1 Introduction
Rice is the staple food for the majority of the world's population and is grown under a diverse range of agro-climatic conditions in the globe. Rainfed rice cultivation is associated with major biotic and abiotic stresses that reduce productivity according to the ecosystem. Among abiotic stresses, submergence and water logging are the major constraints for low productivity of the ecology. Submergence due to flash flood is a major problem of rice production for the rainfed lowland rice ecosystem of eastern India, which spreads over around 16 Mha [1] in the country. Complete submergence for one to two weeks followed by intermittent water logging up to 50 cm occur frequently in the region. In some parts of it, farmers grow moderately tolerant traditional varieties possessing submergence and low elongation ability, which produce little due to their low tillering ability, long droopy leaves, and susceptibility to lodging and poor grain quality. In the majority of areas, high-yielding but submergence-intolerant rice varieties are being taken up; as a result, farmers suffer huge losses caused by periodic flash floods during the monsoon season. Recently, the extent of submergence stress has increased due to extreme weather events such as unexpected heavy rains that have inundated wider areas across many regions, rise in sea level, rise in temperature and more changes in weather parameters that may adversely affect crop yield [2–4]. Submergence tolerance and elongation ability are two opposite strategies by which rice adapts to flood depending upon the nature of flooding [5]. The ethylene response factor genes snorkel 1 and snorkel 2 allow rice to elongate in response to water logging while Sub1A allows it to survive in case of flash flood [6–8]. More sustainable and permanent solutions are needed to overcome this problem. Hence, improved varieties are needed that combine high grain yield with submergence and water logging tolerance along with in-built tolerance to major diseases and insect pests.
Sequencing of the Sub1 region in an FR-13A derived line revealed the presence of three genes encoding putative ethylene responsive factors (ERF), Sub 1A, Sub 1B, and Sub 1C. But Sub 1A was subsequently identified as the major determinant of submergence tolerance. It was also observed that Sub 1C alleles were associated with tolerance; however, it was not known whether the Sub 1C allele had any effect on the level of tolerance. More recently, the Sub 1A gene has been successfully introgressed through marker-assisted backcrossing (MAB) into a popular high yielding variety ‘Swarna’ [9]. Swarna Sub1, the first example of submergence-tolerant mega variety, is recently released in submergence-prone areas of Odisha and Uttar Pradesh states in India. These results highlighted the opportunity to develop additional high-yielding varieties that are adapted to other regions or environments. Therefore, the lowland rice cultivars of the eastern region of the country were screened for identifying donors for submergence and water logging tolerance for subsequent use in the breeding program. Besides, molecular markers were validated for identifying ideal marker(s) to be used in a marker-assisted breeding program for submergence tolerance.
2 Materials and methods
Submergence and elongation screening was performed in the screening tanks of Crop Production Division, Central Rice Research Institute (CRRI), Cuttack, Odisha, India during the years 2011 and 2012. The genotypes comprising commonly cultivated cultivars in the lowland rice ecology of eastern India including resistant check genotypes (Supplementary Table 1) were collected from CRRI gene bank. For submergence screening, 90 lowland genotypes along with five susceptible check varieties (IR42, Satyabhama, Tapaswini, Annada and Satabdi) were seeded directly in pots that were submerged in the tanks on the 20th day after seedling, with two replications. A water depth of 1 m was maintained in the screening tanks during the submergence period. When the susceptible check showed maximum leaf damage, i.e. after about 14 days of complete submergence, the tanks were de-submerged and the surviving plants were scored on the 14th day after recovery. The genotypes were scored using the Standard Evaluation System (SES) for rice developed by International Rice Research Institute, Manila, Philippines. The same set of genotypes was screened for elongation ability. The initial heights of the three-week-old seedlings were measured and then 5 cm of rising water were added to the tanks per day up to a 1-m water depth and maintained for 7 days before draining. After draining, the final plant heights of 10 plants per entry were measured. The elongation abilities of the tested genotypes were calculated on the basis of the elongation of the genotypes compared to the best elongating local check, Jalmagna, and the best non-elongating check, IR42 [10] (Table 1).
