ± 5 °C and 90% relative humidity. Primordia appeared after a further 15 days of growth, and basidiocarps were harvested 7 days later and weighed [25].
P. ostreatus basidiospores were collected by sporal print on a glass Petri dish, previously sterilised in autoclave (1 h at 121 °C).
A spore suspension was prepared in 1 ml sterile physiological salt solution (0.9% NaCl). Sporal concentration was estimated by counting in a Thoma chamber on optical microscopy.
2.3 Mating test
The basidiospores suspension was plated on PDY agar medium after appropriate dilution. Vegetative mycelium colonies were examined by phase-contrast microscopy for clamp connections, the appearance of colony characteristics specific for dikaryon. Colonies lacking clamps were subcultured in PDY agar slants at 28 °C and inoculated in pairs on 2% malt extract agar plates, so that their mycelia would fuse. Compatible monokaryons were identified by the production of clamp connections.
2.4 Culture conditions in liquid culture
2.4.1 Condition 1 (C1), for spent media preparation
Mycelium of variety P. florida was grown in 1 l shaken flasks (125 rpm) containing 300 ml of GYM (Glucose, Yeast extract, Mineral solution) formulated as follow: 10 g/l glucose; 3,8 g/l yeast extract (Difco) 2 g/l H2KPO4; 0.5 g/l MgSO4 7H2O; 0,1 g/l CaCl2 2H2O; biotin 10 mg/l; thiamine 10 mg/l and 10 ml of mineral stock solution (0.5 g/l MnSO4 5H2O; 1 g/l NaCl; 0,1 g/l FeSO4 7 H2O; 0.1 g/l CoCl2 6 H2O; 0,1 g/l ZnSO4 7 H2O; 0,01 g/l CuSO4 5 H2O; 0,01 g/l AlK(SO4)2; 0.01 g/l H3BO3; 0.01 g/l NaMoO4 2 H2O); final pH5. Except where indicated, all chemicals were obtained from Sigma Chemical Co. 5-day-old culture were homogenized by Ultra-Turrax® T25 Basic interconnected with S18N-19G dispersing tool (3 flashes of 30 seconds at 24,000 rpm separated by 30 seconds of stand-by) and 1 ml of homogenate was transferred in 1-l flasks containing 300 ml of GYM broth. The cultures were grown in shaken flasks at 125 rpm and incubated at 28 °C in the dark for 17 days.
2.4.2 Condition 2 (C2), for hybrid strain growth
Submerged cultivation was carried out in 100 ml Erlenmeyer flasks containing 30 ml of PDY with copper sulphate (final concentration 150 μM) on rotary shaker (125 rpm). The flasks were inoculated with four agar plugs (8 mm diameter) cut from the actively growing part of the colony on a Petri dish and incubated for at least 17 days at 28 °C in the dark.
2.5 Liquid–liquid extraction of spent media
Extractions were performed on P. florida samples by adding ultra pure chloroform (Carlo Erba reagents) to 300 ml of P. ostreatus harvested growth medium (condition 1) using a 1:1 v/v ratio. The mixture was subjected to horizontal and rotary shaking for 2 min. The procedure was repeated twice for each sample. After 10 min decantation, organic phase was removed and concentrated up to 1000 times using a Heidolph Laborota 4000 rotary evaporator. The liquid-liquid extraction was used to prepare conditioning solution SM7, SM10, SM13 and SM16 (spent medium 7, 10, 13 and 16 days old, respectively).
2.6 Conditioning by spent medium solutions
The liquid-liquid extraction of 300 ml spent GYM medium were concentrated up to 1000 times, sterilized by filter membrane (cut-off 0,22 μm, Millipore®) and used to condition 300 ml of basal medium of a fresh growth (C1). Conditioning of GYM basal medium was performed using extracted spent medium solutions supplemented at the time of inoculation.
2.7 Protein, biomass and glucose concentration determinations.
Protein concentration was determined using the BioRad protein assay kit (BioRad, Hercules, California), following the manufacturer's instructions, with bovine serum albumin as standard. Biomass was dried by drying oven at 65 °C overnight and estimated gravimetrically. Glucose concentrations were determined by the glucose oxidize method [26]. Each assay was performed in triplicate.
2.8 Enzyme assays
Phenol-oxidase activity was assayed at 25 °C using 2,2′–azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) as substrate [27]. The assay mixture contained 2 mM ABTS and 0.1 M sodium-citrate buffer, pH 3,0. Oxidation of ABTS was followed by absorbance increase at 420 nm (ɛ = 36,000 M−1 cm−1) for 1 minute. Enzyme activity was expressed in UI. All measurements were repeated at least in triplicate.
