1 Introduction
Plants, including potato, respond to environmental stresses with an array of biochemical and physiological adaptations, which involve complex multiple signaling pathways, genes and gene products. Regulating the expression of stress-related genes is one of the most effective regulatory pathways for plants to adapt to adverse environments [1]. A mass of genes has been reported that allow plants to tolerate and overcome unfavorable circumstances [2–5].
In plant genomes, approximately 7% of the coding sequences are assigned to transcription factors (TFs) [6], and many of them are immediate-early and abiotic stress-responsive genes [7], which have been known to play crucial roles in response to important abiotic stress factors, including drought, high salinity, high osmolarity, extreme temperature, and phytohormone [8,9].
Sakuma et al. [10] classified AP2/ERF transcription factors into five subfamilies: AP2 (APETALA2), RAV (related to ABI3/VP1), DREB (dehydration-responsive-element binding protein), ERF (ethylene-responsive factor), and others, according to the number and similarity of the DNA-binding domains (DBD). ERFs play significant roles in the regulation of abiotic- and biotic-stress responses. ERE (ethylene-responsive element) binding factor (ERF) proteins (formerly known as ERE binding proteins [EREBPs]) were isolated as GCC box binding proteins from tobacco (Nicotiana tabacum) [11], and their expression pattern was investigated in detail [12]. ERE binding proteins contain a highly conserved DNA binding domain (designated as the ERF domain) [13] consisting of 58 or 59 amino acids [11]. The AP2 domain (ERF domain) was centered on the base amino acid sequence AAEIRD**RR*R*WLGT*DTAEEAA where the underlined WLG amino acids were diametrically required for AP2/ERF domain binding to DNA and the * represented non-specifically conserved amino acids [10,14]. Pti4 was an ERF transcription factor that was first isolated by its interaction with the kinase Pto, which conferred resistance to Pseudomonas syringae pv. tomato expressing the avirulence gene AvrPto [15].
GCC box widely exists in a large number of gene promoters. ERF can interact with the GCC box in the promoter of genes and activate downstream gene expression. The analysis of expression profile in the transgenic plants with ERF104 gene overexpression evidenced that the expression level of 534 genes raised more than 3-fold changes with the strongest induction (∼1000 fold) for two PDF1.2 genes, and the 1-kb upstream regions of genes up-regulated > 10-fold were enriched in GCC elements [16]. Moreover, the ERF proteins can regulate the biosynthesis of metabolites, such as wax [17], ethylene [18], jasmonate [19], nicotine [20], and gibberellin [21,22]. The result from overexpressed AtERF98 in a Col-0 background plants in Arabidopsis indicated that AtERF98 played an important role in regulating the biosynthesis of ascorbic acid [23].
At present, some ERF genes in plants have been profiled; however, their function was not well explored. In this study, five StERF genes (ERF1-4 and Pti4) in potato were completely identified and characterized, which suggested that StERFs played important roles in potato.
2 Materials and methods
2.1 Plant materials and growth conditions
Potato (Solanum tuberosum L.) cultivar ‘Zihuabai’ was used in the experiment. Plants were grown in in vitro culture in a glass bottle (7 cm diameter and 9 cm height) at (21 ± 2) °C under a 16/8 h light/dark photoperiod on 50 ml MS media containing 3% (w/v) sucrose
2.2 Phylogenetic analysis
Full-length sequences of five StERF genes were originally retrieved from NCBI GenBank (http://www.ncbi.nlm.nih.gov/genbank), and the GenBank accession No. as follows: StERF1 (JN125860), StERF2 (JN711505), StERF3 (JN125857), StERF4 (JN125859) and Pti4 (EU851735). Protein sequences were obtained from potato genome resource (http://potato.plantbiology.msu.edu/index.shtml). Arabidopsis ERF genes examined herein were designated AtERF1-5 and were previously noted by Nakano et al. [14]. The phylogenetic tree was generated by ClustalX2 using default parameters of the neighbor-joining method in MEGA (version 5.0). The StERFs subcellular localization was analyzed using PSORT (http://www.psort.org/). The StERFs signal peptide (SP) was analyzed using Signal P4.1 Serve (http://www.cbs.dtu.dk/services/SignalP/). The domain of the transcription factor was analyzed using the Pfam online data (http://pfam.xfam.org/family/).
