1 Introduction
Individual housing represents an impoverished physical and social environment for gregarious animals, which may have deleterious effect on behavior and brain [1–4]. For instance, social deprivation has deleterious effects on the mammalian pre-frontal cortex or its avian homolog, the nidopallium caudolaterale [2]. In songbirds, individual housing also leads to the development of stereotypies (repetitive, unvarying, and apparently functionless behaviors) that correlate with signs of altered brain striatal functioning [5]. However, individual housing is commonly used in behavioral neuroscience protocols because it facilitates the acquisition of individual data [6–10]. The zebra finch (Taeniopygia guttata) is a songbird widely used as a model system for the study of vocal learning, vocal production, and auditory perception [11–13]. Although the zebra finch is a highly social species, male zebra finches are commonly housed in individual cages or soundproof booths for weeks in order to record their songs or undergo neurophysiological experiments in laboratories [14–17]. In their native environment, the sub-arid zones of Australia, zebra finches form year round groups of dozens to hundreds of birds and establish life-long pair-bonds [18]. In this species, social interactions with pair-mate and group-mates occur through intense vocal communication [18–24], but also largely through physical interactions that can be both affiliative [e.g., grooming (allopreening), perching in close contact (clumping), rubbing each other beaks (beak fencing)] or aggressive (e.g., pecking, chasing, aggressive beak fencing) [18,25–28]. Such physical interactions are essential to pair-bond establishment and socialization [18,28,29]. Zebra finches that experience physical separation from their social partners with or without maintained acoustic contact exhibit an increased plasmatic corticosterone level [14,27]. This increase in physiological stress hormone suggests that suppression of physical social contact is a potent stressor in this species and confirms that cage-mates are social stimuli that normally elicit affiliation [30]. The zebra finch is a model of choice to study the effect of housing conditions resulting in long-term deprivation of socialization on vocal behavior and brain activity.
In birds that learn their vocalizations by imitation of a conspecific tutor, song ontogeny and auditory sensitivity are highly influenced by the acoustic environment experienced by the bird [13,31]. Young birds would for instance develop hybrid songs if they are given two successive tutors during the sensory period of learning [32]. The acoustic environment of adult birds also influences their perception of sounds. More precisely, it changes the tuning properties of neurons in the caudomedial nidopallium, a telencephalic auditory area that selectively responds to conspecific vocalizations [4]. Although the importance of the characteristics of the acoustic environment has been described in both young and adult birds, housing condition variations (single versus group housing) have never been considered as potential parameters of the acoustic environment. Here, we tested whether group or individual housing influences the quantity and quality of vocalizations emitted by male zebra finches.
Evidence across vertebrates shows that evolutionarily conserved brain circuits in limbic regions are implicated in the motivational processes of social behaviors [33–35]. Because of its role in animal social behavior, the so-called “social behavior network” (SBN) could be deeply affected by a lack of social interactions due to poor housing conditions. The SBN is mainly constituted of midbrain areas (ventral tegmental area and periaqueductal gray, substantia grisea centralis in birds) and, in the forebrain, of the medial extended amygdala (medial amygdala—formerly nucleus taeniae [36,37]—and medial bed nucleus of the stria terminalis [BSTm] in birds), the preoptic area (POA), the anterior hypothalamus (AH), the ventromedial hypothalamus (VMH), and the lateral septum (Sl). Studies of the functional activity of these regions have identified several overlapping subnetworks implicated in agonistic, reproductive, or maternal behaviors [34]. No particular region is involved in a specific behavior, but the balance of activity within each area and across all the areas of the network seems to determine the behavioral output [34]. In birds, studies of immediate early gene (IEG) expression revealed that distinct but overlapping neural networks of the SBN are active during aggressive encounters [38,39] and during reproductive behaviors [37,40].
Functional activity of the SBN has mainly been described in reproductive and agonistic contexts during short encounters implicating a small number (two to three) of individuals, like resident-intruder confrontation in territorial species, male–male competition for access to females or male–female courtship [30,33,37–47]. Little is known about the effect on SBN activity of repeated exposure to social stimuli due to social living, or lack thereof [30,33,46,48].
In this article, we hypothesize that physical restraint and lack of social interactions derived from individual housing conditions should change the activity across the network. To analyze the impact of physical and social stress due to individual housing on vocal behavior and brain activity, we compared the quality and quantity of the vocal output, and the basal activity of septo-hypothalamic regions of the SBN of zebra finches in two housing conditions commonly used in behavioral neuroscience protocols:
- • birds housed in adjacent individual cages with visual, auditory, and olfactory, but no tactile contact, that result in physical restraint and absence of physical interaction;
- • communally-housed birds interacting freely within a social group.
Brain activity was assessed using immunoreactive nuclei density of the IEG Zenk (egr-1). Because brain structures of the SBN are constituted of several neural populations that differ both in the neuro-peptides they express [49] and in the way they respond to social stimuli [30], we conducted double labelling of Zenk and VT (vasotocin) to further identify the active neural populations. Indeed VT cells could be an interesting cell population to follow because, on the one hand, their activity has been associated with the perception of social stimuli eliciting affiliation in the BSTm [30], the display of aggressive behaviors in POM [38,50–52] and possibly housing conditions in the BSTm [53], and on the other hand, VT receptors have been implicated in the avian stress response [54]. In the present study, brain activity was then related to behavioral activities of birds like physical and vocal interactions.