2.9 Native polyacrylamide gel electrophoresis
Polyacrylamide gel electrophoresis (PAGE) was carried out at alkaline pH under non-denaturing conditions. The resolving and stacking gels contained 9% and 4% acrylamide, respectively. The buffer solution used for the resolving gel contained 50 mM Tris-Cl (pH 9.5), and the buffer solution used for the stacking gel contained 18 mM Tris-Cl (pH 7.5). The electrode reservoir solution contained 25 mM Tris-Cl and 190 mM glycine (pH 8.4). Gels were stained to visualize laccase activity by using ABTS as substrate, in sodium citrate buffer 0.1 M pH3. Samples containing 0.015 laccase unities were loaded on each lane.
3 Results and discussion
3.1 Analysis of Pleurotus ostreatus growth model in liquid culture
Avoiding false positive induction derived from biotransformation of unknown compounds (generally present in complex media), P. ostreatus var. florida was grown in a semisynthetic medium containing glucose as main carbon source, and yeast extract, as nitrogen source. As shown in Fig. 1, three phases of the basal growth, forming the typical behaviour of filamentous fungi in liquid cultures, have been detected. Starting from the inoculation time (t = 0 day), the lag phase was displayed for 2 days. During this phase, no relevant glucose consumption in the medium was detected. Increasing of glucose consumption rate occurred in the trophophase up to the complete depletion of the main carbon source (7th day). During the same time, the fungal culture reached highest cell density, measured as mycelial dry weight (7.2 ± 0.5 g/L). However, between the 5th and the 7th fermentation day, the rapid increase of glucose uptake and the decrease of growth rate indicate the beginning of the idiophase, when the anabolic pathways of the cultured fungus are altered to produce different biomolecular compounds (secondary metabolites). In fact, the beginning of exponential growth is related to the start-point of glucose consumption, while cellular lyses (Fig. 2) in the last phase of growth should be a consequence of carbon starvation.

Pleurotus ostreatus 17 days fermentation profile in basal condition: extracellular laccase activity, secreted protein concentration, glucose consumption and biomass increasing are reported as U/ml (), 10* mg/l (); g/l (), g/l () respectively.

Light microscopy observation of fungal pellet at the 5th (A) and the 12th (B) day of growth in liquid basal medium. In Fig 2B, it is possible to appreciate vacuolization and cell lyses. Bar, 12 μm.
Time course analysis of extracellular laccase activity production was carried out and the production profile was evaluated in parallel with the fungal growth. As previously reported [28], laccase synthesis does not appear to be related only to the hyphal growth, because the enzyme activity does not parallel biomass production (Fig. 1). Laccase activity peaked on the 5th and the 13th day, reaching 4000 and 3000 U/L respectively, and decreased dramatically thereafter. The first laccase production increment, rising up to the 5th day, is probably related to the development of fungal biomass in liquid culture, whereas the latter is probably connected to the idiophase phenomena, where activation of secondary metabolism and cellular autolysis occurs (Fig. 2).
Laccases were analyzed by native PAGE and stained with ABTS. Analysis of samples withdrawn from the media at different growth times indicates that the activity is associated mainly to the production of three isoenzymes POXA3, POXA1B and POXC [4], as reported in Fig. 3. The same isoenzymatic pattern was observed in correspondence of the two maximum production levels, although the band intensities changed during the time course: detectable levels of POXA1B and POXA3 activity production were only found in correspondence with the first maximum of laccase production (4–5th day), while no significant difference in the relative amount of POXC isoenzyme was detected at different times of growth (4th, 5th and 13th day).
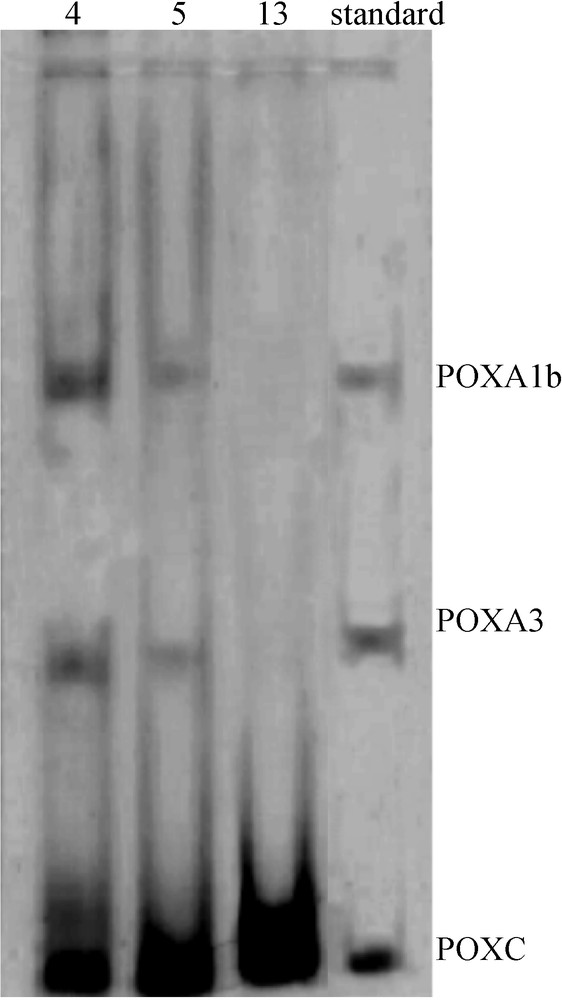
Zymograms of laccase isoenzymes in the basal condition (C1). Samples containing 0.015 U of laccase activity collected at different times (4th, 5th, and 13th day) were used.