2.3 Hormone and stress treatments
For plant hormone treatment, four-leaf-stage plants were grown in solid MS media, then the plants were sprayed with 100 μM MeJA and 100 μM ethephon (5 ml), respectively, and the control was sprayed with distilled water. For abiotic stress, four-leaf-stage plants were grown in liquid MS media as described above, then the liquid media were removed followed by adding 50 ml of liquid MS media containing 200 mM NaCl and 20% PEG6000, respectively, and by adding distilled water as the control. Plant leaves were collected after treatment (1 h, 2 h, 6 h and 12 h) and immediately frozen in liquid nitrogen, then stored at –80 °C. Every experiment was repeated three times.
2.4 RNA isolation, cDNA synthesis, and gene expression analysis
Sprouted seed tubers (about 100 g weight) of potato cultivar ‘Zihuabai’ were planted in pots with three replicates in greenhouse under natural light conditions in Gansu Agricultural University. Leaves, stems, flowers and roots were harvested from 38-day potato plants, and tuber from 60-day potato plants, and then stored at –80 °C until use. Total RNA was isolated using Trizol Reagent (Invitrogen, #15596026) according to the manufacturer's instructions and digested with DNase I (TaKaRa, #2270A). Five micrograms of total RNA were used for cDNA first strand synthesis according to the manufacturer's instructions of One Step PrimeScript® cDNA Synthesis Kit (TaKaRa). The default cycling conditions (3 min at 94 °C and 35 cycles of 94 °C for 30 s, 55 °C for 1 min, 72 °C for 1 min and 10 min final extension at 72 °C) were performed using T100TM Thermal Cycler, BIO-RAD PCR System. The PCR products were electrophoresed on a 1.0% agarose gel. The StERF-specific primers used in RT-PCR were listed in Table 1. All samples were compared with the endogenous reference gene ef1a.
Gene-specific primers for RT-PCR and qRT-PCR.
| Gene | Primers for RT-PCR | Primers for qRT-PCR | |
| StERF1 | F | ATGCCCGTCCGGAAAAAT | GGGAAGGCCATAGTGATTGT |
| R | CTAGAAACACAAAGGGGCAAT | CAGATGGAGGTGGAAAGTTCA | |
| StERF2 | F | ATGCGGAGAGGTAGAGCAGC | CAAAGCATCAACCCTAACGA |
| R | TCACCGTTCCAACGACAG | CCACTGAAAGACTCCACCGTA | |
| StERF3 | F | ATGGATTCTTCTTCACTAGATATGATA | CGGAGATTCGTGATCCAACT |
| R | TCATTCGACCATTTCTCCTCT | TGAGCCTAAATGCTGCTCTG | |
| StERF4 | F | ATGAGAAGAGGCAGAGCAACTC | AGAAGAGGCAGAGCAACTCC |
| R | TCAAAGACATAGTGCTGTGCAG | TTCCTAACTCCACGAAACCTAATC | |
| Pti4 | F | ATGGATCAACAGTTACCACCG | CTCTTAGCGTCGGATGGTC |
| R | TTAAATGACCAATAGTTGATGGACA | TCTTCCCTTCGGTGTTTCAG | |
| ef1a | F | CAAGGATGACCCAGCCAAG | CAAGGATGACCCAGCCAAG |
| R | TTCCTTACCTGAACGCCTGT | TTCCTTACCTGAACGCCTGT |
For expression analysis of StERF genes under stress treatments, qRT-PCR was performed in 20-μl reaction mixtures with the SYBR® Premix Ex Taq™ II Kit (TaKaRa, #DRR047A) and 10 μM of each primer. The ef1a was as an internal control gene. The gene-specific primers were listed in Table 1. Reactions were conducted on the ABI3000 System (Applied Biosystems 3000 Real-Time PCR) using the default cycling conditions (1 min at 95 °C and 40 cycles of 95 °C for 5 s, 58 °C for 34 s, 72 °C for 1 min). 2–△△Ct method was used to calculate relative expression levels. The relative expression data used in the figure represented means ± SE of three biological replicates.
