1 Introduction
Understanding the processes that govern biodiversity patterns is challenging and implies using a broad, multi-level approach involving local factors and biogeographical histories. Major theories concerning species diversity [1] provide a limited understanding of the role played by the interspecific interactions underlying the observed patterns. In species-rich neotropical rainforests, tank bromeliads (Bromeliaceae) are keystone species in that they offer suitable habitats to terrestrial, semi-aquatic, and aquatic organisms. Their interlocking leaves form wells, or phytotelmata, which collect rainwater and debris, providing nutrients for the bromeliad itself and for an aquatic food web consisting of organisms ranging from bacteria to vertebrates [2,3]. In these natural aquatic microcosms, habitat size and complexity (i.e. water volume and leaf display) and food quality/quantity at the base of the food web are the main determinants of the structure of the aquatic communities [4]. Because they are highly replicated in nature, these microcosms also represent a relevant model for addressing questions about diversity patterns [2,5].
Ants are conspicuous insects in tropical rainforests where they constitute the largest fraction of the animal biomass [6]. They have commonly developed facultative associations with many plants that are thus protected from herbivores while, in turn, the ants receive a trophic reward (i.e. extrafloral nectar or food bodies) [6,7]. In more evolved systems, ‘myrmecophytes’ provide food and nesting spaces (i.e. domatia) to some ant species that protect them from herbivores and frequently provide them with nutrients (myrmecotrophy), something which is detrimental to epiphytic myrmecophytes [6,7]. Also, some epiphyte species, including tank bromeliads, are involved in complex associations with arboreal ants known as “ant-gardens”, in which workers collect and incorporate the seeds of selected epiphyte species into their nest (myrmecochory). Ant species with different habitat preferences in terms of sun exposure determine the location of their associated epiphytes by installing their nests in certain parts rather than others of the tree crowns. In so doing, these ants indirectly drive the main environmental constraints on the aquatic habitat, thus mediating the structure and functioning of the aquatic community [8–10]. These plants can benefit from myrmecotrophy, protection from herbivores, and, in some cases, pollination [7–10].
In contrast, most relationships between ants and tank bromeliads are facultative, with ants sheltering in all kinds of cavities provided by the bromeliads [11,12]. In this case, ants do not act as pollinating or dispersal agents for the bromeliads and, so, do not determine their position in trees with the ensuing consequences for their aquatic communities. Still, ants can act as ecological engineers in bromeliad rosettes through the construction of nests and the accumulation of feces and debris [11].
We hypothesized that the presence of nests of the trap-jaw ant Odontomachus haematodus Linnaeus between the aerial parts of the leaves of the tank bromeliad Aechmea aquilega (Salib.) Griseb. indirectly influences the structure of the macroinvertebrate aquatic communities living there by changing the environmental constraints at the scale of the bromeliad. In this context, we predicted that the presence of an ant colony would be followed by significant modifications to key habitat determinants, and that those modifications permit the explanation of observed patterns in the macroinvertebrate aquatic communities.
2 Materials and methods
This study was conducted in Sinnamary (05°22′39″N; 52°57′35″W), French Guiana, along a dirt road bordered by a secondary forest. We selected 13 mature, terrestrial A. aquilega (this species can be terrestrial, lithophilic or epiphytic) of a similar size and exposure to incident radiation (this was verified using hemispherical photographs and the image processing software Gap Light Analyzer), which were harvested and then placed separately into sealed plastic bags for transport to the laboratory. The wells in each plant were emptied by sucking the water out using pipettes. The volumes of water (WV, mL) were measured and the water kept for invertebrate sampling. The different vegetative traits of each individual were measured to characterize habitat size: plant height (PH) (i.e., distance from the insertion of the outer leaves to the top of the crown); plant width (PW) (i.e., the mean of two 90° measurements of the maximum distance between the tips of two opposite leaves); the number of green leaves (NL); the number of wells constituting the phytotelm (NW); leaf display as the proportion of horizontal (HL) and vertical leaves (VL); and the length (LL) and width (WL) of the longest leaf.
