1 Introduction
The phenotypic wild animal polymorphism reflects an adaptation capacity of populations as a result of selection pressure and provides species stability. Also, a polymorphism could be considered as a mechanism regulating the “population-environment” system and maintaining genetic variation [1]. However, the understanding of the adaptive significance of most of these polymorphisms is still limited.
The fluctuating asymmetry (FA) of morphological traits is known as an indicator of both environmental and genomic stress, including pollution, toxins, parasites, inbreeding, directional population selection, deleterious gene combinations, and chromosome balance alteration [2–5]. FA is estimated as a random deviation from the perfect symmetry of bilateral traits. Symmetric parts of bilateral traits are controlled by the same group of genes [6,7]. Also, the formation of the first left–right asymmetry and of both anterior–posterior and dorsal–ventral axes in Xenopus laevis L. and zebra-fish embryos is initiated in the early embryo [8,9] and involve the expression of the transcription factors controlling cell type specification and formation of tissue and organs [10,11]. Thus, both sides share the same environment during the development study, and therefore a perfectly symmetrical phenotype can be expected. So, FA may be used as a marker of an individual developmental instability in humans and animals [7,12,13].
The genotype developmental stability (DS) is viewed as an index of adaptation and coadaptation and is considered as a result of the genetic complexes equilibrium gotten during species (population) evolution [12,13]. The karyotype of some individuals of Rana temporaria L. has a supernumerary (B) chromosome [14,15]. The Bs are known to promote chiasma frequency during meiosis, to increase recombination and to cause a breakup of the coadapted gene complex [16]. Thus, a genotypic disequilibrium due to the Bs is expected to decrease in developmental homeostasis.
The vast majority of brown frogs display the dorsal V-shaped spotting and a large variety of pigment pattern across the populations [17–20]. Generally, most quantitative traits are polygenic and characterized by a persistent variability [1] that limits the selection of proper phenes for both population structure estimation and asymmetry analysis. Since a phene shape is axis associated, as well as FA evaluation in the population is usually to be much more useful and reliable than individual ones [6], so we consider it possible to use it as an index of population genetic structure and developmental stability.
In the present paper, the possible significance of B chromosome as one of the factors disturbing DS, and its relation with the V-shaped spot asymmetry pattern occurrence in populations of R. temporaria L. are examined. Such an insight may be valuable for the design of future more thorough studies.
2 Materials and methods
2.1 Sampling
One hundred and nineteen adult frogs R. temporaria L. have been collected from seven localities in Belarus during the breeding season from 1986 to 2002 (Fig. 1). The studied species has an extensive distribution in this area and usually inhabits meadows and flood plain of streams close to black alder or mixed coniferous forests. Four sites were radiocontaminated due to the Chernobyl accident in 1986 [21]:
- • site 1: lakeside zone of the Zaslavl Reservoir near v. Ratomka, about 15 km to the north of Minsk (53° 58’ N 27° 20’ E);
- • site 2: Rybtsy, Minsk reg., flood plain of the Svisloch stream (53° 39’ N 27° 48’ E);
- • site 3: mixed forest border and drained peat swamp, westward of Ivenets town, Minsk reg. (53° 54’ N 26° 40’ E); 137Cs–370 kBq/m2; 90Sr < 5 kBq/m2;
- • site 4: v. Veprin, Mogilev reg. the watershed plateau (53° 58’ N 27° 01’ E); 137Cs–1202,5 kBq/m2; 90Sr–10,0–55,5 kBq/m2;
- • site 5: t. Cherikov, Mogilev reg. the flood plain on the left bank of the Sozh River (53° 33’ N 31° 23’ E); 137Cs–177,6 kBq/m2; 90Sr–3,7 kBq/m2;
- • site 6: v. Paluzh, Mogilev reg. (53° 20’ N 30° 32’ E); 137Cs–1554 kBq/m2; 90Sr–44,4 kBq/m2;
- • site 7: Oreshkovichi, Minsk reg., meadow of the right bank of Svisloch stream (53° 23’ N 28° 22’ E).