Taking into account the whole data acquired during the time course analysis, secondary metabolism activated in the idiophase could be associated to the second maximum of laccase production, although a cause-effect correlation cannot be formulated at this stage. It has been previously reported that P. ostreatus produce several natural compounds during fermentation in submerged culture during the idiophase [29,30]. The reasons why fungi produce secondary metabolites are still unknown and most of these molecules have not been credited with a biological role [30]. Although no comparative analyses about dynamic variation of exo-metabolites during fermentation in submerged culture was performed, it is evident that under conditions of nutrient limitation, morphological alterations and mycelium changes variations in secondary metabolism dynamically occur. Moreover, many evidences strongly indicate that Pleurotus spp. displays the ability to synthesize lignin-related compounds [31,32]. These extracellular metabolites could regulate laccase expression similarly to other chemical-related inducers [32], as ferulic acid [33].
3.2 Increasing of laccase expression in Pleurotus ostreatus by spent medium solution
In order to investigate the above-mentioned hypothesis, metabolites excreted by P. ostreatus were extracted from the fungal fermented broth, starting from the beginning of the idiophase (7th day). Spent media solution derived from the culture broth at 7, 10, 13, and 16 days (SM7, SM10, SM13 and SM16, respectively) were used to condition liquid fermentation of P. ostreatus grown in basal condition (C1).
Analyses were performed monitoring biomass growth, total secreted protein and laccase production profiles after adding spent medium solutions at the inoculation time (t = 0 days). Conditioning growth media by SM10, SM13 and SM16 caused an increase of laccase activity up to 5 folds in correspondence of the first peak at the 4th and the 5th day (Fig. 4), whereas no induction of laccase expression was induced at the 13th day. No significant variation of the other parameters was observed (p < 0.005).

Laccase activity per litre of culture in Pleurotus ostreatus growth medium (C1) in absence (line A) and in the presence of SM7 (line B), SM10 (line C), SM13 (line D) and SM16 (line E).
No relevant difference in the enzymatic pattern of samples collected at 4th, 5th and 13th day, from basal and conditioned growths was observed. Such data indicate that the presence of spent medium solutions in liquid culture affects general mechanisms of laccase expression and/or secretion and that the increase of laccase activity at the 4th and the 5th day does not depend on the over-expression of a specific isoenzyme. This behaviour does not correspond to the laccase expression trend observed in the basal growth during the second phase of enzyme production (late part of the idiophase). As a fact, activity dramatically decreases after the 13th day. After the 13th day, when the last phase of carbon starvation stress takes place, different mechanisms could deactivate/repress any biological synthesis, including laccase expression. As a fact, quiescence or lyses of hyphae could cause an insensitivity of the fungal cells to the presence of any inducer.
Moreover, conditioned fungal cultures result not responsive to the induction during the idiophase, while a laccase production increase during the trophophase occurs. Probably, chemicals contained in the spent medium solutions, that were supplemented at the time of inoculation, were metabolized or degraded by the fungal enzymatic activities.
3.3 Increasing of laccase expression in Pleurotus ostreatus by classical breeding
The breeding strategies of new varieties of industrially useful fungi like P. ostreatus are defined by the breeding objectives and the legal constraints imposed to the breeding technology used. This last aspect is of the greatest importance in the case of wild-type microorganisms which are considered edible, GRAS and eco-compatibles. In the framework of local and international legislations, in fact, the use of genetically modified microorganisms (GMMs) in industrial bioprocesses could increase the waste disposable cost and preclude the potential conversion of biomass in bioproducts as animal fodder (European Communities Guidance notes for risk assessment outlined in annex 3 of council directive 90/219/EEC on the contained use of genetically modified microorganisms). This prevents the use of genetic-engineering based technologies for breeding. Consequently, our work was focused on classic breeding, in order to evaluate the capability of this technique to improve safe strains for laccase production. As reported in literature [34], heterozygosity in genes responsible for laccase expression triggers high variability of enzyme production and increases average production in basidiospores derived monokaryons obtained from a single dikaryotic strain. In fact, certain monokaryotic isolates produce much higher titres of enzymes than the parental strain.