2 Materials and methods
2.1 Subjects
Two groups of male adult zebra finches (T. guttata; with wild type plumage, more than 90 days of age) were set up for this study: a “social” group and an “isolated” group. Prior to the experiment, all birds (19 males) were born and reared in mixed groups (groups containing young and adults of both sexes) in communal flight-rooms of around 60 birds in our aviary. For each group, males were captured and enrolled in the experiment on the same day. Whereas birds of the social group (n = 9) could freely interact in a 1-m3 cube cage for 10 weeks, birds of the isolated group (n = 10) were individually housed in adjacent cages (40 × 35 × 25 cm) for seven weeks so that they could only visually and acoustically interact. Seven to 10 weeks of time housed together is largely above the time necessary to form social bonds in this species [18,55,56]. Moreover, functional memories of social acoustic signals (songs) can form and affect the response of the immediate early gene zenk after as little as 3 h of passive exposure [18,55,56]. Individual cages represent common housing conditions used in behavioral neuroscience protocols [14,16,57,58]. Each group was acoustically and visually isolated from each other. All other housing conditions were the same for the two groups, before and during the experiment: 14L/10D photoperiod, food (seeds and fresh vegetables) and water provided ad libitum; temperature maintained between 22 and 25 °C. In the cage of the social group, food was available at five feeders and water was available at four water troughs, scattered on walls and on the floor. So, competition for food or water was unlikely in the social group, and indeed, was never observed. Experiments were performed under the authorization No. 42-218-0901-38 SV 09 (ENES Lab, Direction départementale des services vétérinaires de la Loire). The study was carried out in accordance with the French and European legislation regarding experiments on animals.
2.2 Behavioral observations of the social group
The nine male birds of the social group were sacrificed the same week, from the 77th to the 80th day after the group constitution, at the rate of two or three (last day) birds per day. This particular week (from the 77th to the 80th day) social interactions of each bird were recorded for 30 min, 30 to 60 min after the onset of light and 60 to 90 min prior to its sacrifice. A trained observer (JEE) sat quietly behind a black curtain provided with a 20 × 10-cm window and monitored all the social interactions of the two or three birds that will be sacrificed on that particular day. The identity of each bird was assessed by a unique combination of color rings. To characterize the social interactions of each bird, we considered several behaviors [18]:
- • affiliative behaviors: allopreening (the focal bird cleaned the feathers of another one), clumping (the focal bird perched side by side with a group-mate, often erecting its feathers), following behavior (the focal bird moved from perch to perch, always remaining close to the same group-mate during the whole period of observation, i.e. 30 min), courtship dance (series of perching movements associated with courtship in male);
- • aggressive behaviors: chase (the aggressor supplanted a cage-mate), threat (the threatening bird lowered its head and pointed its bill in an aggressive manner toward a group-mate that it generally displaced), peck (the aggressor pecked another bird), aggressive beak fence (two opponents jabbed their close bill at the head and the bill of the other one).
2.3 Behavioral data analysis
Two outcome variables were derived from the behavioral interactions recorded: the number of aggressive encounters (total number of aggressive behaviors that the bird performed or was victim of) and a score reflecting affiliation. This latter score varied from 0 to 4 and gained 1 point whenever the bird performed at least one of the affiliative behaviors described above [18].
We calculated Spearman correlation coefficients between these two behavioral scores and:
- • the different parameters reflecting brain activity (see below, n = 7, brain histology data were not available for two subjects);
- • the plasma level of testosterone (see below, n = 7, hormone data were not available for two subjects).
2.4 Acoustic recordings of birds in social group and individual cages
Isolated birds could not physically interact as birds of the social group, but males in both groups could exchange vocalizations. To monitor vocal activity, groups were acoustically recorded for about 90 min on the days of sacrifice (social group: four days; isolated group: five days) and on the preceding weeks (social group: seven days dispatched between the 65th day and the 75th day of the experiment; isolated group: 28 days dispatched between the 8th and the 43rd day after isolation of birds in individual cages). The recordings began 30 min after the onset of light, using two microphones (Sennheiser, MD42) hanged from the room ceiling, 20 cm from two different sides of the cage (social group) or group of cages (isolated group) and connected to a stereo recorder (Marantz PMD670, sampling frequency 44.1 kHz).
2.5 Acoustic data analysis
Vocalizations were automatically extracted from recordings using custom-written computer programs. These programs are written in Python language (http://www.python.org/) using open-source libraries for either wave files manipulation, Fourier and Spectral analysis and scientific calculus. The set of scripts used in this paper is freely available upon request to the authors and is fully described in [59].
Briefly, this set of programs allows for accurate detection of birds’ vocalizations among hours of recording in a batch process. The detection itself is a pipeline of three treatments: first, sound events are detected based on an energy threshold, then a second treatment refines the bounds of the computed slice and a third treatment simply merges overlapping slices. All those treatment phases yield, at the end of the process, a list of triplet (start, end, duration) for each sound detected in the recording. In addition, the programs can extract sound samples.
For the analysis, we used both recordings on days of sacrifice (social group 384 min in four days; isolated group 420 min in five days) and recordings on weeks preceding the sacrifice (social group 635 min in seven days; isolated group 2666 min in 28 days). The number of vocalizations was normalized for each day with the number of individual remaining in the group (sacrifice-preceding days: social group n = 9, isolated group n = 10; days of sacrifice: two birds removed per day, see sacrifice procedure below), and the recording length. Using R [60], the normal distribution and homogeneity of the variances of the data sets were verified using respectively Shapiro's and Bartlett's tests, before conducting parametric non-paired t-tests or non-parametric non-paired Mann–Whitney U-tests to compare the number of vocalizations between the social group and the isolated group and between periods (before vs. during the week of sacrifice). We also looked for an effect of the decreasing number of birds on the mean vocalization rate along the sacrifice weeks with a Kruskal–Wallis test (two birds removed per day).
In addition to the number of vocalizations, the repertoire used by birds of the two groups was also analyzed. Sounds randomly sampled from the recordings at the rate of fifty or twenty five per daily recording (social group: 100 samples in four recordings on weeks preceding the sacrifice, 200 samples in four recordings during the week of the sacrifice; isolated group: 100 samples in four recordings on weeks preceding the sacrifice, 250 samples in five recordings during the week of the sacrifice) were classified according to the spectrograms by the same person (JEE) in four type categories [18]: noise (cage noise, wing noise… etc.), distance call, tet call, and song syllable. The repertoires of vocalizations (% of each vocalization type, noises—26 out of 650 samples—were rejected from the analysis) of the social group and the isolated group were studied using a linear mixed model (LMM) with the vocalization type (three levels: tet call, distance call and song syllable), the group (two levels: social group vs. isolated group), and the period (before the week of sacrifice vs. during the week of sacrifice) as fixed factors and the recording date (17 levels) as a random factor. We considered second-order and third-order levels of interaction effects. To perform LMM, we used the lme() function of the nlme package under R environment [60]. All post hoc tests were done using a t-test with Bonferroni correction.