For expression analysis of StERF genes under hormonal treatment, RT-PCR was used, first with 3 min at 94 °C, followed by 35 cycles of 30 s at 94 °C, 1 min at 55 °C, and 1 min at 72 °C, and a 10-min final extension at 72 °C.
2.5 Analysis of trans-activation activity for StERFs in yeast
The coding regions of StERFs were amplified by PCR with specific primers (Table 1) containing Nde I and Sma I restriction sites, corresponding to those presented in the yeast expression vector pGBKT7 (Clontech, USA), to produce pBD-StERFs. According to ClontechTM Yeast Protocols Handbook instructions, pBD-StERFs, pGBKT7 (negative control), and pGBKT7-53+pADT7-Rec2-53 (positive control) were transformed into yeast strain Y187, respectively. Selection of transformants was done on SD/-Trp media, and the trans-activation activity of each protein was evaluated according to their status of growth and the activity of X-Gal (5-bromo-4-chloro-3-indoxyl- β-d-galactopyranoside).
2.6 GCC-box binding assay for StERFs in yeast
In order to investigate whether StERF was bound to GCC-box in the promoter region of the genes, a yeast one-hybrid system was used. The full lengths of StERFs were fused to the GAL4 activation domain in the vector pADT7 digested with Nde I and Sma I to get pAD-StERFs. A 66 bp single-stranded oligonucleotide sequence (5′-AATTCAAGAGCCGCCACTAAAGAGCCGCCACTAAAGAGCCGCCACTAAAGAGCCGCCACTAGAGCT-3′) and its reverse complementary sequence (3′-GTTCTCGGCGGTGATTTCTCGGCGGTGATTTCTCGGCGGTGATTTCTCGGCGGTGATC-5′), which contained quadruple tandem repeat copies GCC-box (5′-AAGAGCCGCCACTA-3′) (GCC-box was underlined), and cohesive termini of EcoR I and Sac I were synthesized. The mGCC-box (m: mutated) (5′-AAGATCCTCCACTA-3′) was originated from the GCC-box (5′-AAGAGCCGCCACTA-3′) by replacing two G residues with T residues (underlined). The oligonucleotides were annealed as follows: each single-stranded oligonucleotide of 100 μM, mixed at a ratio of 1:1, yielding a final concentration of 50 μM each, was heated to 95 °C for 30 s, 72 °C for 2 min, 37 °C for 2 min and 25 °C for 2 min. After annealing, the double-stranded oligonucleotides were cloned into a pHis2.1 vector linearized using the restriction enzymes EcoR I and Sac I. The resulting pGCC-His2.1 construct, pmGCC-His2.1 and p53-His2.1 (control) vectors were transformed into yeast. Selection of transformants was performed on SD/-Trp media. After PCR confirmation, the minimal inhibitory concentration of 3-amino-triazo (3-AT, Clontech) for yeast was determined [24,25] and the result was 40 mM 3-AT. The pAD-StERFs and pGADT7 vectors were transformed into the yeast strain Y187 containing pGCC-His2.1 and pmGCC-His2.1, respectively. Large healthy colonies were picked and suspended in 0.9% NaCl. The optical density at 600 nm was adjusted to 0.002 (for ∼2000 cells per 100 μl), and 2 μl of cells was dotted on the SD/-Trp/-His/-Leu media with and without 3-AT to assess DNA-protein interactions. The colonies were then allowed to grow for 2–3 days at 30 °C.
3 Results
3.1 Potato StERFs are typical transcription factors
A family of five StERF genes was identified and characterized from potato (Fig. 1, Table 2). Alignment of their proteins revealed that there was high similarity in domain regions, such as the core conserved region of the AP2 domain (Fig. 1A), which was similar to that obtained in previous alignments of ERF proteins from a wide range of land plants [26]. Phylogenetic analysis based on similar domain sequences indicated that StERF2 and StERF4 have a paired relationship, suggesting an ancient duplication, as well as most StERFs had an Arabidopsis orthologue (Fig. 1B and Fig. 1C). Potato and Arabidopsis had orthologous phylogenetic protein pairs (StERF3 with AtERF5). Additionally, potato StERF (Pti4) protein was grouped with two Arabidopsis proteins (AtERF1 and AtERF2). The StERFs subcellular was preliminary located by PSORT (http://www.psort.org/) and the result showed that StERF1-3 and Pti4 were most likely located in the nucleus with possible theoretical probability. StERF4 was located most likely in the chloroplast and then in the nucleus.