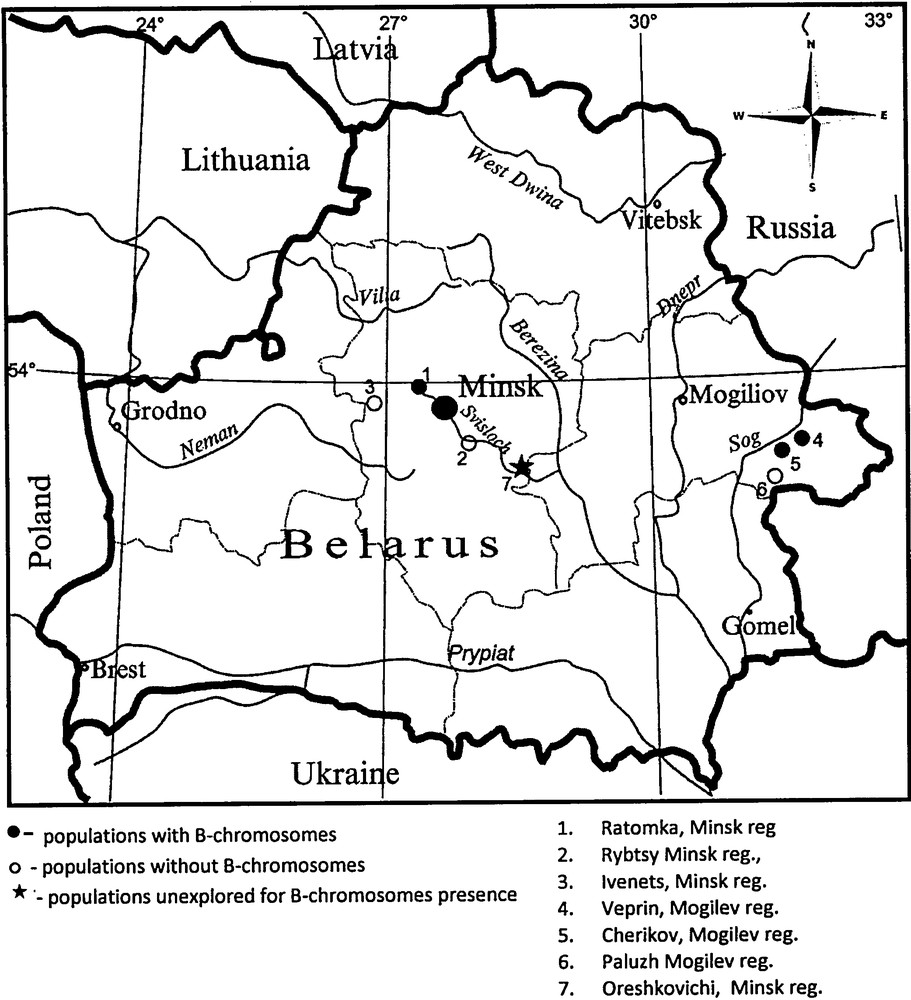
Sampling localities and B-chromosome occurrence in R. temporaria populations.
The live animals were transported to a laboratory, where they were maintained no more than three days.
2.2 Cytogenetic analysis
Chromosome spreads were obtained from direct cytological preparations from bone marrow, according to Schmid [14], with adaptations. Animals received an intraperitoneal injection with 0,3% colchicine (0,1 ml per 1 cm of body length) for 4 h before euthanasia. The bone marrow cell suspension was treated with KCl (0,56%) fixed in methanol:glacial acetic acid (3:1) and dropped on degreased slides at 0° С. The flame-dried slides were stained by Giemsa (Merck).
The occurrence of B-chromosomes at the mitotic metaphase was analysed. A minimum of 30 animals per population and 30 metaphases per animal were examined.