2.6 Histology and immunocytochemistry
2.6.1 Sacrifice
Males of each group were sacrificed on the same week: social group: from the 77th to the 80th day after group constitution; isolated group: from the 53rd to the 57th day after isolation of males. Because the males of the social group established the same-sex pair-bonds described in Elie et al., 2011, we decided to sacrifice birds at the rate of two (both partners of a same-sex pair) or three birds per morning. Two to three hours after the onset of light, the birds were captured, deeply anesthetized by vapors of isoflurane (two successive injections of 200 μL in a 500-cm3 jar, AErrane®, Baxter, Lessines, B), intracardially punctured (1 mL), then perfused with a phosphate buffer saline (PBS) 0.1 M solution (pH 7.4, 20 mL, P-3813, Sigma-Aldrich, Steinheim, Germany) followed by addition of paraformaldehyde (PF) (4% pH 7.4, 20 mL, P6148-500G, Sigma-Aldrich, Steinheim, Germany). The brains were rapidly removed from the skull and postfixed in PF 4% for seven to nine hours at room temperature prior to cryoprotection for at least two days in 30% sucrose–PBS (179949-1KG, Sigma-Aldrich, Steinheim, G) at 4 °C. Then, the brains were frozen in nitrogen and stored at –80 °C before cutting at 14 μm on a cryostat. The frontal sections kept for the study extend from 3 mm behind the rostral point of the brain until the end of the cerebral hemispheres. Tissue was mounted on slides SuperFrost®Plus (H867.1, Menzel GmbH&Co, KG) and equivalent series were either stained with cresyl violet or processed for immunocyto-labeling of Zenk and VT. As we performed four equivalent series, a series contained one sample section every 42 μm.
2.6.2 Circulating testosterone level
Immediately after puncture, blood samples (around 1 mL per subject) were centrifuged at 4 °C, plasma separated and stored at –20 °C. The testosterone concentration was later determined by radioimmunoassay (RIA) using antibodies specific for testosterone. Testosterone was extracted from a 50-μL plasma sample in diethyl-ether and determined in duplicates in one single assay. Duplicate aliquots of the extracts redissolved in 0.01 M phosphate-buffered saline (pH 7.4) containing 0.1% bovine albumin (PBS-BSA) were incubated overnight at 4 °C with ca. 9000 cpm of the appropriate 3H-steroid and anti-serum. Protocols were developed and validated for birds at the CEBC (Centre d’études biologiques de Chizé; CNRS UPR1934) as detailed in Chastel et al. [61].
2.6.3 Brain immunocytochemistry
Brain immunocytochemistry was performed on one of the four series as follows [62–64] (Fig. 1): 10 min in PBS 0.1 M (pH 7.4); 30 min in PBS + 2% BSA (Albumin from Bovine Serum, A2153-10G, Sigma-Aldrich, Steinheim, G) + 0.3% Triton® X-100 (S26-36-23, Sigma-Aldrich, Steinheim, G); three 5 min rinses in PBS; endogenous biotin blockage (Biotin Blocking System, X0590, DakoCytomation, DK); 7–9 h (25 °C) and 14–16 h (4 °C) incubation with guinea pig anti-VT (1:1000, T-5048, Bachem, Ca) and rabbit anti-EGR-1 (1:1000, sc-189, Santa Cruz Biotechnology, Ca); three 10-min rinses in PBS; 1 h (21 °C) incubation with biotinylated goat anti-guinea pig secondary (1:400, sc-2440, Santa Cruz Biotechnology, Ca); three 10 min rinses in PBS; 2 h (21 °C) incubation in goat anti-rabbit secondary conjugated to AlexaFluor 546 (1:750, A11071, Molecular Probes Invitrogen, OR) + streptavidin conjugated to AlexaFluor 488 (1:750, S11223, Molecular Probes Invitrogen, OR). All antibodies were diluted in PBS + 2% BSA + 0.3% Triton® X-100. Sections were extensively washed in PBS before mounting in Sigma Diagnostics® mounting medium (Sigma-Aldrich, Steinheim, Germany). The brains from both conditions were processed in parallel, so differences in cell density could not be attributed to a variation in immunocytochemistry runs.
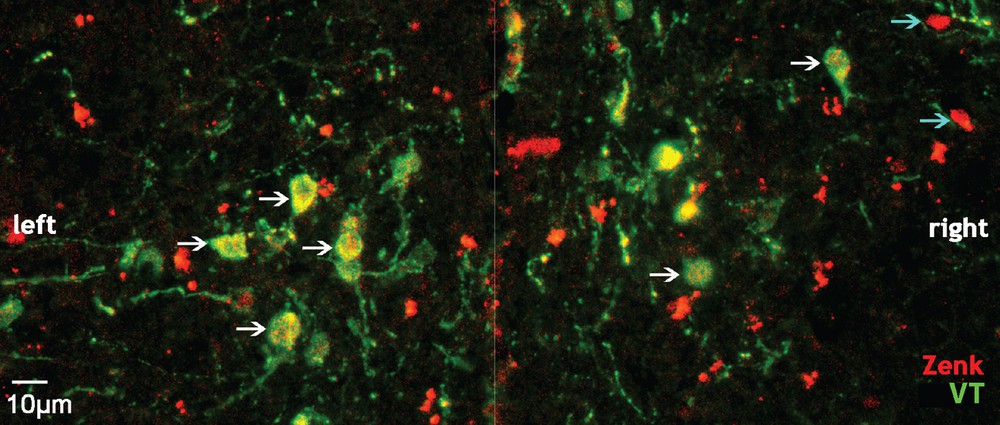
(Color online.) Photomicrograph of labelled cells in the paraventricular nucleus of the hypothalamus (PVN) of an isolated male (coronal view). The white arrows indicate cells co-labelled for Zenk and vasotocin (VT); the blue arrows indicate cells labelled for Zenk only; the white line indicates the midline position.