P. ostreatus var. florida (strain F) and P. ostreatus var. ostreatus (strain O) are the dikaryotic fungi used in the present work. These varieties differ in several morphological and physiological features like size, colour, temperature tolerance, etc. Both strains have been extensively characterized in previous studies for their ability to produce oxidative enzymes [4,35,36], hydrophobins [37,38], and natural compounds [29]. In order to produce new dikaryotic hybrids with increased production capabilities, basidiospores-derived monokaryons, obtained from both strains, were isolated and analysed. Collected spores from the two different basidiocarps were successfully germinated in solid medium. Microcolonies progeny was microscopically analyzed and monokaryotic state was confirmed by the absence of mycelial clamp connections.
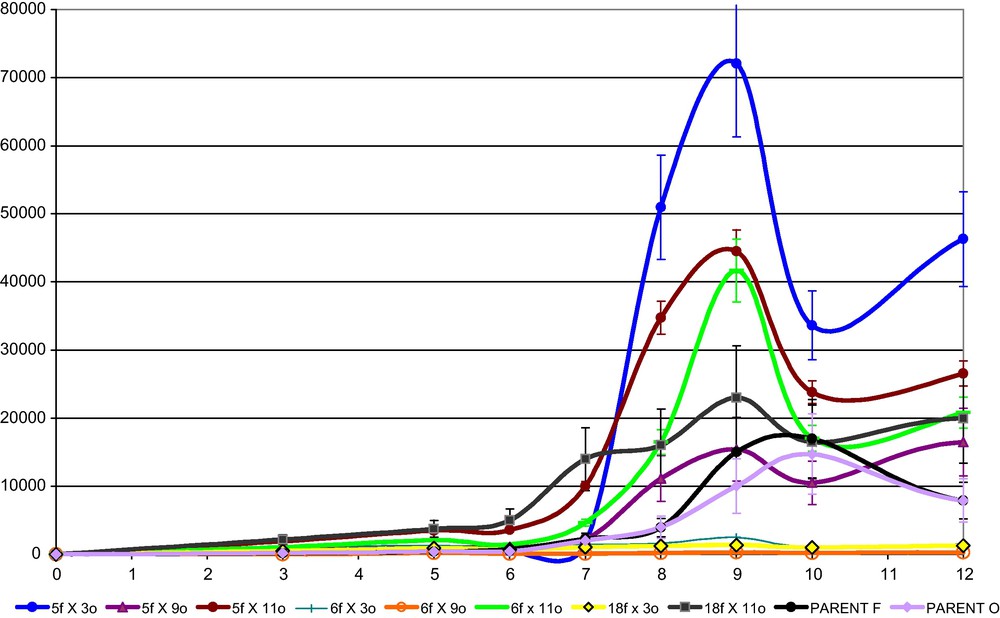
Monokaryotic strains “f” and “o”, isolated among twenty-eight randomly chosen germinating spores, were inoculated in the PDY production medium (C2) in order to enhance laccase production during fungal fermentation. Time courses analysis of extracellular laccase activities showed high variability among the strains and a maximum of production between the 9th and the 10th day of growth (Fig. 5). Three monokaryons from the parental strain F (5f, 6f, 18f) and three monokaryons from the parental strain O (3o, 9o, 11o) exhibit higher or comparable production levels than the two parental strains. As also reported by Eichlerov́a and Homolka [34], the isolates differ also in morphology and growth rate when subcultured on agar media in Petri dishes (data not shown). However, colony appearance, whose morphological patterns are transient, could not be connected with laccase production levels.

Time course of laccase activity secreted by monokaryotic strains “f” and “o” in PDY medium (C2). Enzymatic activity of parental varieties F and O are also reported. Standard deviation < 20%.
Anastomosis induction, followed by formation of clamp connections, indicated compatibility among two strains and the formation of the corresponding dikaryon. All compatible crossings, among the six monokaryotic strains were performed. Crossing selected strains, new dikaryotic hybrids were obtained, excepting for the pairs coming from the same parent (f X f and o X o) and the crossing 5f X 9o, that resulted incompatible (Table 1). These dikaryons were studied for their laccase production yields when grown in production medium (C2). Four hybrids reached laccase expression levels higher than those of compared to the related monokaryons (Fig. 6) up to 70,000 U/L.
