1 Introduction
Animals constantly make decisions: they choose mates, select resources, and decide whether to engage in activities that increase predation risk. These decisions are often based on the perception of chemical or acoustic cues [1,2]. Wisenden and Stacey [3] defined three distinct states that characterise the evolution of these chemical and acoustic signals: ancestral, spying, and communication. In the ancestral state, cues are released by an individual (the sender) but cannot be detected by other individuals (i.e. no receiver). The spying state consists of the perception of a cue by a receiver with no specific response detected by the sender. For example, during mate choice in frogs [4], and fishes [5], satellite males can intercept acoustic signals produced by dominant male use these to their own advantage to successfully mate with females. Similarly, bacteria in biofilms are known to release so called quorum-sensing molecules, which are involved in bacterial cell–cell communication. However, some seaweeds and marine invertebrates (e.g., polychaete Hydroides elegans) can intercept these quorum-sensing molecules to detect a suitable biofilm for attachment or settlement [6]. The communication state was defined as cues that are actively used to transfer information between senders and receivers, and that are used by senders to manage the behaviour of receivers [2]. For example, social interactions in insects are primarily the result of chemical communication. For instance, dung beetle larvae release chemical signals to regulate maternal food provisioning [7]. Though less understood, many vertebrates also use chemicals to communicate and avoid aggressive conspecifics interactions [1]. For example, Tilapia fish use chemical cues to facilitate conspecific recognition, limiting unnecessary confrontations [8]. Determining whether the chemical cues that an individual produces are used by receivers via spying or communication is necessary to understand the importance of these cues in the detection of food, predators, mates, or during social interactions [1–3]. Aquatic organisms, such as fish, often live in environments where visual information is limited but where chemical information abounds. Therefore, fishes, given their long evolutionary history, have had both cause and opportunity to develop sensitive chemosensory systems enabling individuals to respond adaptively to conspecific and predator odours [3]. In coral reef fish, a good sense of smell is also important for detecting and orientating towards suitable reef habitats prior to larval recruitment [9]. However, despite the importance of chemical cues for many coral reef fishes, there is currently no information regarding the behavioural process underlying chemical cue use, namely whether the incorporation of cues is a result of either spying or communication.
Most coral reef fishes have stage-structured life histories, with a largely sedentary benthic stage (usually juveniles and adults), preceded by a pelagic larval stage during which there is often the capacity for long-distance dispersal [10]. At the conclusion of the pelagic phase, larvae must return and settle onto reef habitats to continue their development into juvenile and adult stages (i.e. recruitment). During recruitment, fish larvae are subject to strong selective pressure to choose habitats that will promote their survival and growth [11]. Many species are highly selective between available habitats, basing these choices on the availability of specific substrates, the presence of juvenile or adult conspecifics, and the relative abundance of predators or direct competitors [12–14]. Several studies have highlighted the critical role that larval sensory mechanisms play in habitat selection, including the detection of visual, chemical and auditory cues from conspecifics, habitats, or predators [9,15]. Chemical cues in particular may play an important role in leading larvae back to a reef [10,11]. Chemical cues are used by fish larvae to find a suitable reef habitat [16–20], to find conspecifics at juvenile or adult stage [17,21,22], or to avoid predators [23]. Unfortunately, no information is available about the question in behavioural ecology: the detection of chemical cues emitted by conspecifics is it related to communication or spying in coral reef fish? Yet, determining the nature of chemical cues, and the social recognition mechanisms used to integrate them into behavioural processes (spying or communication), will increase our understanding of a wide variety of topics, such as the evolution of social recognition, drivers of habitat selection, and cognitive processing in larval fishes [2,3,5].