Finally, the amount of organic matter held by the plant was separated into three classes: fine particulate organic matter (FPOM, 1000–0.45 μm in size), coarse particulate organic matter (CPOM, small pieces of fragmented material) and intact, unfragmented leaf litter (LEAF). The organic matter was dried in an oven during two days at 60 °C (results expressed in dry mass). All of these variables are summarized in Table 1.
List of plant characteristics measured on sampled Aechmea aquilega from Sinnamary, French Guiana, summarizing mean absolute values for each of them.
| Plant characteristics | IDa | Mean ± SE | Units |
| Incident radiation | IR | 34.05 ± 4.76 | % |
| Well volumes | WV | 392.15 ± 83.69 | mL |
| Plant height | PH | 94.65 ± 9.70 | cm |
| Plant width | PW | 116.88 ± 10.42 | cm |
| Number of leaves | NL | 20.38 ± 1.03 | – |
| Number of wells | NW | 10.54 ± 1.05 | – |
| Horizontal leaves | HL | 40.58 ± 4.29 | % |
| Vertical leaves | VL | 59.42 ± 4.29 | % |
| Length of the longest leaf | LL | 91.23 ± 8.35 | cm |
| Width of the longest leaf | WL | 6.52 ± 0.26 | cm |
| Fine particulate organic matter | FPOM | 1.86 ± 0.48 | g |
| Coarse particulate organic matter | CPOM | 16.35 ± 3.48 | g |
| Unfragmented leaf litter | LEAF | 22.05 ± 4.17 | g |
a Codes as in Fig. 1a.
Each A. aquilega was then taken apart over a bucket and we carefully cleaned each leaf with a jet of water to collect all of the remaining invertebrates living deeper in the wells. The invertebrates were mostly keyed to morphospecies or species, enumerated and preserved in 70% alcohol. Identifications were made using the Merritt and Cummins larval keys [13] and Lane keys [14] to identify mosquitoes to species level.
The influences of biotic and abiotic determinants on the aquatic communities were investigated through a canonical ordination using CANOCO software (version 4.5). Macroinvertebrate distribution was first analyzed with an initial detrended correspondence analysis (DCA) in CANOCO, allowing us to test the first assumption regarding the use of a linear type ordination method (redundancy analysis; RDA). Specifically, the summary provides the lengths of the gradient for each ordination axis. These measurements represent the beta diversity in community composition (or the extent of taxa turnover) along gradients of newly created ordination axes. In order to use an RDA, the longest gradient along axis 1 should not exceed 3.0 [15]. Forward selection was employed to test which of the environmental variables explained a significant (P < 0.05) proportion of the species variance. The significance of explanatory variables was tested against 499 Monte Carlo permutations.
3 Results and discussion
Seven out of the 13 A. aquilega sampled sheltered O. haematodus colonies (i.e. brood present), while the six others did not shelter ant colonies at all. When present, O. haematodus colonies occupied most of the leaf axils, packing sand and twigs in the wells to nest above the water level and using dead leaves to fit out their nest chambers. In one sample, a small Crematogaster brasiliensis Mayr colony co-occurred with O. haematodus. Due to its small size, this C. brasiliensis colony was not taken into account in the subsequent analyses.
The 13 A. aquilega contained a total of 5895 aquatic macroinvertebrate individuals belonging to 30 taxa (Table 2). The length of the longest gradient resulting from the DCA was 2.43 (< 3.0; see [15]) along axis 1, permitting us to conduct an RDA to highlight the role of each environmental variable. Axes 1 and 2 accounted equally for 48.8% of the variance in total taxa and in the taxa–environment relationship (Fig. 1a and b); eigen values for axes 1 and 2 were 0.27 and 0.21, respectively. Taxa–environment correlations were equal to 1 for both axes. Forward selection identified two variables explaining a significant amount of the taxa variance (bold arrows in Fig. 1a): the number of wells (NW), which also accounted for the greatest proportion of the total canonical eigen values (19%; F = 2.58; P = 0.002), and FPOM (P = 0.034).