(Color online.) StERF alignment and phylogenic relationships. (A) Protein sequence alignment of the AP2 DNA-binding for StERFs was shown with a sequence consensus. (B) Neighbor-Joining tree of StERF proteins based on alignment of the AP2 domain with support values shown out of 1000 bootstrap replicates. (C) Neighbor-Joining tree of StERF and Arabidopsis ERF (AtERF) proteins based on alignment of the AP2 DNA-binding domain with support values shown out of 1000 bootstrap replicates. (D) Full-length of protein sequence for alignment. Masquer
(Color online.) StERF alignment and phylogenic relationships. (A) Protein sequence alignment of the AP2 DNA-binding for StERFs was shown with a sequence consensus. (B) Neighbor-Joining tree of StERF proteins based on alignment of the AP2 domain with support values shown ... Lire la suite
StERF gene description.
| Gene name | Chromosome/position (Build v4.03) |
Gene model | Size (amino/acids/bp) |
| StERF1 | chr12(6424671∼6425243) | Sotub12g011770.1.1 | 190/573 |
| StERF2 | chr07(55079184∼55080640) | Sotub07g028960.1.1 | 240/1457 |
| StERF3 | chr05(47308711∼47310174) | Sotub05g024660.1.1 | 296/1464 |
| StERF4 | chr10(1121218∼1122800) | Sotub10g005470.1.1 | 223/1583 |
| Pti4 | chr05(47324479∼47325451) | Sotub05g024670.1.1 | 230/973 |
The signal peptide of StERFs was predicted by Signal P4.1 Serve [27]. The result showed that all the average values of C, Y,and S were less than 0.5, so five StERFs were likely not to have a signal peptide and belonged to non-secretary protein. In addition to nuclear localization, transactivation activity was another defining feature of a transcription factor. We used a yeast one-hybrid system to examine the transcriptional activity of StERFs. A fusion protein of GAL4 DNA-binding domain with StERFs was expressed in yeast cells to assay their ability to activate transcription from the GAL4 sequence. The result demonstrated that StERFs can promote yeast growth in the absence of Trp with X-gal activity; however, the control vector pGBKT7 did not activate (Fig. 2). These data confirmed that the function of StERFs was as a transcriptional activator in yeast.
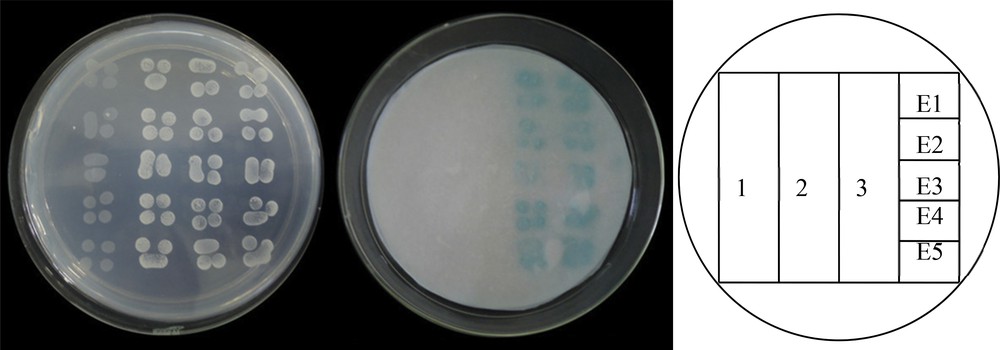
(Color online.) Analysis of the transactivation activity of StERF. StERF and GAL4 DNA-binding domain fusion protein were expressed in the yeast strain Y187. The transactivation activity was revealed through the expression of the lacZ reporter gene (β-galactosidase activity). Vectors pGBKT7 and pGBKT7-53 + pGADT7-Rec2-53 were expressed in yeast as a negative and a positive control, respectively. The yeast streak was cultured on SD/-Trp. (1: Y187; 2: pGBKT7; 3: pGBKT7-53+ pGADT7-Rec2-53; E1: pBD-StERF1; E2: pBD-StERF2; E3: pBD-StERF3; E4: pBD-StERF4; E5: pBD-Pti4).