In this study, we investigated if (a) chemical cues are “passively” received by larval fishes (“spying”) or if (b) there is an “active” effort on the part of the juvenile or adult fishes to communicate these cues at recruitment (“communication”). As it was not possible to analyze and identify the chemical structure of signals emitted into the water, a series of three experiments were conducted using a two-chamber choice flume. These experiments tested whether (1) larval fishes are attracted to the odour of conspecifics and if so, if they can (2) distinguish between “communication” and “spying” cues, and (3) if responses vary depending on if cues are produced by either juvenile or adult conspecifics. With our protocol, there is a difference in the cues between the two channels which is right only if significant differences are found between water containing “spying cues” and water containing potential communication cue (if any). Thus, a communication cue would be only a significant choice compared to unexposed ones (spy).
2 Methods
2.1 Study site and fish capture
Larval fish were collected using crest nets [24,25] set off the west coast of Moorea Island (17°30′58.85′′S, 149°55′26.77′′W) in October 2009 and January 2010. Among all larvae captured, seven target species were collected in high abundance (60 larvae per species): five pomacentrids (Stegastes fasciolatus, Chromis viridis, Chrysiptera leucopoma, Dascyllus aruanus, Pomacentrotus pavo), one apogonid (Apogon novemfasciatus) and one acanthurid (Ctenoachaetus striatus). Larvae were captured at night, just prior to entering the lagoon to recruit [24] and thus had no prior experience of reef habitats (i.e. naïve larvae).
Larvae were collected at dawn, transferred to the laboratory, and subsequently maintained in aquaria (0.3 × 0.2 × 0.2 m) until 2100 hours when experiments were begun [17,26]. Prior to initial experiments and between all subsequent experiments, each fish was maintained in individual aquaria supplied with flow-through seawater from the adjacent lagoon, and free of added artificial or natural habitats.
Conspecifics, used as cue transmitters, were either (a) larvae reared in aquaria for 15 days (juveniles) or (b) individuals captured with hand nets in the Moorea lagoon (adults). Aquaria contained live coral throughout the 15-day period, with juveniles fed a mixture of rotifers and brine shrimp over this period.
2.2 Sampling protocol of the choice flume
The responses of larvae to chemical cues from conspecifics were tested in a 2-channel choice flume (Fig. 1). Two tanks were connected with the choice flume by pipes, one per channel, to create a constant gravity-driven flow (1 L/min). Dye tests confirmed laminar water flow in each channel, with water from both channels mixing in the downstream compartment.

A diagram of the 2-channel choice flume (60 × 10 cm; water depth, 5 cm). Two tanks (20 × 10 cm; water depth: 30 cm) were simultaneously connected with the two upstream channels (A and B) of the choice flume by pipes to create constant gravity-driven flow into each channel at 1 L/min (flow rate and laminar flow equal between the two channels). Dye tests showed laminar water flow in each channel and showed that water was mixed homogeneously in the downstream compartment. Then, water exited the flume between the two panels, past a net barrier that prevented fish from escaping. Larvae were initially placed in the downstream end of the choice flume, then could either stay in the downstream compartment or move toward the two upstream channels.
For each test, a single larva was placed into the centre of the downstream end of the choice flume (downstream compartment) for a 1 min acclimatization period (a net prohibited the larva from swimming into the upstream channels). At the end of acclimatization period, the net was removed and a 4 min test period began. The test was finished when the larva stayed more than 25 s in an upstream channel (A or B, Fig. 1) or after a 4 min period in which the larva made no choice (larva moving between channels or staying in the downstream compartment). This sampling protocol was validated by previous experiments conducted by Lecchini [18,26,27]. Moreover, preliminary tests conducted on five to eight larvae of each species showed that at least 80% of the larvae from each species swam between channels, and thus would come into contact with both odors before making a decision.
2.3 Choice flume experiments to detect chemical abilities of fish larvae
Using this protocol, three experiments were conducted using 20 larvae per species per experiment. Firstly, we determined the distribution of side choice exhibited by larvae when exposed to chemically identical artificial seawater in both channels to discount the presence of an innate side preference (i.e., control test) (Exp. 1). Then, we tested whether larvae can distinguish between, and exhibit a preference for, the odour of conspecifics that had been exposed to larvae (suggesting communication) or conspecifics that had not been exposed to larvae (suggesting spying). If no preference were observed, this would suggest that the chemical cues did not differ between treatments. This test was repeated twice, once using the odour of juvenile conspecifics (Exp. 2) and again with the odour of adult conspecifics (Exp. 3).