List of the aquatic macroinvertebrate morphospecies and species occurring in Aechmea aquilega from Sinnamary, French Guiana. Abundance refers to the real number of individuals per taxon. Note that all Coleoptera and Diptera were found at the larval stage. Taxa with a relative abundance > 1% and/or a percentage of occurrence ≥ 60% are highlighted in bold.
| Order | Family | Sub-family | Morphospecies/species | Abundance | Taxa IDa |
| Coleoptera | Dytiscidae | Dytiscidae sp.1 | 1 | DYT1 | |
| Dytiscidae sp.2 | 1 | DYT2 | |||
| Elateridae | Elateridae sp. | 1 | ELAT | ||
| Elmidae | Elmidae sp. | 11 | ELMI | ||
| Hydrophilidae | Hydrophilidae sp.1 | 66 | HYD1 | ||
| Diptera | Brachycera sp.2 | 2 | BRA2 | ||
| Brachycera sp.3 | 37 | BRA3 | |||
| Brachycera sp.4 | 9 | BRA4 | |||
| Brachycera sp.5 | 11 | BRA5 | |||
| Brachycera sp.6 | 10 | BRA6 | |||
| Brachycera sp.10 | 2 | BRA10 | |||
| Brachycera sp.11 | 3 | BRA11 | |||
| Culicidae | Culicinae | Culex pleuristriatus | 283 | CULE | |
| Wyeomyia pertinans | 1318 | WYEO | |||
| Toxorhynchites haemorrhoidalis | 76 | TOXO | |||
| Ceratopogonidae | Ceratopogoninae | Bezzia sp.1 | 543 | BEZ1 | |
| Bezzia sp.2 | 6 | BEZ2 | |||
| Ceratopogoninae sp.2 | 29 | CER2 | |||
| Ceratopogoninae sp.3 | 4 | CER3 | |||
| Ceratopogoninae sp.4 | 2 | CER4 | |||
| Forcipomyiinae | Forcipomyiinae sp.2 | 332 | FOR2 | ||
| Forcipomyiinae sp.4 | 1 | FOR4 | |||
| Forcipomyiinae sp.5 | 1 | FOR5 | |||
| Chironomidae | Chironominae | Chironomini spp. | 267 | CHIR | |
| Limoniidae | Limoniinae | Limoniinae spp. | 960 | LIMO | |
| Psychodidae | Psychodinae | Psychodinae sp. | 2 | PSYC | |
| Telmatoscopus sp.1 | 227 | TEL1 | |||
| Tabanidae | Tabanidae spp. | 6 | TABA | ||
| Haplotaxida | Naididae | Aulophorus superterrenus | 1274 | AULO | |
| Pristina spp. | 127 | PRIS |
a Codes as in Fig. 1b.
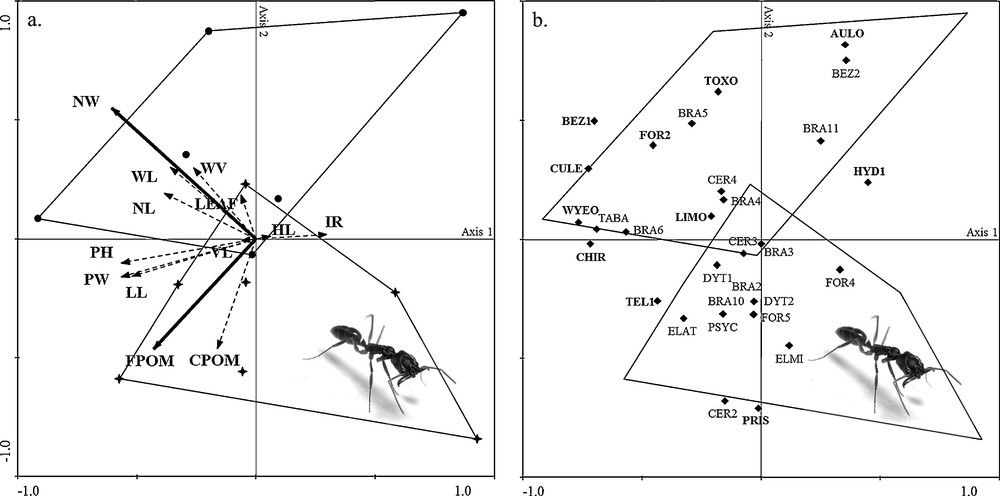
Redundancy analysis biplots showing: a: Aechmea aquilega sheltering Odontomachus haematodus colonies (black crosses, n = 7) and those without any ant colonies (black dots, n = 6). Environmental variables are represented as vectors and named as such in the text. Arrows show the gradients, and their lengths correspond to the strength of the variables in the ordination space. Bold arrows highlight environmental variables explaining a significant (P < 0.05) amount of taxa variance. Thin, dashed arrows represent non-significant environmental variables; b: aquatic macroinvertebrate taxa are projected onto the same ordination space (black diamonds) and identified by taxa ID as in Table 2. Taxa with a relative abundance > 1% and/or a percentage of occurrence ≥ 60% are highlighted in bold. Envelopes consisting of black crosses and black dots in (a) are reported in (b) to show taxa distribution among the two groups.