2.7 Histological data analysis
2.7.1 Brain histology
For each individual, structures locations were finely determined using the series stained with cresyl violet. We bilaterally quantified Zenk-ir (Zenk-immunoreactive) nuclear profiles and VT-ir (VT-immunoreactive) cells within the paraventricular nucleus of the hypothalamus (PVN) and four zones of the social behavior network [33]: medial preoptic area (POM), ventromedial hypothalamus (VMH), medial bed nucleus of the stria terminalis (BSTm), lateral septum (Sl) (Fig. 2).
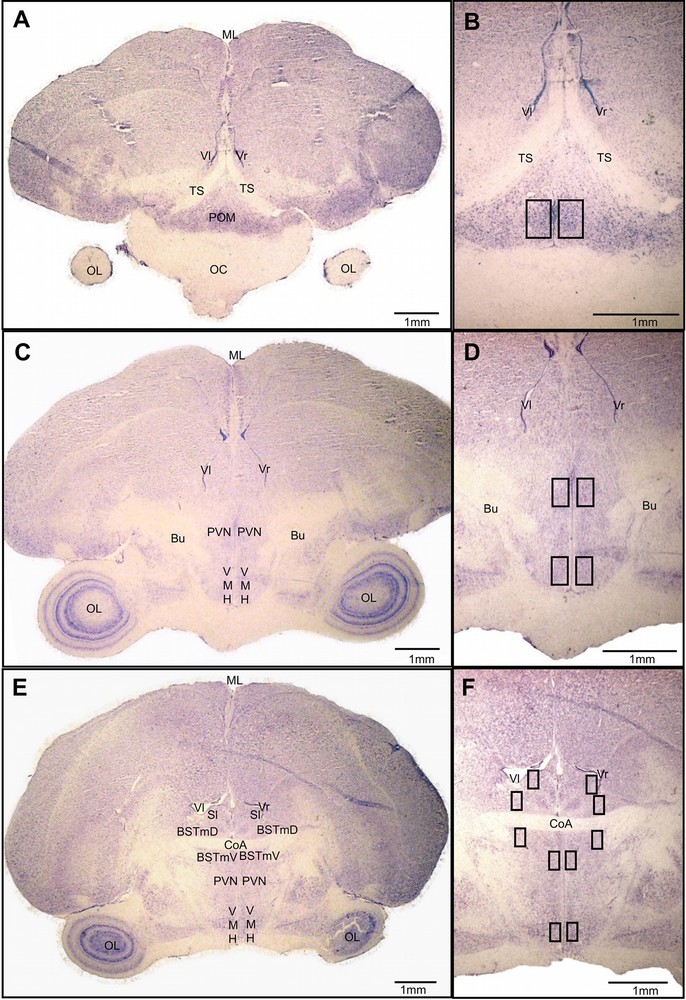
(Color online.) Social behavior network structures (A, C, E) and location of analyzed areas (B, D, F). Coronal sections stained with cresyl violet. Along the rostro-caudal axis (A to E): POM was quantified below the TS (A, B), PVN and VMH at the level of the Bu (C, D), and BSTm, PVN, VMH and Sl around the CoA (E, F). BSTmD: dorsal part of the bed nucleus of the stria terminalis; BSTmV: ventral part of the bed nucleus of the stria terminalis; Bu: bundle or fasciculus prosencephali lateralis; CoA: anterior commissure; ML: medial line; OC: optic chiasma; OL: optic lobe; POM: medial preoptic area; PVN: paraventricular nucleus of the hypothalamus; Sl: lateral septum; TS: tractus septomesencephalicus; Vl: left ventricle; Vr: right ventricle; VMH: ventromedial hypothalamus.
POM: data were quantified at the level of the preoptic area region that lies ventromedial to the tractus septomesencephalicus (see Fig. 2A and B) (4–10 sections per individual spaced 42 μm apart).
VMH and PVN: we examined zones of the VMH and PVN that were situated below the fasciculus prosencephali lateralis (or bundle) (see Fig. 2C and D) and below the anterior commissure (see Fig. 2E and F) (9–16 sections per individual interspaced by 42 μm). The position of the PVN was delineated based upon the distribution of VT-ir neurons [65].
BSTm: the data were analyzed in two zones, the first one above the anterior commissure and the second one below this same tractus (see Fig. 2E and F) (3–6 sections per individual interspaced by 42 μm).
Sl: we quantified immunoreactive cells in the ventro-lateral region of the lateral septum at the level of the anterior commissure, where VT fibers are the densest (see Fig. 2E and F) [38] (4–6 sections per individual interspaced by 42 μm).
For each zone of interest (6), two photomicrographs were generated in each section (3 to 16 depending on the brain area and on the subject) and in each hemisphere, one for Zenk-ir nuclei and another for VT-ir cells using a Leica DMLB microscope provided with Fluo HBO100 W and a Nikon camera (Coolpix 4500). Then, for all these locations, in all subjects, we used the same 9.5 × 10−2 mm2 box outline of the area to be quantified. The box was placed as indicated on Fig. 2B, D, E using anatomical landmarks. All Zenk-ir nuclei and VT-ir cells were quantified inside the box. Cells co-labeled for Zenk and VT were quantified by superimposing monochrome pictures of Zenk-ir nuclei and VT-ir cells when cells were too numerous and/or densely packed; otherwise, they were quantified without the use of photomicrographs. Depending on the subject and on the brain area quantified, this protocol provides us with 3 to 16 sample-counts of Zenk-ir nuclei and VT-ir cells per hemisphere. For the BSTm, although data were quantified in two zones per hemisphere (dorsal and ventral), they were further analyzed considering both zones together. So, instead of 3 to 6 sample-counts per hemisphere, this yielded 6 to 12 sample-counts.