3.2 Differential expression of StERF genes in different potato tissues
The results of RT-PCR showed that StERF1-4 and Pti4 expressed with varied degrees in different plant tissues including leaf, stem, flower, root, and tuber (Fig. 3). There was no expression or very weak of StERF1-4 and Pti4 in tuber, and no expression of StERF3 in flower. StERF2 was relatively stable in tissues, except tuber. The expression levels of StERF4 were higher in leaf, flower and root than in stem and tuber. Generally, the expression levels of StERFs were consistent among the examined plant tissues. However, StERF1 and StERF4 in flower and StERF4 in leaf showed preferential tissue expression (Fig. 3).

StERF expression patterns were profiled in various potato tissues including leaf, stem, flower, tuber and root by RT-PCR analysis. Leaves, stem, flower and root were obtained from 38-day-old potato plants, and tuber from 60-day potato plants. The ef1a gene was as an internal control.
3.3 StERF transcript levels were regulated by hormones and abiotic stresses
Potato plants (four-leaf-stage) were treated with 200 mM NaCl and 20% PEG6000 for 1 h, 2 h, 6 h and 12 h, respectively. The result from qRT-PCR showed that the expression of StERF genes (except StERF4) was induced and reached the highest level after 6 h under NaCl treatment. StERF5 was strongly induced (1.7- to 6.5-fold) (Fig. 4A). StERF1, StERF3 and StERF4 showed no induction at 1 h, but StERF1 was highly induced (4- to 5-fold) after 6 h of treatment, whereas StERF3 and StERF4 showed weaker levels (1.5- to 2-fold) of induction at 2 h and 6 h (Fig. 4A). For drought stress treatment with PEG6000, all of StERF genes were strongly induced (4.3- to 14.6-fold) and reached their highest at 1 h (Fig. 4B). There was a rapid induction after treatment with PEG6000 and then a rapid decrease compared to NaCl treatment. StERF3 was more strongly induced (14.6-fold) and decreased more rapidly than others.
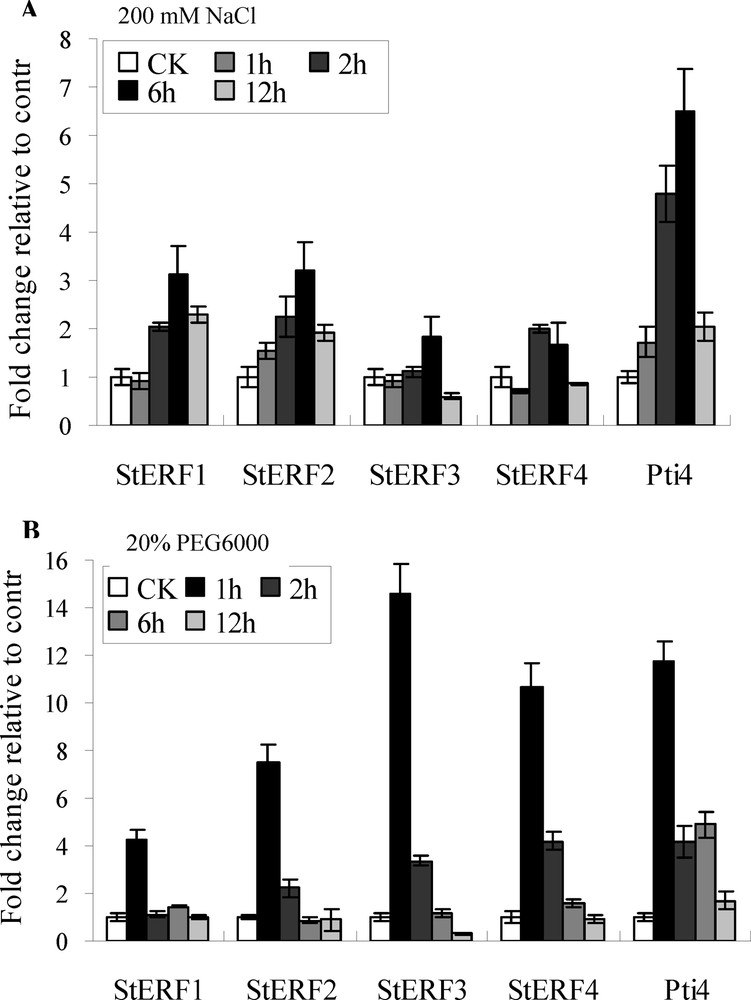
Expression of StERF genes under stress treatment. Relative expression in four-leaf-stage plant leaves of StERFs in response to salt and drought stress treatment versus non-treated controls. CK was the control treated with distilled water. The ef1a was as internal control gene. Pti4 was an ERF factor. (A) qRT-PCR of salt (200 mM NaCl) treatment. (B) qRT-PCR of drought stress (20% PEG6000) treatment. Data presented as a mean ± SE (three biological replicates).