Molecular markers used for genotyping lowland rice genotypes of the Sub1 gene cluster.
| Sl No | Primer name | Oligonucleotide primer sequence |
| 1 | RM8300 (F) | 5’ GCT AGT GCA GGG TTG ACA CA 3’ |
| RM8300 (R) | 5’ CTC TGG CCG TTT CAT GGT AT 3’ | |
| 2 | AEX (F) | 5’ AGG CGG AGC TAC GAG TAC CA 3’ |
| AEX (R) | 5’ GCA GAG CGG CTG CGA 3’ | |
| 3 | Sub 1 A203 (F) | 5’ CTT CTT GCT CAA CGA CAA CG 3’ |
| Sub 1 A203 (R) | 5’ AGG CTC CAG ATG TCC ATG TC 3’ | |
| 4 | Sub 1 BC 2 (F) | 5’ AAA ACA ATG GTT CCA TAC GAG AC 3’ |
| Sub 1 BC 2 (R) | 5’ GCC TAT CAA TGC GTG CTC TT 3’ | |
| 5 | Sub 1 C173 (F) | 5’ AAC GCC AAG ACC AAC TTC C 3’ |
| Sub 1 C173 (R) | 5’ AGG AGG CTG TCC ATC AGG T 3’ |
Gene-based and intragenic Sub1 DNA markers developed by Septiningsih et al. [11] based on the DNA sequences published by Xu et al. [6] and available in the NCBI database (http://www.ncbi.nlm.nih.gov/) were used for genotyping the lowland cultivars. Lowland genotypes were initially identified using two markers tightly flanking the Sub1 region and were subsequently confirmed by further genotyping using gene-based and intra-genic markers. For this, three markers were used within the Sub1A gene, one each within Sub1B and Sub1C (Table 2) for genotyping studies. Genomic DNA was isolated from 10-day-old seedling using the CTAB method following Murray and Thompson [12]. DNA amplification was performed in a Gradient Thermal Cycler (Verity, Applied BioSystems) with a reaction volume of 20 μL containing 1.5 mM of tris HCL (pH 8.75), 50 mM KCL, 2 mM MgCl2, 0.1% TrotonX-100, 200 μM each of dATP, dCTP, dTTP, dGTP, 4 pmol of each forward and reverse primers, 1 unit of Taq polymerase and 30 ng of genomic DNA. The reaction mixture was initially denatured for 4 min at 94 °C and then subjected to 35 denaturation cycles of 1 min at 94 °C, to annealing for 1 min at 55 °C, and to extension for 1 min at 72 °C, and then to a final extension for 10 min at 72 °C. Aliquots of 10 μl of DNA products from PCR amplification were loaded in a 2.5% agarose gel containing 0.8 μg/mL of ethidium bromide for electrophoresis in 1X TBE (pH 8.0). At least one lane was loaded with a 50 bp DNA ladder. The gel was run at 60 V (2.5 V/cm) for 4 h and photographed using a Gel Documentation System (SynGene). Data were scored for analysis on the basis of the presence or the absence of the amplified products for each genotype–primer combination. The data entry was done into a binary data matrix as discrete variables. The data were analysed and the similarity matrix was constructed from binary data with Jaccard's coefficients, and the dendrogram was generated with the algorithm of the unweighted pair group Method with Arithmetic Average (UPGMA), using FreeTree software [13,14], and the dendrograms were visualized by Treeview 32 software [15]. Principle component analysis (PCA) analysis [16] was used to estimate the Euclidean distance between genotypes and the correlation between the variables. These analyses were performed using SAS programs [17]. The association study between the Sub1 markers and phenotyping parameters was done with Tassel 5 [18].