Statistical analyses were performed using R [60] on the brain results of seven individuals of the social group and 10 individuals of the isolated group. For each subject, we computed the mean numbers of Zenk-ir and VT-ir cells per mm2 from the cell counts in the 3 to 16 sections (depending on the region considered), first in each region of interest (POM, VMH, PVN, BSTm and Sl) and then considering all the studied structures. In the same manner, the mean number of co-labeled cells and the mean percentage of VT-ir cells expressing Zenk were calculated, for each subject, first in each one of the three areas containing VT-ir cells (PVN, BSTm and POM), and then taking altogether these three areas. A paired Wilcoxon test comparing mean subject values between the two hemispheres did not reveal any significant difference whatever the location or the parameter, so we pooled data of the two hemispheres. These latter mean subject values were compared between the two experimental groups using Mann–Whitney U-tests. Because the values of the isolated birds sacrificed on the last two days differed from others, we divided the isolated group for further analysis into two subgroups: birds sacrificed early (the three first days) vs. late (the two last days). These two subgroups were compared with a Mann–Whitney U-test. Finally, correlation of activity of areas of interest was estimated by calculating the pair-wise Pearson correlation coefficients (Σ[(Xi − mean(X)) × (Yi − mean(Y))]/[SD(X) × SD(Y)]) on the density of Zenk-ir cells, that of VT-ir cells, and that of co-labeled cells.
2.7.2 Level of circulating testosterone
The levels of circulating testosterone were compared between the two groups (social group n = 9, isolated group n = 10) by a Mann–Whitney U-test using Statistica 6.1 software, StatSoft, Inc. We also calculated the Spearman coefficients of correlation between the concentration of testosterone and any of the four variables reflecting the brain state and the activity described above, for 15 of the subjects (the histology data of two subjects from the social group and blood samples of two other subjects of the social group were missing).
3 Results
Whereas males from the social group freely socialized in their common cage, isolated males only interacted acoustically and were not given the opportunity to interact physically with their neighbors. Free socialization resulted in the establishment of a complex social structure in the social group: males established stable same-sex pair-bonds that were characterized by a high level of affiliative interactions and an absence of aggressive behaviors between partners. In 10 min, a male participated on average in 0.78 allopreening bout with his partner [median (0.25–0.75 interquartile range): 0.78(0.67–1)] and engaged his other group-mates in 4.56 aggressions [4.56(1.89–17.44)] (see Elie et al., 2011 for a full description of the social structure of this same-sex group). Thus, the social environments of the two groups were remarkably different.
3.1 Effect of individual housing on vocal behavior
Individual housing modified acoustic interactions of birds compared to group housing. The relative proportions of vocalizations (repertoire) used by males were different between social group and isolated males (F2,26 = 11.4, P < 10−3; Fig. 3A), but the period (before vs. during the week of sacrifice) had no effect on the repertoire used neither in the social group, nor in the isolated group (second-order level of interaction: F2,26 = 0.35, P = 0.71; third-order level of interaction: F2,26 = 1.76, P = 0.19). Isolated males sang more (60.6 ± 14% vs. 36.9 ± 11.9%; t-tests with Bonferroni correction: P < 0.01) but tended to emit fewer tet calls (10.4 ± 6.5% vs. 29.9 ± 18.7%; t-tests with Bonferroni correction: P = 0.055) compared to birds of the social group.

Effect of physical isolation on group vocalizations. A. Repertoire use is different between birds in isolated and social condition (F2,26 = 11.4, P < 10−3). Isolated males sang more but emitted less tet calls than males living in social condition (t-test with Bonferroni correction, P < 0.01). No effect of the period could be detected for either group (second-order level of interaction: F2,26 = 0.35, P = 0.71; third-order level of interaction: F2,26 = 1.76, P = 0.19). B. Vocalization densities in social and isolated groups during the weeks preceding the sacrifice and on the sacrifice week. Before the sacrifice week, isolated birds vocalized more than males of the social group (*, U-test, P = 0.01). Their vocalization density was not perturbed on days of sacrifice (Ø, U-test, P = 0.32). On the contrary, birds of the social group increased their vocalization density (***, t-test, P < 10−3) and vocalized more than isolated birds on sacrifice week (**, t-test, P < 0.01).
Moreover, on weeks preceding sacrifice, isolated birds vocalized more than birds living in social group (U28,7 = 37, P = 0.01; Fig. 3B). This difference reversed on days of sacrifice (two birds were removed each day). Whereas isolated birds did not change their rate of vocalizing (U28,5 = 91, P = 0.32; Fig. 3B), males living in social group increased their density of vocalizations above the level of isolated birds (t-test isolated vs. social groups: t7 = 3.67, P < 0.01; t-test social group before vs. on weeks of sacrifice, t9 = −6.53, P < 10−3; Fig. 3B). But in both groups, the mean vocalization rate was not significantly influenced by the decreasing number of individuals along the sacrifice week (social group: KW3 = 3, P = 0.39; isolated group: KW4 = 4, P = 0.41).
3.2 Effect of individual housing on Zenk-activity
Although isolated birds could not physically interact and vocalized differently from birds living in social group, their brain activity was not significantly different in the septo-hypothalamic regions analyzed in this study (all structures pooled: U10,7 = 31, adjusted Z = −0.39, P = 0.7) except in the BSTm. In this limbic region, isolated birds presented significantly more Zenk-ir nuclear profiles (expressed as Zenk-ir nuclei per mm−2) compared to males in social group (U = 16, Z = −2.08, P = 0.04; Fig. 4A). In the four other analyzed areas (POM, PVN, VMH and Sl), the variability between individuals within each group was high and no difference between groups was detected (POM: U = 30, Z = −0.49, P = 0.62; PVN: U = 28.5, Z = −0.66, P = 0.51; VMH: U = 34, Z = 0.1, P = 0.92; Sl: U = 20, Z = −1.71, P = 0.09). However, the Zenk-ir cell density in the PVN of the isolated group tended to be higher than in the social group (isolated group: 9.75 ± 6.56; social group: 0.53 ± 0.27; Fig. 4A). Only one bird of the social group presented Zenk-labeled cells in the Sl region (with only 1.05 cells·mm−2) compared to five isolated birds (mean ± SE on these five individuals: 81.28 ± 44.75). In these three regions (BSTm, PVN and Sl), isolated birds that presented an increased Zenk-activity were the males sacrificed on the last days (Fig. 4B). The four isolated males sacrificed on the last two days had significantly more Zenk-ir cells in the BSTm and Sl compared to the six males sacrificed on the first three days (BSTm: U6,4 = 0, Z = −2.65, P < 0.01; Sl: U = 0, Z = −2.73, P < 0.01, Fig. 4B). Thus, the decreasing number of individuals along the sacrifice week influenced Zenk-activity in isolated birds, but not in social group.