For spying cues, 5 conspecifics (either juvenile or adult) were placed for 3 h in a 10-L aquarium (filled with artificial seawater) without visual contact with fish larvae. For communication cues, five conspecifics (either juvenile or adult stage) were placed for 3 h in a 10-L aquarium (filled with artificial seawater) with visual contact with conspecific larvae in another aquarium set up one centimetre away. Thus, in experiments 2 and 3, larvae were offered a choice between water containing the odour of conspecifics that had observed larvae vs. water containing the odour of conspecifics that had not. Observations of juvenile and adult conspecifics in aquaria suggested that they behaved differently when they had visual contact with larvae, appearing to swim faster than when visual cues were absent and often swimming close to the larval aquarium. Differences in behavioral responses could alter the chemical signature produced by conspecifics. Thus, the “spying” and “communication” effect as defined in our experimental design is an indirect testing of these behavioral responses.
For each individual trial, a larva could either stay in the downstream compartment or swim in one of the two channels (Fig. 1). Following each trial, the choice flume was emptied and washed with freshwater; and the position of channels filled with spying or communication cues were switched.
2.4 Statistical analysis
For each species, a χ2 test was conducted to compare the observed distribution of larvae (Exp. 2 or Exp. 3) to a baseline distribution (number of larvae in each compartment during the Exp. 1) in order to determine if larvae were attracted to conspecifics cues from juveniles or adults. If was an attraction was indicated, a second χ2 test with a correction of Bonferroni test for multiple tests was conducted comparing the observed distribution of fish larvae in the channel filled with spying cues vs. the channel filled with communication cues in order to determine if larvae exhibit a preference for the odour of water containing spying vs. communication cues.
3 Results
In the control experiment (Exp. 1), all species exhibited either a homogenous distribution between all three choices in the flume (e.g., C. leucopoma: left channel: 6 larvae; right channel: 8; downstream compartment: 6; χ20.05,2 = 0.40, P = 0.96), or they did not make a decision, with the majority remaining in the downstream compartment (e.g. S. fasciolatus, left channel: 4 larvae; right channel: 2; downstream compartment: 14) (Fig. 1a). These results confirmed that the choices made by larvae were not influenced by physical aspects of the flume system, most importantly confirming that there was no innate left/right channel preference in any species tested.
When juvenile “spying” vs. “communication” cues were compared (Exp. 2), only C. viridis and A. novemfasciatus showed a significant attraction to the channels containing the “pure” cue sources (Fig. 1b). For example, 60% of C. viridis were attracted by the channel filled with communication or spying cues. C. viridis larvae were then significantly attracted by conspecifics cues (χ20.05,2 = 15.7, P < 0.001), but did not distinguish between communication and spying cues (χ20.05,1 = 0.01, P = 0.9). Similarly, A. novemfasciatus larvae were attracted by conspecifics cues (χ20.05,2 = 28.1, P < 0.001), but did not distinguish between communication and spying cues (χ20.05,1 = 0.3, P = 0.6).
When channels were filled in with water cues obtained from adults (Exp. 3), C. viridis, P. pavo, D. aruanus and A. novemfasciatus showed a significant attraction (Fig. 1c). C. viridis, P. pavo and A. novemfasciatus were attracted by conspecific cues (χ20.05,2 = 54.6, P < 0.001; χ20.05,2 = 39.5, P < 0.001; χ20.05,2 = 49.8, P < 0.001, respectively), but did not distinguish between communication and spying cues (χ20.05,1 < 3.84, P > 0.05). For example, 40% of C. viridis larvae were attracted by the channel filled with communication cues and 50% by the channel filled with spying cues. In contrast, D. aruanus larvae were attracted by conspecific cues (χ20.05,2 = 10.8, P = 0.005) and preferred communication cues (χ20.05,1 = 4.0, P = 0.04).