Phenotyping summary of submergence tolerance and elongation ability of the lowland rice genotypes.
| Sl. | Name of the genotypes | Submergence tolerance | Elongation ability | Remarks | |||
| % survival after desubmergence | SES Score | IPh | FPh | DPh | |||
| 1 | TJ 87-1 | 0 | 9 | 35 | Dead | – | S |
| 2 | TJ 06-1 | 0 | 9 | 35 | Dead | – | S |
| 3 | CR3835-1-7-2-1-1(TJ107) | 0 | 9 | 36 | Dead | – | S |
| 4 | TJ 87-2-3 | 0 | 9 | 34 | Dead | – | S |
| 5 | CR2687-3-3-1-1-1(TJ58) | 0 | 9 | 33 | Dead | – | S |
| 6 | TJ 12-1-4-1 | 0 | 9 | 35 | Dead | – | S |
| 7 | TJ 54-3 | 0 | 9 | 34 | Dead | – | S |
| 8 | CR2682-1-1-5-1-1(TJ 115) | 0 | 9 | 33 | Dead | – | S |
| 9 | TJ 137-1 | 0 | 9 | 36 | Dead | – | S |
| 10 | TJ 150-1-1 | 0 | 9 | 36 | Dead | – | S |
| 11 | TJ 120-2 | 0 | 9 | 35 | Dead | – | S |
| 12 | TJ 14-1 | 0 | 9 | 35 | Dead | – | S |
| 13 | TJ 193-1 | 0 | 9 | 34 | Dead | – | S |
| 14 | TJ 17-1-1 | 0 | 9 | 36 | Dead | – | S |
| 15 | TJ 1-3-1 | 0 | 9 | 37 | Dead | – | S |
| 16 | TJ 14-1-3 | 0 | 9 | 36 | Dead | – | S |
| 17 | Reeta | 30 | 9 | 35 | 52 | 17 | S |
| 18 | Mahalaxmi | 25 | 9 | 33 | Dead | – | S |
| 19 | Sabita | 76 | 5 | 29 | 109 | 80 | HE |
| 20 | Agonibora | 90 | 5 | 30 | 35 | 22 | MT&LE |
| 21 | Panikekoa | 40 | 9 | 32 | 113 | 81 | HE |
| 22 | Khoda | 100 | 1 | 35 | 105 | 70 | T&ME |
| 23 | Dinesh | 17 | 9 | 36 | 125 | 89 | HE |
| 24 | Mahsuri | 4 | 9 | 33 | 101 | 71 | ME |
| 25 | Nalini | 42 | 9 | 28 | 55 | 27 | LE |
| 26 | Hanseswari | 64 | 7 | 31 | 113 | 82 | HE |
| 27 | Matangini | 0 | 9 | 29 | Dead | – | S |
| 28 | Niraj | 26 | 9 | 34 | 115 | 81 | HE |
| 29 | Raspanjar | 18 | 9 | 36 | 121 | 85 | HE |
| 30 | Purnendu | 40 | 9 | 31 | 111 | 80 | HE |
| 31 | Pooja | 16 | 9 | 30 | 52 | 22 | LE |
| 32 | Ravana | 42 | 9 | 35 | 101 | 66 | ME |
| 33 | Jalmagna | 6 | 9 | 32 | 113 | 81 | HE |
| 34 | Boitalpakhia | 85 | 5 | 35 | 95 | 60 | MT&ME |
| 35 | Nangalamunda | 2 | 9 | 36 | 96 | 60 | ME |
| 36 | Padmanath | 0 | 9 | 28 | 62 | 34 | MS |
| 37 | Sumit | 22 | 9 | 28 | 53 | 25 | S&LE |
| 38 | Gangasiuli | 88 | 5 | 32 | 60 | 28 | MT |
| 39 | Kalaputia | 93 | 5 | 30 | 48 | 18 | MT |
| 40 | Kusuma | 87 | 5 | 28 | 50 | 22 | MT |
| 41 | Dharitri | 71 | 7 | 29 | 52 | 23 | S&LE |
| 42 | Utkala Prava | 0 | 9 | 35 | 77 | 42 | S&ME |
| 43 | Sabitri | 70 | 7 | 30 | 50 | 20 | MS |
| 44 | Varsadhan | 41 | 9 | 34 | 115 | 81 | HE |
| 45 | Marisal | 40 | 9 | 32 | 94 | 62 | S&ME |
| 46 | Sarala | 42 | 9 | 28 | 59 | 31 | S&LE |
| 47 | Ranjit | 32 | 9 | 30 | 58 | 28 | S&LE |
| 48 | Gayatri | 77 | 5 | 28 | 50 | 22 | MT&LE |
| 49 | Polai | 4 | 9 | 36 | 117 | 81 | HE |
| 50 | Durga | 60 | 7 | 28 | 109 | 81 | S&HE |
| 51 | Padmini | 0 | 9 | 28 | Dead | – | S |
| 52 | Ambika | 40 | 9 | 31 | 113 | 82 | HE |
| 53 | Atiranga | 80 | 5 | 34 | 108 | 74 | MT&ME |
| 54 | Chakaakhi | 43 | 9 | 29 | 59 | 30 | S&LE |
| 55 | Naveen | 16 | 9 | 28 | Dead | – | S |
| 56 | IET 20220 | 53 | 7 | 33 | 94 | 61 | ME |
| 57 | IR 42 | 15 | 9 | 28 | Dead | – | S |