2.3 Phenotypic analysis
All individuals were examined for the presence of V-shaped spotting and its structure; only live animals were used (Fig. 2). V-shaped spotting was estimated by the form and number of elements according to R. Novitsky [20]. The pattern composed of one element at both sides of the axis or of one continuous element was considered as a simple V-shaped spot (Fig. 3A). The asymmetry was estimated as the difference (up to 50%) in size of components on both sides of the axis (Fig. 3B).

V-shaped and spotting patterns.

V-shaped pattern forms. A. Simple forms. B. Complex forms.
The regular V-shaped spot was represented predominantly by dark grey or black patterns. V-shaped spotting variety assumes persistent trait variability. The more complex variants may consist of a greater number of pigmented elements, including those superimposed on the background. Sometimes they are completely absent. Such disappearance of quantitative traits is observed at a breakdown of coadapted gene complexes [1].
2.4 Statistical analysis
To compare differences between populations where different V-shaped spotting patterns occur, a Chi-square test by Yates correction at P = 0.05 was used [22].
3 Results
Animals from six natural Belarus populations except for those collected in site 7 have been examined for the presence of B-chromosome. The karyotype of R. temporaria L. has 26 chromosomes. A supernumerary chromosome (Fig. 4) was found in three populations from sites 1, 4, and 5. The maximal Bs frequency was up to 25% and was observed in site 4. The results are presented in Table 1. Intra- and interindividual mosaicisms for Bs were observed in all populations, and the maximum number of Bs per cell ran to seven [23]. Observations of B-chromosome dynamic in the population of Cherikov (site 5) over a four-year period have shown an increase in Bs frequency from 15% to 23.5% [23,24].
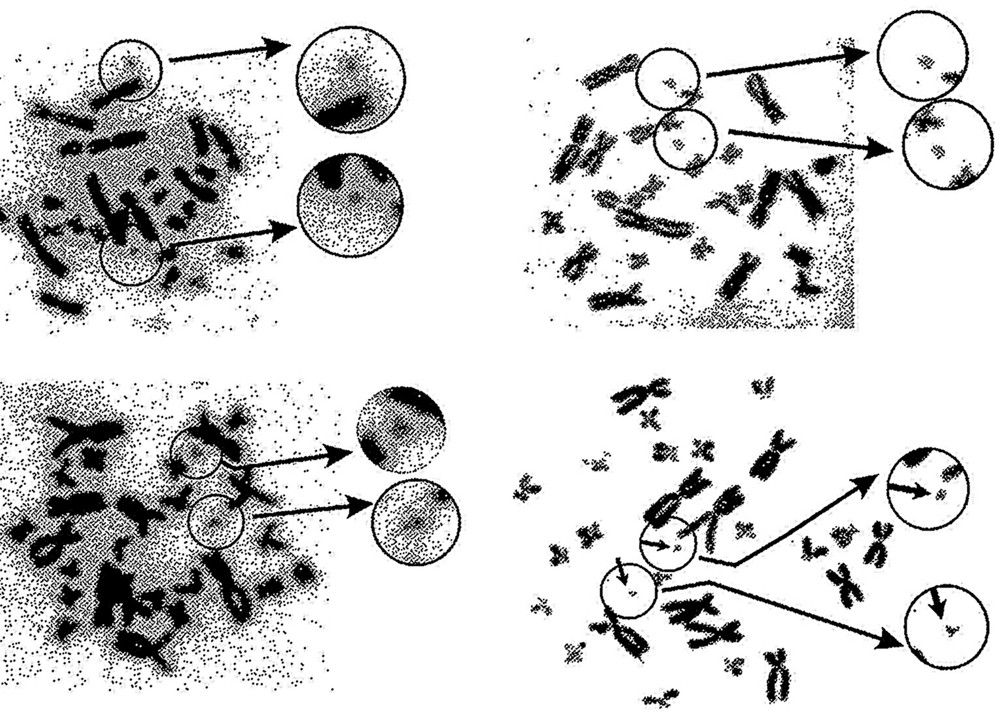
B-chromosomes in R. temporaria karyotype: 2n = 26 + 2.