4 Discussion
The findings of this study suggest that, while larvae are attracted to conspecific chemical cues (four of the seven species tested), they generally do differentiate between “spying” and “communication” cues. However, it does appear that some species have greater sensitivity to conspecifics at specific life stages. While this study used a negative control (blank seawater), no positive control was included (seawater plus odor of heterospecific or odor of conspecific in the two channels). Therefore, any preference for unmixed cue in channels may simply reflect the existence of an olfactory gradient or presence of ammonia or other general physiological byproducts not unique to conspecifics. Nevertheless, previous papers have suggested that the four species attracted by conspecifics cues in the present study (C. viridis, P. pavo, D. aruanus and A. novemfasciatus) can differentiate between the odor of conspecifics and heterospecifics [17,18].
The level of social organisation that a species exhibits may determine whether or not the larvae are attracted to conspecifics. Among the five pomacentrid species tested, C. viridis, P. pavo, and D. aruanus exhibited an attraction to conspecifics cues. Yet, all three species are gregarious, generally forming group territories with both juveniles and adults [28,29]. In contrast, C. leucopoma and S. fasciolatus larvae were not attracted to any conspecific cues. Juvenile and adult S. fasciolatus are aggressive, territorial and solitary [30] and adults will actively attack conspecifics [29]. Likewise, juvenile and adult C. leucopoma are generally solitary, occasionally occurring in pairs or trios [31]. Social organisation may also explain the variable chemical attraction to conspecific cues in the non-pomacentrids tested, namely the attraction to cues in A. novemfasciatus and the lack of any attraction in C. striatus. Newly settled A. novemfasciatus school with juveniles and adults on reefs at Moorea, while newly settled C. striatus are solitary [28]. For a settling larvae of a species with a strong social structure, using chemical cues to locate older conspecifics may be the best strategy for quickly locating essential resources and reducing mortality risk while for non-schooling species alternative cues, i.e. those that originate directly from preferred habitats or food resources, may be used for this purpose. However, the most efficient chemical cues to locate older conspecifics would be emitted by the adults (Fig. 2). P. pavo and D. aruanus larvae were more sensitive to adult cues. C. viridis and A. novemfasciatus larvae were sensitive to both adults and juveniles cues. No fish species tested was only sensitive to juvenile cues. These results suggest that chemical cues of immature individuals (juveniles) would be treated by perceivers (larvae) as less informative than those of adults, possibly due to the greater vulnerability of juveniles to reef predators, their lack of experience on the reef, and/or due to incomplete development of physiological process in juveniles that become more perceptible in adults. Similar hypotheses have also been used to explain ontogenetic variation in mammalian alarm cue production, with alarm vocalizations of immature individuals often treated by perceivers as less provocative than those of adults [32].