| 58 | CR MAS 2232-85 | 26 | 9 | 29 | 54 | 25 | S&LE |
| 59 | J/C (Jalamani) | 65 | 7 | 30 | 110 | 80 | MT&HE |
| 60 | Savitri Sub1 | 100 | 1 | 29 | 43 | 14 | T |
| 61 | Ciherang Sub1 | 85 | 5 | 30 | 45 | 15 | MT |
| 62 | IR 64 Sub1 | 100 | 1 | 28 | 44 | 16 | T |
| 63 | Swarna Sub1 | 100 | 1 | 29 | 45 | 16 | T |
| 64 | Samba Mahsuri Sub1 | 90 | 5 | 26 | 43 | 17 | MT |
| 65 | FR 13 A | 100 | 1 | 30 | 45 | 15 | T |
| 66 | Asthapari | 0 | 9 | 35 | 78 | 43 | ME |
| 67 | Matiaburush | 18 | 9 | 34 | 112 | 77 | ME |
| 68 | Mandakini | 0 | 9 | 30 | Dead | – | S |
| 69 | CN 344 | 0 | 9 | 30 | 75 | 45 | ME |
| 70 | Golak | 0 | 9 | 34 | 69 | 35 | ME |
| 71 | Nadiaphula | 12 | 9 | 35 | 112 | 76 | ME |
| 72 | Moti | 81 | 5 | 32 | 59 | 27 | MT&ME |
| 73 | Panidhan | 0 | 9 | 31 | 78 | 47 | ME |
| 74 | Rupasala | 0 | 9 | 35 | 78 | 43 | ME |
| 75 | IC 567993 | 77 | 5 | 32 | 112 | 80 | MT/HE |
| 76 | IC 568008 | 88 | 5 | 28 | 53 | 25 | MT |
| 77 | IC 568009 | 100 | 1 | 29 | 48 | 19 | T |
| 78 | IC 568038 | 83 | 5 | 28 | 55 | 27 | MT |
| 79 | IC 568039 | 55 | 7 | 30 | 55 | 25 | S&LE |
| 80 | IC 568838 | 0 | 9 | 29 | Dead | – | S&LE |
| 81 | IC 568839 | 54 | 7 | 30 | 53 | 23 | MS&LE |
| 82 | IC 568842 | 100 | 1 | 29 | 57 | 28 | T |
| 83 | IC 568921 | 84 | 5 | 35 | 66 | 31 | MT&ME |
| 84 | IC 568038 | 9 | 9 | 29 | 110 | 81 | HE |
| 85 | Hatipanjar | 35 | 9 | 25 | 75 | 50 | ME |
| 86 | Matiaburua | 35 | 9 | 29 | 84 | 55 | ME |
| 87 | Panindra | 40 | 9 | 30 | 72 | 42 | ME |
| 88 | Sudha | 0 | 9 | 30 | Dead | – | S |
| 89 | Amulya | 38 | 9 | 30 | 78 | 48 | ME |
| 90 | Satyabhama | 0 | 9 | 28 | 47 | 19 | S |
| 91 | Pyari | 0 | 9 | 32 | 66 | 34 | LE |
| 92 | CR Dhan 300 | 100 | 1 | 29 | 46 | 17 | T |
| 93 | Tapaswini | 20 | 9 | 30 | 48 | 18 | S |
| 94 | Annada | 0 | 9 | 36 | Dead | – | S |
| 95 | Satabdi | 0 | 9 | 30 | 43 | 13 | S |
| LSD at5% | 10.3 | – | 11.6 | 20.1 | – | – | |
| CV% | 11.4 | – | 10.7 | 11.7 | – | – |
To study the effect of marker combinations with respect to their discrimination ability of tolerant and susceptible genotypes, clustering analysis was performed taking the amplicons together. The combinations of markers were selected on the basis of their location with respect to Sub1 gene cluster. RM8300, being located at one extreme end (downstream of Sub1A gene) (Supplementary Fig. 1), was fixed constant, and other markers added one by one to the combination to form 2, 3, 4, and 5 marker combinations. After that, RM8300 was excluded from the combination and a direct marker ‘AEX’ was fixed constant and then the next markers like Sub1A203, Sub1BC2 and Sub1C173 were added one by one to the combination. Similarly 12 combinations were decided to start the analysis (Supplementary Table 2). The total numbers of tolerant genotypes, tolerant plus elongating, elongating, susceptible plus elongating or susceptible genotypes were considered to be 100% individually for analysing the discrimination ability. The dendrograms were scored for their clustering ability with a similarity index of 50–60%.