The result from RT-PCR for expression of StERF genes under hormone treatment showed that the expression of StERF1, StERF3, StERF4 and Pti4 was repressed at 1 h and 12 h under ethylene treatment (1 mM ethephon), while StERF2 showed little to no alterations (Fig. 5A). Expression analysis of 100 mM MeJA treatment showed that the expression of StERF1 was not changed, while the expression of StERF2, StERF4 and Pti4 after 1 h treatment, and StERF4 was completely inhibited (Fig. 5B).
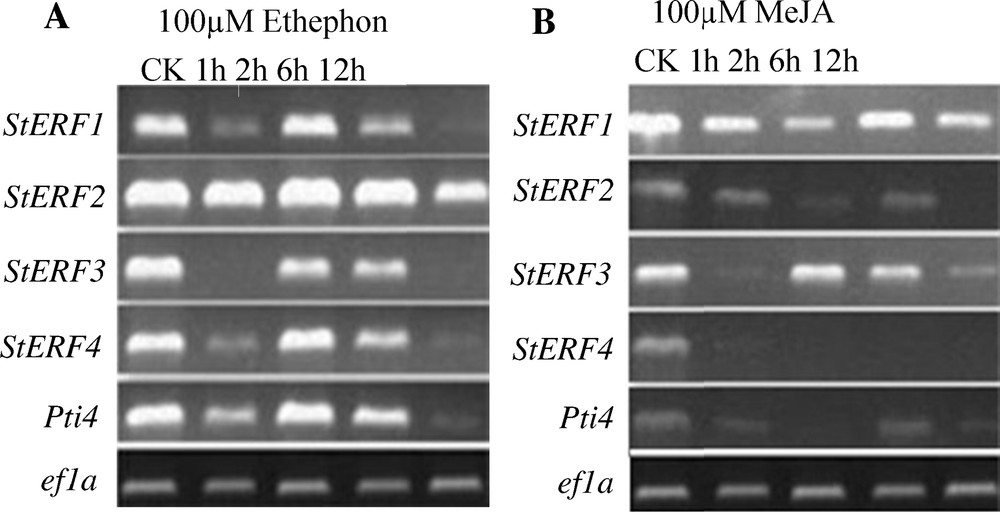
Expression of StERF genes under hormones treatment. Relative expression in four-leaf-stage plant leaves of StERFs in response to hormone treatment versus non-treated controls. (A) RT-PCR of ethylene (100 μM ethephon) treatment. (B) RT-PCR of methyl jasmonate (100 μM MeJA) treatment. Data presented for RT-PCR from a representative sample of experiments, with the ef1a gene serving as an internal control.