Occurrence of B-chromosomes and V-shaped spots pattern variants in R. temporaria populations.
| Population | The total number of analysed frogs | B-chromosome frequency (%) | V-shaped patterns | |
| 1 element (simple form) | 2 and more elements (complex form) | |||
| 1 Ratomkaa | 101 | 17,27 | 53 | 48 |
| 2 Rybtsy | 61 | – | 45 | 16 |
| 3 Ivenets | 190 | – | 79 | 111 |
| 4 Veprina | 170 | 25 | 121 | 49 |
| 5 Cherikova | 264 | 14,95 | 188 | 76 |
| 6 Paluzh | 40 | – | 30 | 10 |
| 7 Oreshkovichi | 193 | – | 90 | 103 |
a Bs carrier populations.
Across all studied populations, a phenotypic structure of R. temporaria represents a considerable variation; V- shaped pattern structure, spot distribution and pigmentation vary and did not depend on the presence of B-carriers. The results are summarized in Table 1.
In spite of the variety in the phenotypic structure, populations 4, 5 and 6 as well as 1 and 7 have not shown significant differences in spotting frequency and distribution (Table 2). However, the population of site 2 differed considerably from those located up and down Svisloch river (site 1 and site 7), but did not differ by all the parameters from the remote population inhabiting site 6 (Table 2). This can be explained by the fact that the populations from sites 1 and 7 and those from sites 4, 5 and 6 belong to two separate geographical populations, respectively. Moreover, the animals from sites 4 and 5 hibernate at the same stream. The animals from site 3 represented an asymmetric V-shaped spotting similar to that of frogs from site 1, situated at a distance of about 60–70 km, which allows us to suppose that animals from sites 1, 2, 3, and 7 are primordially of the same geographical population, resulting in two different ones through isolation. However, the population of site 2 differed considerably from those located up and down Svisloch river (site 1 and site 7), but differed in no way from the remote population inhabiting site 6 (Table 2).
Significance of differences between populations χ2(P) with Yates correction; in the left lower part of the table, simple form patterns, in the right upper part, complex form patterns.
| Site | 1a | 2 | 3 | 4a | 5a | 6 | 7 |
| 1a | 6.35 (0.011) | 2.7 (0.098) | 8.58 (0.0029) | 10.6 (0.0011) | – | – | |
| 2 | – | 17.8 (0.000) | – | – | – | 12.6 (0.0004) | |
| 3 | – | 17.8 (0.000) | – | – | 13.5 (0.0002) | 1 (0.37) | |
| 4a | 8.58 (0.0029) | 0.05(0.8) | 30.6 (0.000) | 0.01 (0.9) | – | – | |
| 5a | 10.6 (0.0011) | 0.06 (0.8) | 38.84 (0.000) | 0.01 (0.91) | – | – | |
| 6 | 5.11 (0.0238) | 0.01 (0.9) | – | 0.08 (0.77) | 0.09 (0.758) | – | |
| 7 | 0.7 (0.4) | – | 1 (0.37) | 21.3 (0.000) | 27.2 (0.000) | 9.57 (0.002) |
a Bs carrier populations.
Then, interpopulational differences in spotting (Table 3) were observed; adjacent populations have demonstrated different spot pattern compositions and a large variety in the spot's shape and structure. Moreover, a positive correlation between the presence of B-carriers in the populations of sites 1, 4 and 5 and a predominance of asymmetric V-shaped spotting have been noted (Table 3). Also, the symmetric patterns’ distribution in most of the populations (in spite of the presence or the absence of Bs) is for a simple form (Table 3) benefit; the last one (predominantly with a grey pattern) consists of one element on the each side along the symmetry axis. Unfortunately, in some cases the sampling on such patterns is insufficient for comparison by χ2. Also, variable simple forms patterns predominate in the B-carrier populations (sites 1, 4, 5). The patterns consisting of a large number of pigmented elements were prevalent among asymmetric samples (Tables 3 and 5). It is interesting that in the population from site 5, presented by the most numerous sampling, almost all B-carriers (10 out of 12) manifested a simple symmetric V-shaped spot (Fig. 3).