3 Results
3.1 Phenotyping
The phenotyping results (Table 3) for plant survival indicated that nine out of 95 genotypes showed survival of 100% with a SES score of 1, which may be either due to submergence tolerance or have escaped as elongating type. The cultivars showing a high percentage of survival were FR13A, Khoda, CR Dhan 300, Swarna Sub1, Savitri Sub1, IR64 Sub1, IC-568009, SambhaMahsuri-Sub1 and IC-568842. As regards our results concerning elongation ability, the elongating types can be classified into three groups. Genotypes with a difference in plant height readings (final and initial) > 80 cm were considered as highly elongating, a difference in plant height readings of 50–79 cm as moderately elongating, and a difference in plant height readings of 21–49 cm as low elongating, whereas values below 20 cm can be considered as non-elongating among the materials of lowland ecology. The phenotypic variation for elongation ability showed that fifty-one genotypes were of the elongating type, comprising high elongation (15) moderate elongation (22), and low elongation (14) genotypes. The genotypes with high elongation ability were Panikekoa, Sabita, Dinesh, Hanseswari, Niraj, Raspanjar, Purnendu, Jalmagna, Varshadhan, Polai, Durga, Ambika, J/C(Jalamani), IC-567993, and IC-568930. After exposing the genotypes to a rising flooding situation under control facility, the moderately elongating types identified were Khoda, Mahsuri, Ravana, Boitalpakhia, Nangalmunda, Padmanath, Utakala Prava, Marisal, Atiranga, IET 20220, Asthapari, Matiaburush, CN344, Golak, Nadia Phula, Moti, Panidhan, Rupasala, Hatipanjar, Matiaburua, Panindra, and Amulya. The genotypes classified as low elongating type were Aghonibora, Nalini, Pooja, Sumit, Dharitri, Sarala, Ranjit, Gayatri, Chakaakhi, CRMAS 2232-85, Pyari, IC-568039, IC-568839 and IC-568838. Jalmagna and other highly elongating genotypes elongated very quickly and survived up to a water depth of 1 m with exposing the leaves above the water surface, while IR42 and other four susceptible checks did not survive. A considerable variation for both elongation ability and submergence tolerance was observed in the studied genotypes. Twenty-seven genotypes could not elongate significantly in response to the rising water level or do not possess the trait submergence tolerance, hence these could not survive and were susceptible to submergence. The elongation ability results clearly depict that genotypes with no elongation ability but with high percentage of plant survival during submergence tolerance phenotyping are to be taken as belonging to the submergence-tolerant type. Hence, the genotypes FR13A, Khoda, CR Dhan 300, Savitri Sub1, IR64 Sub1, IC-568009 and IC-568842, which exhibited high submergence tolerance (score 1), were used as donors in the breeding program. Genotypes with moderate tolerance to submergence coupled with low to moderate elongation ability may also have a breeding importance for lowland ecology. From these results, it was observed that 51 genotypes showed some elongation ability, out of which 36 displayed low to moderate levels of elongation. There were nine highly tolerant genotypes found for submergence tolerance. Surprisingly, only one landrace ‘Khoda’ is observed to possess both high submergence tolerances with moderate elongation ability. Thirteen genotypes were found to possess moderate tolerance to submergence. Eight genotypes, i.e. Boitalpakhia, Gayatri, Atiranga, Aghonibora, Chakaakhi, Moti, IC-567993 and IC-568921, possessed both moderate elongation ability and moderate tolerance to submergence.
Association of marker alleles with phenotypic traits using the GLM model.
| Trait | Marker name | F value | P value | R2 |
| DPh | RM8300 | 2.755728 | 0.100466 | 0.030364 |
| DPh | Sub1A203 | 0.035782 | 0.850402 | 4.06E-04 |
| DPh | AEX | 1.968391 | 0.164137 | 0.021879 |
| DPh | Sub1BC2 | 3.466901 | 0.065946 | 0.037903 |
| DPh | Sub1C | 0.020355 | 0.886877 | 2.31E-04 |
| SES score | RM8300 | 5.031372 | 0.027267 | 0.051324 |
| SES score | Sub1A203 | 9.845192 | 0.002281 | 0.095728 |
| SES score | AEX | 1.199654 | 0.276219 | 0.012735 |
| SES score | Sub1BC2 | 40.1919 | 8.18E-09 | 0.301759 |
| SES score | Sub1C | 0.010906 | 0.917051 | 1.17E-04 |
| Survival% | RM8300 | 1.874195 | 0.174293 | 0.019755 |
| Survival% | Sub1A203 | 12.49389 | 6.38E-04 | 0.118432 |
| Survival% | AEX | 1.592999 | 0.210054 | 0.016841 |
| Survival% | Sub1BC2 | 36.72614 | 2.87E-08 | 0.283105 |
| Survival% | Sub1C | 0.018017 | 0.893514 | 1.94E-04 |
3.2 Genotype-by-trait biplot analysis
The phenotyping data for submergence tolerance and elongation ability were used to generate a genotype-by-trait biplot diagram (Fig. 1) for analysing the lowland genotypes for the first two principal components. The top left (first) quarter contained 26 genotypes that were susceptible to submergence. Some of the susceptible varieties with moderate survival percentage fell in the second quarter, i.e. the right top quarter. This quarter contained all the tolerant genotypes except Khoda, which showed moderate elongation ability, hence, grouped under the third quarter (right bottom) that included mostly the high elongating genotypes and some of the moderately tolerant and moderate elongating types. The fourth quarter contained the moderate elongating types and three high elongating types (Polai, Raspanjar, Dinesh) with low survival. The encircled area consisted of six moderately tolerant and seven tolerant types, of which Sambamashuri Sub1, IR64 Sub1, IC-568842 (tolerant) and Kalaputia, Gayatri (moderately tolerant) are a little bit away from others.