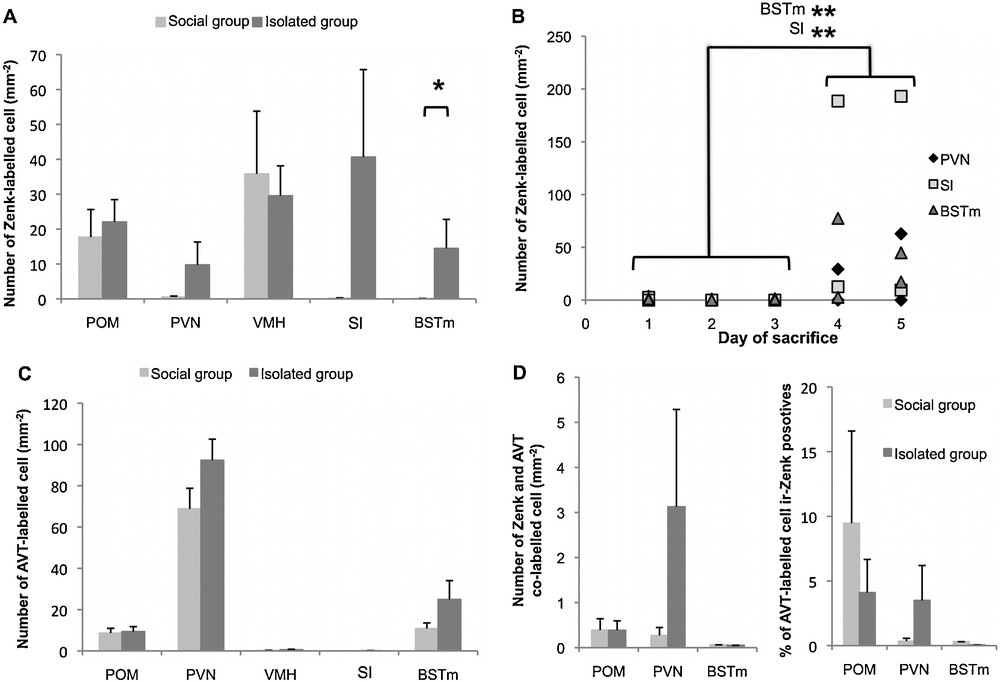
Effect of physical isolation on Zenk immunoreactivity (A, B, D) and vasotocin (VT) cell density (C) in medial preoptic area (POM), paraventricular nucleus of the hypothalamus (PVN), ventromedial hypothalamus (VMH), lateral septum (Sl) and medial bed nucleus of the stria terminalis (BSTm). A, C & D mean ± SE; B: individuals’ values of isolated birds. Light grey: social group; dark grey: isolated birds. Out of the five studied structures, an effect of physical isolation on the density of Zenk-ir cells was detected in the BSTm and in the Sl. The increase in Zenk-ir cell density relative to socially housed birds in these two areas was significant in the isolated birds sacrificed on the last two days (A & B, U-test, * P < 0.05, ** P < 0.01). These Zenk-ir cells did not co-localized with VT (D).
Consistent with previous description [51], we found VT-ir cells in three regions: POM, PVN and BSTm (Fig. 4C). Variability within group was high and we did not find any significant difference in the density of VT-ir cells between the two groups in any of the three regions (POM: U10,7 = 34.5, adjusted Z = 0.05, P = 0.96; PVN: U = 23, Z = −1.17, P = 0.24; BSTm: U = 23, Z = −1.17, P = 0.24). Even when we considered the VT system as a whole, pooling the results obtained for the three structures, we did not find any significant effect of physical isolation (U = 19, Z = −1.56, P = 0.12). There was also no difference neither in the number (Fig. 4D) nor in the percentage (Fig. 4D) of VT and Zenk co-labeled cells between isolated and social groups in the three regions (co-labeled cell number: POM: U = 35, Z = 0, P = 1; PVN: U = 29, Z = −0.68, P = 0.49; BSTm: U = 34, Z = 0.17, P = 0.86; percentage of VT-ir co-labeled cells: POM: U = 33.5, Z = 0.18, P = 0.86; PVN: U = 32, Z = −0.36, P = 0.72; BSTm: U = 33, Z = 0.35, P = 0.73). Nevertheless, quantification of co-labeled cells indicated that the brain activity showed in the previous section in the BSTm of isolated birds was due to a non-VT cell population (Fig. 4D). No co-labeled cell could be detected in the BSTm of 9 out of the 10 isolated birds, whereas we did observe co-labeled cells in PVN and POM of the same individuals (Fig. 4D), thus validating our labeling protocol. Then the significant difference between isolated birds and birds in social group in the number of Zenk-ir cells in the BSTm is due to non-VT cells. The increased activity in the PVN of isolated birds sacrificed on the last two days was due to both VT and non-VT cells, since around half of the Zenk-ir cells were also immuno-responsive for VT (Fig. 4A and D).
Finally, we examined covariance in Zenk-revealed activity of the studied areas of the SBN and the PVN. Isolated birds showed a high number of significant co-variations (r > 0.7) between the studied structures (Fig. 5). The pattern of high covariance among this neural network was different between the two groups of males. Whereas isolated birds showed activity correlation values superior to 0.7 between PVN and Sl/BSTm/VMH as well as between VMH and POM/Sl and finally between BSTm and Sl (Fig. 5), such a high correlation value was observed only between PVN and VMH as well as between POM and Sl in birds living in social group (Fig. 5).

(Color online.) Effect of physical isolation on covariance activity pattern as revealed by the density of Zenk-ir nuclei. Upper panels present pair-wise Pearson correlation coefficients between studied structures [ventromedial hypothalamus (VMH), medial preoptic area (POM), paraventricular nucleus of the hypothalamus (PVN), medial bed nucleus of the stria terminalis (BSTm), lateral septum (Sl)] or structures restricted to active VT cells population [POM vasotocin (VT), PVN VT]. Diagrams below represent high (r > 0.7) values of correlation coefficients with arrows. Plain arrows, high covariance in Zenk-activity; dotted arrows, high covariance in Zenk-activity in VT-ir cells; grey arrows, high covariance in density of VT-ir cells.