Symmetry and asymmetry V-shape spot pattern frequencies in R. temporaria populations.
| Population | Symmetry | Asymmetry | ||||
| Structure (number of elements) | ||||||
| Total | 1 element (simple form) | 2 and more elements (complex form) | Total | 1 element (simple form) | 2 and more elements (complex form) | |
| 1. Ratomkaa | 40 (39.6%) | 34 | 6 | 61 (60.4%) | 19 | 42 |
| 2. Rybtsy | 45 (73.8%) | 39 | 6 | 16 (26.2%) | 6 | 10 |
| Ivenets | 91 (47.9%) | 49 | 42 | 99 (52.1%) | 30 | 69 |
| 4. Veprina | 66 (38.8%) | 57 | 9 | 104 (61.2%) | 64 | 40 |
| 5. Cherikova | 108 (40.9%) | 106 | 2 | 156 (59.1%) | 82 | 74 |
| 6. Paluzh | 29 (72.5%) | 28 | 1 | 11 (27.5%) | 2 | 9 |
| 7. Oreshkovichi | 89 (46.1%) | 59 | 30 | 104 (53.9%) | 31 | 73 |
a Bs carrier populations.
Significance of the differences in the number of elements of V-shape spot asymmetry between populations (χ2(P) value with Yates correction).
| Sites | 1 | 2 | 3 | 4 | 5 | 6 |
| 1 | ||||||
| 2 | 16.4 (0.0000) | |||||
| 3 | 1.5 (0.21) | 11.4 (0.0007 | ||||
| 4 | 0.00 (0.98) | 20.6 (0.000) | 2.6 (0.1) | |||
| 5 | 0.01 (0.91) | 20.1 (0.000) | 1.9 (0.16) | 0.1 (0.73) | ||
| 6 | 11.1 (0.0009) | 0.1 (0.9) | 7.0 (0.008) | 8.0 (0.045) | 11.8 (0.0006) | |
| 7 | 0.89 (0.34) | 14.3 (0.0003) | 0.06 (0.8) | 1.68 (0.19) | 1.03 (0.31) | 8.2 (0.0042) |
4 Discussion
We assume that the large variability in the phenotypic structure is caused rather by the evolution factors and the environmental impact than by geographic isolation. Merrell [25] has supposed the dorsal pigmentation and spotting polymorphisms in wild population of Rana pipiens to be a complex trait influenced by both genetic factors and environmental effect. Also, colour polymorphism in the populations of strawberry poison frog, Dendrobates pumilio, is rather strongly associated with the genetic distance than with geographic isolation [26].
The studied populations inhabiting sites 3, 4 and 5 and displaying V-shaped asymmetric patterns prevalence were exposed to radiocontamination [21]. As we know, stressful factors are suggested to influence recombination rate and developmental homeostasis [27]. Thus, a higher level of FA was documented in populations of mice A. flavicolis, A. agrarius and frogs Rana esculenta complex subjected to anthropogenic impacts such as chemical and radiation pollutions [28–30]. In addition, an increase in FA was reported for a stickleback (Pungitius pungitius) population living in stressful environments (high predation, isolation, and loss of genetic variation) [31]. This is compatible with our present results.
B-chromosome was shown to disturb development stability and to be correlated with deviations in the number of asymmetrical characters in yellow-necked mouse Apodemus flavicollis [32,33]. Also, an increase in a recombinant progeny issued from B-bearing parents as a result of the effects of Bs on chiasma frequency in paternal A-chromosomes has been observed in Eyprepocnemis plorans and A. Flavicollis [34,35]. As demonstrated by the analysis of grasshopper populations of the South American species Dichroplus elongates [36] and of the Moroccan species E. plorans [37], there is a close relationship between B-accumulation and chiasma occurrence; the more B-chromosome drive among the populations, the more intensive is the increase in chiasma frequency. Unfortunately, we do not know how long B-chromosomes have persisted in a frog's genome at a moment of our study: could they drive through generations or into the populations without B-carriers, or could they be eliminated from populations. However, our observations in site 5 for a four-year period showed that Bs frequency increases from 15% to 23.5% [24].