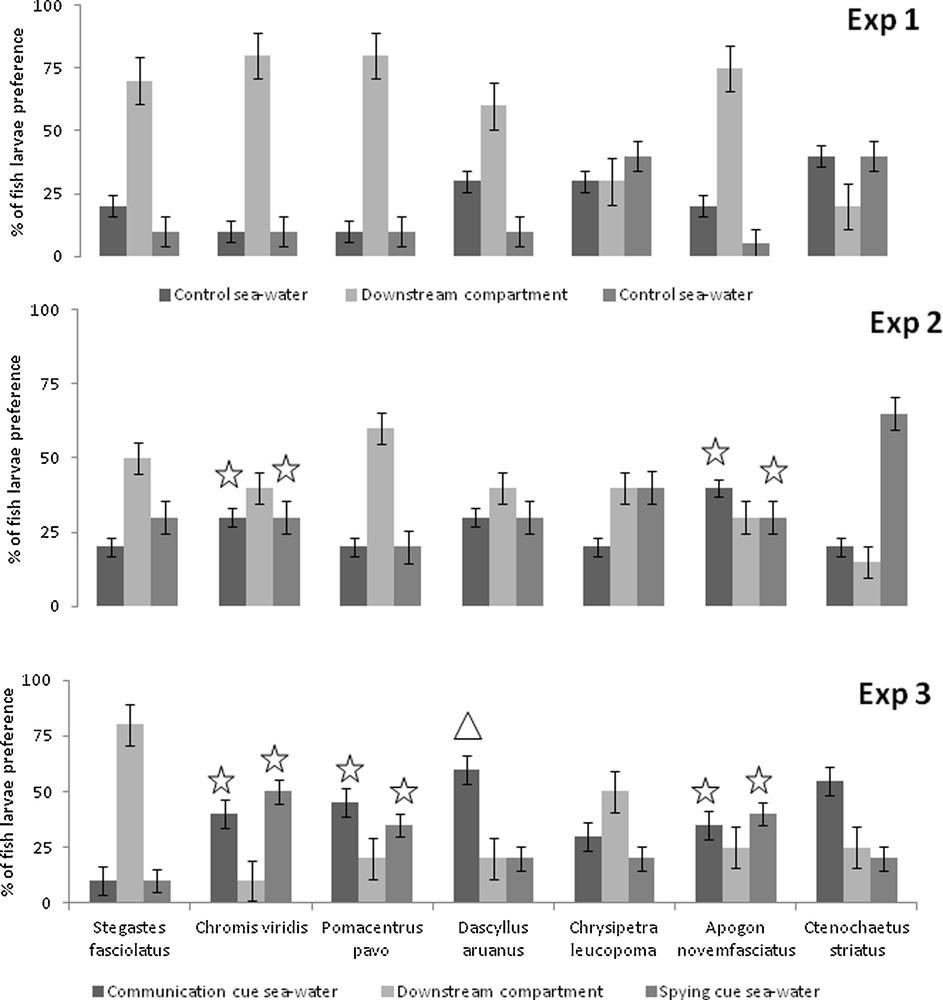
Olfactory cue preferences of larval reef fishes based on two channel choice flume trials. In the control expriment (Exp 1), the two channels were filled with control sea water. In the experiment with juvenile cues (Exp 2) or adult cues (Exp 3), the two channels were filled with water containing either “communication” or “spying” cues from juvenille or adult conspecifics. The downstream compartment represents the percentage of larvae which showed no preference for either cue: χ2 test comparing the observed distribution (juveniles or adults exp.) to a baseline distribution (control exp.) identified a significant difference between distributions (P value < 0.05): χ2 test comparing the observed distribution of larvae in channel filled with “spying” cues vs. in channel filled with “communication” cues identified a significant difference between distributions.
While three of the study species did not distinguish between cues, one species (D. aruanus) exhibited a preference for the odour of conspecifics that had visual contact with larvae (i.e. a communication cue). The larvae of some species (including D. aruanus [33]; Lecchini, pers. obs.) colonize the reef in successive waves of larval cohorts that settle during the night. Hypothetically, the first wave of larval cohorts could recruit onto habitats occupied by juvenile and adult conspecifics using habitat-specific cues [18]. Settling larvae may provoke an active or passive behavioural modification in conspecifics that increases the attractiveness or potency of their odour, facilitating the recruitment of further larval cohorts. Nevertheless, this hypothesis needs both field and experimental validation to determine whether conspecific behaviour changes once larvae begin settlement and whether this in turn alters the molecular structure of their odour molecules.
5 Conclusion
The ability of animals to gather information about their social and physical environment is essential for their ecological function. In coral reef fishes with pelagic larval stages, recruitment to appropriate habitats is essential [34]. However, the unlikelihood of encountering these often rare habitats at recruitment by chance [11] means that the identification and incorporation of multiple sources of sensory information is essential for locating places to settle and ultimately reside as adults [15]. While the adaptive benefits of social recognition mechanisms, such as spying or communication, at larval recruitment remain unclear, the results of our study suggest conspecific chemical cues can be used by coral reef fish larvae to locate appropriate habitats. However, the nature of these cues may vary, with one of the seven species tested (D. aruanus) potentially modifying their odour to increase its attractive to fish larvae.