3.4 StERF can specifically bind to the GCC-box element in the promoter region of stress-related genes
Previous studies have shown that AtERF can bind to the cis-element of GCC-box [28]. In order to investigate whether the five StERFs of potato can bind to the GCC-box element of the promoter region of the stress-related genes, the full-length ORFs of StERFs were fused to the GAL4 activation domain of the vector pADT7. The fused construct (pAD-StERFs) was co-transformed with the pGCC-His2.1/pmGCC-His2.1 constructs containing quadruple tandem repeats of GCC-box or mGCC-box into the yeast strain Y187 (Fig. 6). Although all yeast cells harboring different constructs could grow on SD/-Trp without 3-AT, those with pGCC-His2.1 and pmGCC-His2.1 did not grow in the presence of 40 mM of 3-AT (Fig. 6, upper panel). However, cells only co-transformed with pAD-StERF and pGCC-His2.1 grew normally in the presence of the minimal inhibitory concentration (40 mM) of 3-AT. Moreover, the growth of transformants containing constructs lacking StERF was completely inhibited (Fig. 6, lower panel), which demonstrated that all pAD-StERF fusion proteins were able to bind to a 66-bp oligonucleotide that contained the wild-type GCC-box sequence (AAGAGCCGCCACTA), whereas binding activity was abolished when both G residues within the GCC-box were replaced with T residues (AAGATCCTCCACTA), suggesting that StERFs could only bind to the GCC-box cis-element.
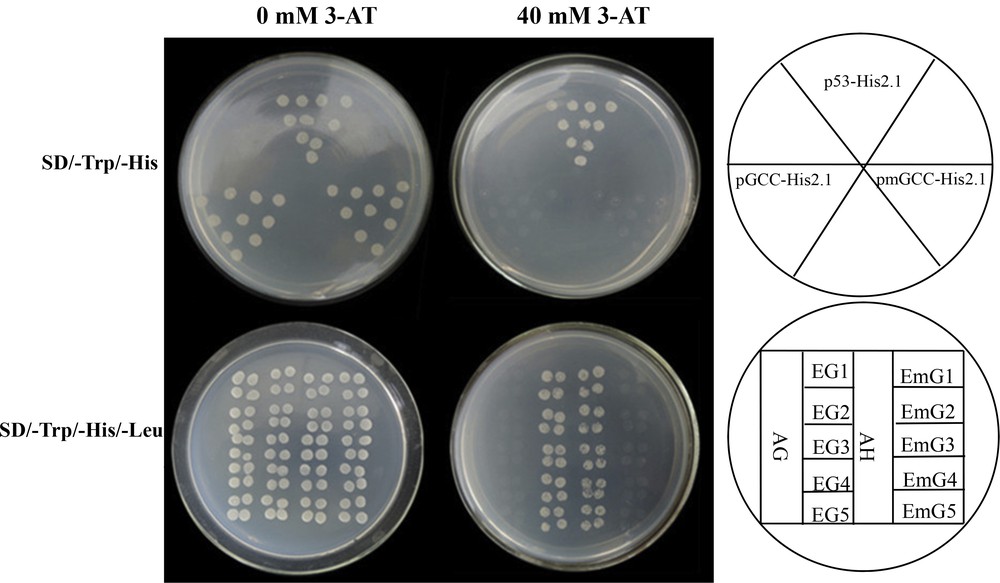
(Color online.) Yeast one-hybrid assay for binding of StERF to the GCC-box domains. AH and AG was expressed in yeast as positive and negative controls, respectively. The minimal inhibitory concentration of 3-AT for the pGCC-His2.1 and pmGCC-His2.1 yeast strain was 40 mM. The photograph showed the growth of yeast cells on SD/-Trp/-His or SD/-Trp/-His/-Leu medium with (40 mM) or without 3-AT. (AG: pGADT7 + pGCC-His2.1; AH: pGAD-Rec2-p53 + p53-His2.1; EG1: pAD-StERF1 + pGCC-His2.1; EmG1: pAD-StERF1 + pmGCC-His2.1; EG2: pAD-StERF2 + pGCC-His2.1; EmG2: pAD-StERF2 + pmGCC-His2.1; EG3: pAD-StERF3 + pGCC-His2.1; EmG3: pAD-StERF3 + pmGCC-His2.1; EG4: pAD-StERF4 + pGCC-His2.1; EmG4: pAD-StERF4 + pmGCC-His2.1; EG5: pAD-Pti4 + pGCC-His2.1; EmG5: pAD-Pti4 + pmGCC-His2.1). Masquer
(Color online.) Yeast one-hybrid assay for binding of StERF to the GCC-box domains. AH and AG was expressed in yeast as positive and negative controls, respectively. The minimal inhibitory concentration of 3-AT for the pGCC-His2.1 and pmGCC-His2.1 yeast strain was ... Lire la suite
4 Discussion
ERF is ubiquitous in plant and plays a crucial role in a wide range of processes, including developmental process, hormonal signal transduction, regulation of metabolic pathways, and response to biotic and abiotic stresses. StERF1-StERF4 and Pti4, as members of ERF family in potato, contained an AP2/ERF domain and likely activated the expression of related genes by binding to GCC box cis-element. In the present study, the full-length cDNAs of StERF genes were obtained and their initial localization and function analysis has been carried out by bioinformatic software. The results indicated that StERF1-StERF3 and Pti4 distributed in nucleus, since nuclear import of transcription factors was instrumental to their transcriptional activity. In addition to nuclear localization, transactivation activity was another defining feature of a transcription factor. We found that all five StERFs had the transcriptional activity using yeast one-hybrid system.