3.3 Effect of individual housing on testosterone level
Preventing physical interactions did not influence the level of circulating testosterone. As revealed by radioimmunoassay on blood samples, birds of both groups had similar plasma concentrations of testosterone (social group: 134.28 ± 21.6 pg/mL; isolated group: 262.29 ± 61.57 pg/mL; U10,9 = 30, adjusted Z = −1.22, P = 0.22). The level of circulating testosterone could not be correlated with the activity of any of the SBN brain structures studied here (Table 1).
Coefficients of Spearman correlation calculated between the level of circulating testosterone and any of the four variables measuring brain activity in the five studied brain structures.
| POM | PVN | BSTm | VMH | Sl | |
| Zenk-ir nuclei number | −0.269 | −0.064 | 0.179 | −0.396 | 0.325 |
| VT-ir cell number | −0.304 | −0.182 | −0.064 | −0.395 | 0.309 |
| Co-labeled cell number | 0.375 | 0.028 | 0.000 | ||
| % of VT-ir cell co-labeled with Zenk | 0.329 | 0.021 | 0.000 |
3.4 Correlation between physical interactions and SBN activity or testosterone level of birds living in social group
Variability in brain activity and testosterone level in the social group can be related to the behaviors recorded on days of sacrifice. The number of aggressive encounters was positively correlated with the level of circulating testosterone and negatively correlated with the density of VT cells detected in POM (concentration of testosterone: Fig. 6A, r = 0.89, n = 7, P < 0.01; VT-ir cells: Fig. 6B, r = −0.77, n = 7, P < 0.01). The score reflecting affiliation (composite score reflecting allopreening, courting, clumping or following behaviors of males) was positively correlated with both the density and the proportion of VT-ir cells co-labeled with Zenk in POM, PVN and BSTm (total density of co-labeled cells: Fig. 6C, r = 0.86, n = 7, P < 0.02; total percentage of VT-ir cells co-labeled with Zenk: Fig. 6D, r = 0.86, n = 7, P < 0.02).
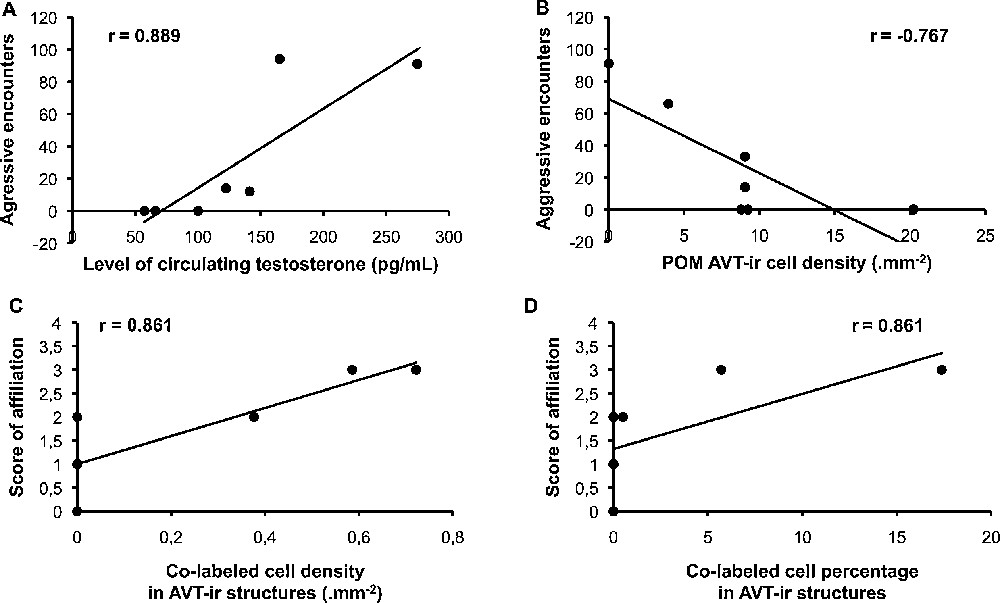
Correlation between behaviors, and brain activity or hormonal state in birds of the social group. On the one hand, the more birds engaged in aggressive interactions, the highest their level of circulating testosterone was (A, P < 0.01), but the lowest the density of vasotocin (VT)-ir cells was in medial preoptic area (POM) (B, P < 0.01). On the other hand, the more birds engaged in affiliative behaviors, the highest the density (C, P < 0.02) and percentage (D, P < 0.02) of co-labelled cells were in POM, paraventricular nucleus of the hypothalamus (PVN), and medial bed nucleus of the stria terminalis (BSTm).
4 Discussion
In the present paper, we compared the vocal behavior and SBN activity of male zebra finches maintained in two types of housing conditions commonly used in behavioral neuroscience protocols: birds housed in individual cages (isolated group) or birds communally-housed in aviary (social group). Individual cages not only restrained birds in their movements, but also prevented physical interaction necessary to normal socialization. The aviary on the other side allowed males of the social group to establish stable same-sex relationships (fully described in [28]).
4.1 Impact of individual housing on vocal behavior
On weeks before the period of sacrifice, we showed that isolated birds vocalized more and used a different repertoire than the social group birds. Confirming our previous results [59], isolation influences vocal communication between birds even if vocal and visual contacts are maintained. Additionally, modification of the vocal output of the social group during the sacrifice period seems to point to an effect of the perturbation of social links on vocal communication. Whereas isolated birds did not change their vocalization rate on the days they were sacrificed, birds in social group did. This increase in vocal output of group-living birds could be due to group-mate departure, although partners of the same same-sex pair were always sacrificed at the same time. Catching a subject in a communal cage is certainly more stressful for the group-mates than removing an individual cage, but the change in vocal output could also reflect the perturbation of social links previously established by birds in the social group, aside from the same-sex pair-bonds. Whatever the reason, differences in vocalization rate and in vocal repertoire between isolated birds and communally-housed males indicate that housing conditions (social interactions, physical restraint…) are key factors that determine the acoustic environment.