Genotype-by-trait biplot analysis of 95 lowland rice genotypes for the first two principal components. (The numbers in the figure represent the serial number of the genotypes listed in Table 2).
3.3 Cluster analysis
Five markers, namely RM8300, AEX, Sub1A203, Sub1BC2, and Sub1C173, were used to screen the 95 lowland genotypes for Sub1 gene cluster. The discrimination ability of the markers, either singly or in combination, for submergence tolerance was determined by clustering the genotypes and by constructing the dendrogram on the basis of amplification pattern of the genotypes with the markers. It was hypothesized that the marker or combination of markers that can group the tolerant and susceptible genotypes into different clusters should be considered to be the best marker or marker combination. The number of clusters obtained and the percentage of tolerant (T), tolerant plus elongating (T + E), elongating (E), susceptible plus elongating (S + E) and susceptible (S) genotypes present in each cluster for all the combinations is depicted in Supplementary Table 2.
RM8300, one of the closest simple sequence repeat (SSR) markers downstream of Sub1A locus, was able to cluster 71.43% of tolerant (T) and 44.44% of tolerant with elongation ability (T + E) into one group, whereas Sub1BC2 clubbed 77.78% of tolerant with elongation ability and 71.43% of tolerant types. The AEX and Sub1C173 marker could not show discrimination among the genotypes with respect to the traits. The gene specific marker Sub1A203 showed the best grouping among the genotypes, including all the tolerant and tolerant with elongation ability ones in one cluster (Fig. 3a). But it also included 33.33% E, 41.67% S + E and 36.67% S genotypes. Hence, different possible marker combinations were tried out.
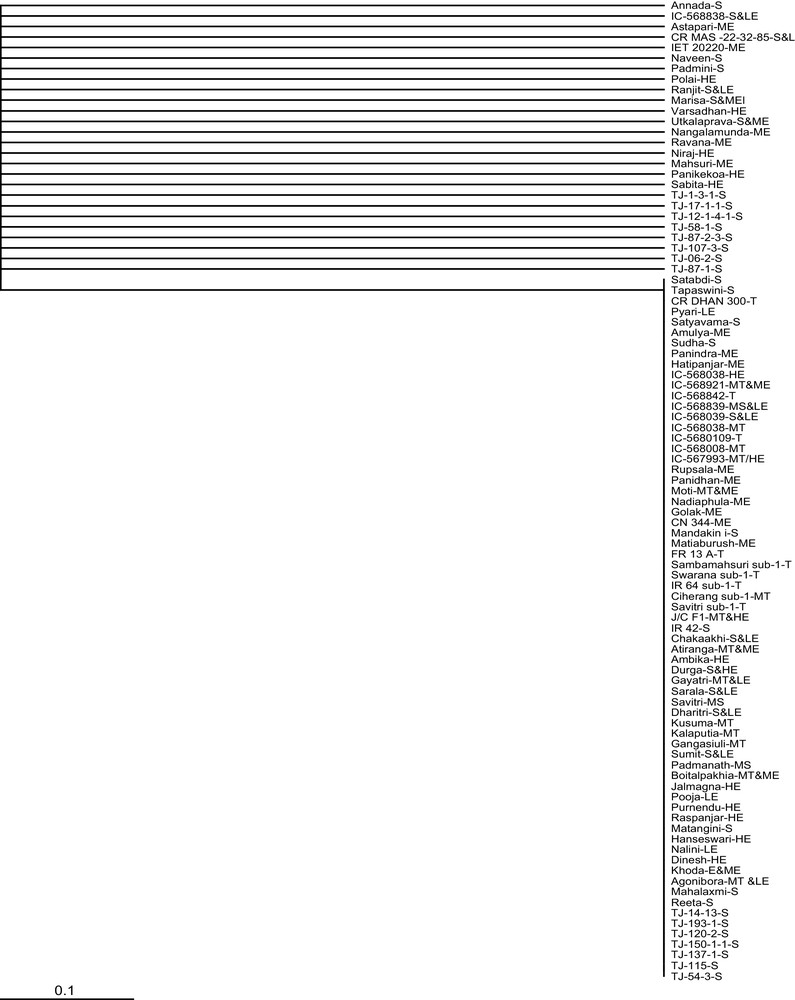

a: dendrogram representing 95 rice genotypes grouped based on submergence tolerance using Sub1A203 marker; b: dendrogram representing 95 rice genotypes grouped based on submergence tolerance using Sub1A203 + Sub1BC2 marker combination.