AP2/ERF family members shared a conserved AP2/ERF DBD of approximately 60 amino acid residues referred to as the GCC-box binding domain (GBD). The NMR solution structure of the AP2/ERF DBD of ERF1 in complex with GCC-box DNA (5′-TAGCCGCCA-3′) revealed that the AP2/ERF DBD contained an N-terminal, three-strand anti-parallel β-sheet that recognized a target sequence and a C-terminal α -helix, which was packed parallel to the second beta-strand [29].
MBP-AtERF fusion proteins were able to bind to a 16-bp oligonucleotide that contained the wild-type GCC-box sequence (AGCCGCC), whereas binding activity was abolished when both G residues within the GCC-box were replaced by T residues (ATCCTCC) [28]. Moreover, a series of oligonucleotides were generated in which each nucleotide within the GCC-box was separately substituted with a T nucleotide. These oligonucleotides were used to assess the relative binding activities of each AtERF in comparison to the wild-type GCC-box sequence. The results showed that any mutations within the GCC box reduced the binding ability of the AtERFs, indicating that GCC box was the optimal sequence for AtERF binding among the mutants investigated [28]. By virtue of its ERF domain, Pti4 can bind the sequence GCCGCC (GCC box) and regulate the expression of several GCC box-containing genes [30]. A similar result was obtained in the yeast one-hybrid experiment in the research.
Transcription factors can bind to either enhancer or promoter regions of DNA to regulate the expression of genes [31]. Our results showed that these five StERFs of potato could bind to the GCC-box cis-element, but not bind to mGCC-box (Fig. 6). Most transcription factors acted either as activators or repressors [32]. Different ERF proteins had distinct roles in stress response by regulating the expression of specific downstream genes. It can enhance plant tolerance to abiotic stress by regulating the expression of detoxification enzyme genes [33]. Under drought stress, the higher level of ABA made greater decrease in water loss and maintained the better growth of root to get more water from the soil [34]. StERFs were strongly induced by drought stress in this study, which suggested that they may be involved in response to drought stress.
The ascorbic acid level was approximately 1.6- to 1.7-fold higher in AtERF98 overexpressed (OX) lines than in the WT, which indicated that AtERF98 played an important role in regulating the biosynthesis of ascorbic acid in Arabidopsis [23]. In contrast, the mutants of aterf98-1 and aterf98-2 played a role in reducing transcript levels for pivotal genes, and the expression of other synthesis genes was not affected [23]. Moreover, the results of another study demonstrated that ORA59 (a member of ERF family) was an essential integrator of the JA and ethylene signal transduction pathways and thereby provided a new insight into the nature of the molecular components involved in the cross talk between these two hormones [35]. Our result showed that the expression of StERF genes was regulated by ethylene and MeJA.
In summary, five StERF genes in potato were isolated and characterized, and they were strongly induced by abiotic stress (salt and drought), and some of them were found to be regulated by hormones of ethylene and MeJA. These five StERFs had transcriptional activity and may have multiple regulatory functions in potato plants.
Acknowledgments
We thank Professor Jianzhong Ma (School of Life Science and Engineering, Lanzhou University of Technology) for providing the yeast one-hybrid system and valuable discussion in the experiment technology. This work was supported by Gansu Provincial Key Laboratory of Aridland Crop Science of Gansu Agricultural University (No. GSCS-2012-02), Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20126202110007), and International Science & Technology Cooperation Program of China (No. 0102014DFG31570).