Envelopes drawn around those A. aquilega sheltering O. haematodus colonies (black crosses) and those that did not (black dots) showed a clear subdivision along axis 2 (Fig. 1a). This subdivision points out that, as hypothesized, ants (here O. haematodus) can influence the structure of the phytotelm community by altering the environmental determinants. Plants associated with O. haematodus contained significantly more organic material (here FPOM), originating at least in part from ant wastes falling into the water. Yet, they displayed a significantly lower number of available wells (NW) due to constructions by workers to keep their nests above the water. These two environmental determinants can be considered a proxy for food availability and habitat size for aquatic fauna [16], resulting in a trade off between an increase in available food and a smaller aquatic habitat.
Concerning the aquatic fauna, Fig. 1b shows the correlations between taxa and environmental variables projected onto the same ordination space. Overall, if we consider taxa with a relative abundance > 1% and/or a percentage of occurrence ≥ 60% (in bold; Table 2, Fig. 1b), the aquatic communities held by A. aquilega without O. haematodus showed a greater abundance of most of these taxa. This is particularly true for the filter feeders represented by mosquito larvae (i.e. Wyeomyia pertinans (Williston) and Culex pleuristriatus Theobald) and a top predator (i.e. Toxorhynchites haemorrhoidalis (Fabricius)). However, two deposit feeders (i.e. Telmatoscopus sp.1 and Pristina spp.), on the contrary, were more abundant in the presence of O. haematodus, likely due to an increase in FPOM. So, the presence of O. haematodus results in a positive bottom-up influence due to a higher quantity of FPOM in the water from ant wastes. It also negatively affects the abundance of top predators, presumably due to less available space resulting from the ant constructions (see also [17]), releasing a top-down pressure that favors lower trophic levels.
Facultative ant–plant associations such as the one presented here imply that the ants select their host plant, as noted for two other Ponerinae [18,19], and that this selection may be related to an imprinting process [20]. Therefore, we encourage future studies to further investigate the driving mechanisms behind nest-site selection along a gradient of ant–plant associations from strict to facultative, and, ultimately, to assess the ecological and evolutionary outcomes for all partners involved in this relationship (i.e. ants, host plants, and the aquatic community).
In conclusion, facultative associations with ants can affect some of the main environmental determinants of the aquatic communities in tank bromeliads. Given the large number of ant species interacting with tank bromeliads and differences in colony size and the nature of these associations (facultative or obligatory), a gradient of environment- to ant-driven community structure can be expected.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
We are grateful to Andrea Yockey-Dejean for proofreading the manuscript, and the “Laboratoire Environnement de Petit Saut” for furnishing logistical assistance. This study has benefited from an “Investissement d’avenir” grant from the “Agence nationale de la recherche” (CEBA, ref. ANR-10-LABX-0025). ST and OD were funded by a PhD scholarship (“Université Antilles–Guyane” for ST; the French “Centre national de la recherche scientifique” and the European Social Fund for OD).