Also, it is known that the intensity of the effect of the B-chromosome depends on the evolutionary status of its polymorphism: B-systems variation and Bs drive were shown in different populations of the different species [38,39]. For instance, the fast spread (for three to four generations) of Bs into the population of wasp Trypoxylon albitarse was observed [40]. A substitution of one B type by another one [41], and a selective drive without accumulation through females for only one of the B-chromosome types but not for the other ones have been documented in the grasshopper E. plorans [42].
On the one hand, the Bs should be eliminated by natural selection because they are dispensable in nature and often are parasitic: they entail an additional stress to the host genome whose response is favoured by selection pressure. Thus, a new B type differing in only one duplication has resulted in the female fertility decrease in E. plorans, that points to selection against B-chromosome accumulation in the genome [43].
However, Bs may have beneficial (heterotic) effects on their hosts and could be maintained in the population without drive [43,44]. So, to avoid elimination through random genetic drift or natural selection, Bs require special combinations of evolutionary forces promoting them in populations [43]. Also, it has been shown that Bs of A. flavicollis are beneficial for their carriers under the more extreme climatic conditions [45,46]. Additionally, the Bs occurrence and their accumulation have been reported in the R. temporaria populations inhabiting a mountain region in Bulgaria [47]. Thus, the accumulation of Bs in the population of Cherikov inhabiting a radio-contaminated area that is also the highest place of this region (100–150 m) corroborates their heterotic role. Therefore, the maintenance of Bs in the populations can signify their contribution to the genetic diversity of species and their selective advantage in changeable environmental conditions.
Then, simple symmetric V-shaped spot patterns present in almost all the B-carriers let us assume that Bs contributes to the genetic variability rather than disturbs a development stability of the B-carriers. It is known that a fluctuating asymmetry is less sensitive than an adaptive modification and it indicates more severe stress. FA increases when a stress has exceeded the homeostatic abilities of an organism. Consequently, if FA remains unchanged in the face of a stressor, it may simply mean that developmental homeostasis was not disturbed [7]. Thus, the predominance of asymmetric patterns in the population could indicate that a population has been subjected to some stressors. At the same time, the prevalence of simple forms and the presence of symmetric patterns in B-carriers of the same population let us suppose that B-chromosome could be considered as of adaptive significance and to make its host resist stress.
Perhaps, a genome balance that is manifested in V-shaped spot pattern symmetry seems to allow B-chromosome to be maintained in the genome, but any genotypic disequilibrium would result in the elimination of Bs animals from the populations at an early ontogenesis stage. Thus, it was found that young animals of A. flavicolis with Bs were preferentially eliminated from the population under stress conditions due to overcrowding [48]. It is known that the presence of B-chromosomes disturbs cell cycle dynamics and causes delays at S and G2, due to the checking of additional genetic material [49]. So, it is possible that the presence of Bs cause coadapted gene complexes disturbance and affects early embryonic and postnatal development stages, including the earliest stages of mesoderm and symmetry axis formation. It might lead to an asymmetry pattern appearance or even the elimination of young B-carriers animals from the population. Additionally, most of the analysed animals inhabiting the contaminated territories had been born before the Chernobyl accident.
5 Conclusions
The analysis of the dorsal V-shaped spot pattern asymmetry in brown frog populations implies that the persistence of additional chromosomes in populations may have different effects on developmental stability in different populations under different environmental conditions. Also, the obtained results suggest that the presence of B-chromosome may be one of the factors disturbing the developmental stability of some individuals. However, it should be taken into account that Bs may confer some selective advantages to their carriers that makes them react differently to environmental changes from non-carriers. Nevertheless, the causative role of Bs remains to be examined. Such an approach can present an additional tool to a battery of available applications for population biomonitoring.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.



Vous devez vous connecter pour continuer.
S'authentifier