The combination of Sub1A203 and Sub1BC2 showed better resolution as compared to all other combinations (Fig. 3b and Supplementary Table 2). Four clusters were obtained, out of which two were major and two were minor. Out of the two major clusters, one consisted of 71.43% T and 77.78% T + E and few susceptible and elongating types, whereas the other cluster included a majority of susceptible (93.33%), susceptible with low elongation ability (75%), and elongating (73.33%) genotypes.
The tolerant genotypes like IC568842, CR Dhan 300 and moderately tolerant genotypes Kusuma, Kalaputia, Gangasiuli, IC 568038 stood consistently away from the other tolerant genotypes in the majority of the clustering cases. Similar conditions were obtained for genotypes Moti, Gayatri, Biotalpakhia, Aghonibora and IC 568921 with moderate tolerance showing elongation ability. Sambamashuri Sub1 and Swarna Sub1 being tolerant also clustered out in few combinations. Similar results were obtained for Atiranga. The tolerant variety Khoda, with moderate elongation ability, could not be clustered along with the other tolerant types in many cases, although some combinations could place it with the other tolerant types.
When the phenotypic results were compared with the amplification pattern with the five employed markers for screening, eight varieties, namely Savitri Sub1, IR64 Sub1, Swarna Sub1, Samba Mashuri Sub1, FR 13A, IC 567993, IC 568008 and IC 568009, were selected as tolerant amongst lowland genotypes. But the land race Khoda could not be included, although it was phenotyped as tolerant for submergence.
3.4 Association of marker alleles with phenotypic traits
The association study between the Sub1 markers and phenotyping parameters showed that the Sub1BC2 marker could be associated, with low survival percentage and high SES score (susceptible score range), with higher F value and low P value for the marker (Table 3), indicating a negative association with submergence tolerance. The AEX and Sub1C marker did not show any significant association, which was evident from cluster analysis also. Only one marker that showed a significant association with survival percentage under submergence was Sub1A203, indicating its association with tolerance to submergence.
4 Discussions
The phenotyping results indicated that nine genotypes were observed to be tolerant with 100% survival, while 13 genotypes were moderately tolerant to submergence. Sub1A203, a direct marker for submergence tolerance, could screen better as compared to markers like AEX, Sub1BC2, and RM 8300. AEX is a specific designed DNA marker with SNP at its 3’ end, and was exclusively designed for rice genotype IR40931 containing the Sub1A allele [11]. Hence, this marker may not be able to differentiate other genotypes, which is evidenced by the present study. The linked microsatellite marker RM8300 is located 300 kb away from the Sub1A allele. So, there is a chance that this marker might not always be able to differentiate the genotypes perfectly for the presence of the Sub1A locus. This marker could group 71.43% tolerant genotypes into one cluster in the present study. The Sub1BC2 marker being an intergenic marker of Sub1B and Sub1C stood next to Sub1A203 in terms of discrimination ability, which is quite obvious. However, some susceptible and elongating types were grouped into tolerant clusters. Hence, the use of these markers with its various combinations was tested for screening of lowland genotypes. Earlier reports suggest that a single Sub1 specific primer was unable to differentiate tolerant and susceptible genotypes for submergence [19]. Singh et al. [20] suggested that the use of a higher number of Sub1 specific primers needs to be tested for identifying new genes/alleles. Hence, the marker combinations were tried for the discrimination of genotypes for submergence tolerance, and it was observed that the Sub1A203 + Sub1BC2 combination could group the genotypes better amongst the combinations considered in the study. Though it could not incorporate more tolerant genotypes into one cluster as compared to the best single markers Sub1A203 and Sub1BC2, it could still eliminate more susceptible and elongating types.