Mating taste between selected monokaryons derived from the parent strain F (5f, 6f and 18f) and O (3o, 9o and 11o).
| 5f | 6f | 18f | 3o | 9o | 11o | |
| 5f | – | – | – | + | + | + |
| 6f | – | – | – | + | – | + |
| 18f | – | – | – | + | + | + |
| 3o | + | + | + | – | – | – |
| 9o | + | – | + | – | – | – |
| 11o | + | + | + | – | – | – |
Laccase activity of Pleurotus ostreatus selected hybrids in PDY culture (C2) after 12 days of growth.
3.4 Laccase induction by new dikaryotic hybrids through spent medium solution
As mentioned above, because laccase production is regulated by multifactorial and mutiallelic expression systems which are dependent on extra- and intracellular regulations [4,39], the effect of SM16 on the partially characterized dikaryotic hybrids was tested. Results indicate that hybrids grown submerged cultures could differentially respond to the autoinduction mechanisms, increasing enzymatic production. As a fact, addition of the inducer solution to the culture broth (C2) at the time of the inoculation increases laccase production levels, for all but one of the selected hybrids, up to 3 times (Fig. 7). Surprisingly, the best laccase producer among the selected hybrids results insensitive to the presence of the inducer. The insensitive strain seems therefore to be deregulated.
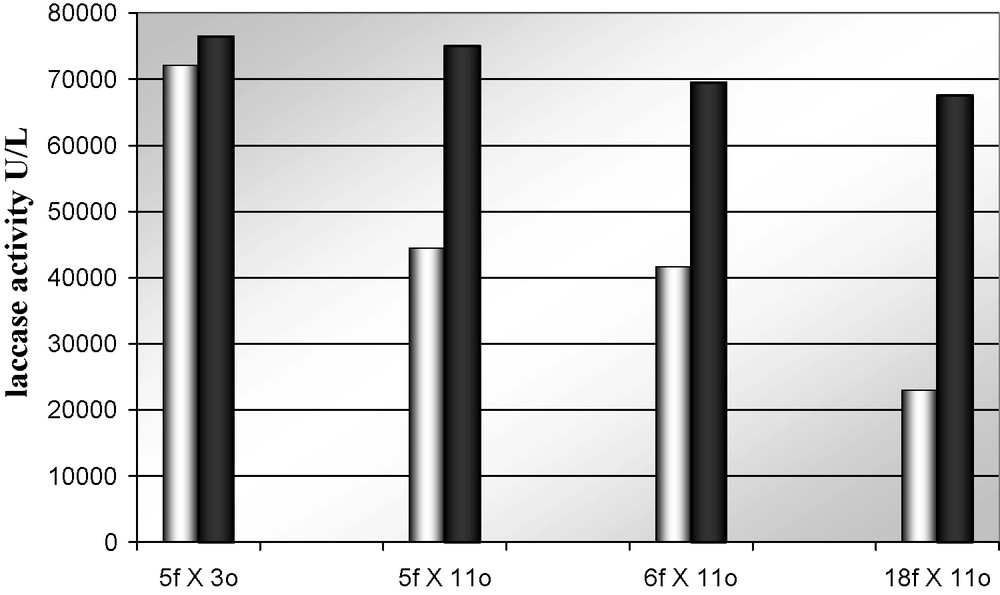
Maximum laccase activity secreted by Pleurotus ostreatus selected hybrids in PDY medium (C2) in absence (light) and in presence (dark) of SM16 laccase inducer. Standard deviation < 20%.
This study has allowed us to get new insights in improving fungal laccases production: classical breeding and autoinduction mechanisms. These represent promising tools for the improvement of fungal fermentation without affecting waste disposable cost that also depend on the safe and ecocompatibility of the whole process.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
This work was supported by the European Commission, Sixth Framework Program (QUORUM, 032811), by grants from the Ministero dell’Università e della Ricerca Scientifica (Progetti di Rilevante Interesse Nazionale, PRIN), from Compagnia di San Paolo, Turin, Italy, project “Sviluppo di procedure di biorisanamento di reflui industriali (BIOFORM)”, from COST Action FP0602 “Biotechnology for lignocellulose biorefineries” (BIOBIO), and from the Ministero Degli Affari degli Esteri di Intesa con il Ministero dell’Università e della Ricerca (Progetti di ricerca di base e tecnologica approvati nei protocolli di cooperazione scientifica e tecnologica bilaterale come previsto dal protocollo bilaterale tra Italia e Turchia).