The cultivars showing high percentage of survival (100%) were FR13A, Khoda, CR Dhan 300, Swarna Sub1, Savitri Sub1, IR64 Sub1, IC 568009 and IC 568842. From the elongation study, it was observed that all these genotypes were of the non-elongating type, except the landrace ‘Khoda’. Hence, it is concluded that genotypes FR13A, CR Dhan 300, Savitri Sub1, IR64 Sub1, IC 568009 and IC 568842 were submergence tolerant: they can tolerate submergence for two weeks, while Khoda may possess both traits for survival. From the genotyping study, it is observed that Khoda showed positive amplification with direct markers AEX and Sub1A 203. Hence, it may be concluded that the landrace contains Sub1 QTL along with low elongation ability for its adaption. This is a rare situation where both traits are present in one genotype. Similar results have been reported by Sarkar et al. [21] and Sarkar and Bhatacharjee [19], in which both submergence tolerance and elongation ability traits are present in few landraces. In this study, thirteen genotypes were found to possess moderate tolerance to submergence. Eight genotypes, i.e. Boitalpakhia, Gayatri, Atiranga, Aghonibora, J/C(Jalamani), Moti, IC567993 and IC568921, were recorded with both characters, i.e. moderate elongation ability with moderate tolerance to submergence. This is also evidenced from the cluster analysis where one major cluster possessed a higher proportion of genotypes with submergence tolerance plus elongation ability. Hence, this is a common adaptive feature seen in the case of the lowland genotypes of eastern India. The occurrences of flash flood is a common feature along with frequent inundations for more than two weeks and may remain up to one month with water depths of 30 to 50 cm. Similar conditions have been described by many scientists [19,21–23], wherein they have described that some rice-growing areas were affected by only flash flood or both flash flood and stagnant flooding in different times or years. Genotyping results using molecular markers indicated that these genotypes possessed Sub1 intragenic and other markers. Hence, natural introgression of the submergence gene may be the reason for tolerance in these moderately tolerant genotypes. These genotypes also possess moderate elongation ability for tolerating accumulation of water for a longer period. These two traits should be in the breeding objectives of rainfed shallow lowland ecology. A standing flood with a water depth above 25 cm may adversely affect growth and survival of modern varieties. It hinders tillering and increases lodging and in some cases causes a severe reduction in crop stand [1,24,25]. Due to the lack of highly yielding varieties with these two traits, the farmers of the eastern region are also cultivating low-yielding landraces possessing moderate elongation ability with submergence tolerance. This is evident from the results of the genotype-by-trait biplot analysis (Fig. 1) by grouping representative genotypes, wherein cultivars like Matiaburua, Gangasiuli, Panindra, Moti and Amulya have both traits and form a sub-group. The other lowland cultivars were also clearly separated into various groups based on tolerance and elongation ability. The tolerance and moderately tolerance ability for submergence with moderate elongating types were clubbed into a single group. The biplot analysis result, when compared with the dendrograms of Sub1 markers, all these tolerant and moderately tolerant genotypes formed a separate sub-cluster, where IC 568842 and Khoda formed a separate sub-cluster.
Phenotyping results for submergence tolerance indicated that 21 genotypes were moderately tolerant to submergence. The molecular analysis of these 21 genotypes using two intragenic markers namely Sub1A203 and AEX indicated the presence of the Sub1A allele (Fig. 2). Submergence tolerance may be due in these genotypes to Sub1A locus. Earlier reports [26–28] have already stated the role of the Sub1A allele. When these tolerant genotypes were analysed using the Su1BC2 marker, which is linked to the Sub1B locus, it was observed that the majority of the genotypes exhibited a specific amplicon. Similar results were also obtained from an association study of marker alleles with phenotypic traits using the GLM model, exhibiting a higher F value and a lower P value for the marker (Table 3). This indicated a trend toward a negative association of Sub1BC2 with submergence tolerance. When all susceptible and tolerant genotypes were analysed, they revealed no association with Sub1C specific bands (Table 3) indicating that the role of Sub1C may be ignored for submergence tolerance. It was noted earlier that a limited expression of Sub1C was associated with tolerance [6]. But Septiningsih et al. [11] reported a non-significant contribution of the Sub1C allele.

Amplification pattern with Sub1A molecular markers. A. Sub1A203; B. Sub1BC2. C. RM 8300. D. Sub1C173. E. AEX with the genomic DNA of 95 rice genotypes (lanes 1to 95 are as per the genotypes listed in Table 2).
The highly tolerant genotypes for submergence, FR13A, Khoda, CR Dhan 300, Savitri Sub1, IR64 Sub1, IC 568009, and IC 568842, may be used as donor parents for flash flood areas through marker-assisted breeding. Landraces and cultivars possessing both submergence tolerance and elongation ability, like Khoda, Boitalpakhia, Gayatri, Atiranga, Aghonibora, Jalmani, Moti, IC 567993, and IC 568921, may be utilized as donors for developing varieties in a region affected by both moderate stagnant water and flash flood areas. Use of Sub1A203, a direct marker is better for differentiating tolerant species from intolerant to submergence species, as compared to AEX, Sub1BC2 and RM 8300. Sub1A203 and Sub1BC2 combinations could group the genotypes better amongst the combinations studied. Besides, it may be inferred that the role of Sub1B and Sub1C in submergence tolerance cannot be ignored.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.