4.2 Moderate impact of individual housing on IEG activity in BSTm, PVN and Sl
As for brain activity, the observed effects are moderate, but we can at least underline that one brain area of the SBN was affected by our experiment: the BSTm, which showed a significantly increased Zenk-revealed activity in isolated males compared to males in social group. One other area of the SBN—namely Sl—and one region associated with the SBN—namely PVN—seemed to be more active in isolated birds compared to birds living in social group. This increased activity in BSTm, PVN and Sl appeared only in the isolated birds sacrificed on the last days. Zenk-revealed activity was also highly correlated across several areas of the SBN in isolated birds, but not in communally-housed birds. To sum up, individual housing with visual, auditory, and olfactory but no tactile contact had moderate effects on SBN activity in male zebra finches, but seems to influence the activity levels in three regions when birds experience the loss of neighbors and also to increase the correlation of activity throughout the studied structures of the SBN. To disentangle the exclusive role of single housing, a future study should investigate neural activity as revealed by IEG in singly and communally-housed birds sacrificed all at once, avoiding the experience of losing neighbors.
These moderate effects of physical isolation on SBN activity were not linked to variations in testosterone level. Isolated birds had testosterone blood levels similar to birds communally-housed. Consistent with previous studies on the implication of testosterone in social behaviors [66–70], the level of testosterone in birds living in social group was correlated with the number of aggressive encounters these birds engaged on days of sacrifice.
BSTm, Sl and PVN are reported to be part of the subnetwork activated when the subject is exposed to a social stress such as the agonistic stimuli emitted by a territorial competitor [39]. Here, we showed that individual housing with maintained auditory and visual contact in a social species increases Zenk-activity and the correlation of activity within this “social stress” subnetwork of the SBN when birds experience loss of neighbors. The increased Zenk-activity in BSTm, Sl and PVN was revealed in isolated birds sacrificed on the last two days. Because the activity levels in the two other studied regions (POM and VMH) were comparable between these four birds and the other birds, we could not attribute this increased Zenk-activity to an experimental artifact. Like a threshold effect, the “social stress” subnetwork was activated only when the isolated birds experienced loss of neighbors and the number of neighbors per male was below four birds, whereas communally-housed birds presented a quieter “social stress” subnetwork independent of the number of remaining group-mates. It is possible that physical interactions with their same-sex partner acted as a social buffer and thus may have dampened the activity of the “social stress” subnetwork in communally-housed birds that experienced loss of group-mates while individually housed males had no access to such physical interactions. The efficacy of social buffering depends both on the degree of affiliation between individuals and the number of companions during the stress event [71]. For instance, several familiar individuals would be needed to induce the same buffering effect as a bonded partner [71]. In the present study, isolated birds began to be stressed when there were only a few birds left. A prediction from the previous hypothesis would be that more companions would be needed to mediate social buffering in isolated birds compared to communally-housed males. The increase in the vocalization rate of communally-housed birds could also reflect social stress. The fact that the vocalization rate per bird remained constant along the week of sacrifice in the social group could suggest that social birds were stressed as soon as the first birds were removed for sacrifice. Then, it is possible that social links established with group-mates acted as a social buffer that changed the way birds responded to loss of group-mates, dampening the activity of the “social stress” subnetwork, but increasing their vocalization rate. In any case, our results give some indications that housing conditions during neuroscience protocols (isolated cages vs. communal housing) could participate in shaping SBN activity and modify vocal behaviors in group-living species. Moreover, these results underline an interesting point for future studies in behavioral neuroscience: removing one or two birds from a colony each morning for an experimental protocol has consequences on the behavior and brain activity of the remaining birds.
The diversity and complexity of the sensory stimuli in the social group certainly participate in the variability of the Zenk-ir responsiveness of the studied forebrain regions. We controlled at least the vocal activity of birds during the week of sacrifice, and we did not see any effect of group-mate departure along the week either in the social group or in isolated birds. Future works should further investigate the within-species variation in SBN activity in relation to the amount of social interactions using a more easily controlled setup. Zebra finches engaged in a pair-bond perform far more social affiliative behaviors (i.e. beak fence, allopreening, clumping) distinct from reproductive behaviors (courting, copulating, singing) with their partner than with other cage-mates, while singly housed birds cannot engage in such behaviors. The present study cannot conclude if the effect on IEG expression in the SBN is due to the stress of losing neighbors in combination with the immediate lack of physical interactions or in combination with the recurrent lack of physical interactions due to long-term single housing. Future work should address that question and could investigate for instance any correlation between the degree of social attachment and social interactions with the activity of the SBN.
4.3 Influence of social behavior occurrences but not housing condition on the activity of VT cells within the SBN
VT cells of the BSTm have been described as valence-sensitive neurons that are selectively activated by social stimuli eliciting affiliation in gregarious species [30]. Presentation through a wire barrier of a same-sex conspecific compared to no presentation increases the c-Fos responsiveness of vasoticin immuno-responsive (VT-ir) neurons of the BSTm in zebra finches. In our experiment, we could expect that birds in isolation, which were able to see and hear same-sex conspecifics through their cage barrier, would have modified Zenk-activity of VT-ir cells in BSTm relative to birds freely interacting with their group-mates. We did observe an increased activity of the BSTm, but in non-VT-ir cells. As we did not use the same IEG as Goodson and Wang [30] and because c-Fos and Zenk are not completely redundant markers of neural activity [40], it is possible that we did not detect the activity of the valence-sensitive cells because they might not express Zenk. Moreover, the presentation of conspecifics through a barrier in Goodson and Wang experiment was a short exposure (90 min), and thus very different from our isolated birds that lived in adjacent cages for seven weeks.
Although oxytocic nonapeptides have been implicated in the flocking and pairing behavior of females, neither mesotocin nor vasotocin seem to influence the propensity of males to be attracted by conspecifics [72,73]. The present study also failed to show an effect of individual housing on the activity of VT cells in the SBN of male zebra finches. Nevertheless, we did find a correlation between the number of affiliative behaviors engaged by males in the social group and the total number of active VT-ir cells across all the studied SBN areas. This could indicate that VT participates in modulating the propensity of males to engage in affiliative physical interactions with their partner.
We also found an inverse correlation between the number of VT cells in the POM and the amount of aggressive behaviors. VT has already been implicated in the promotion of aggressive behaviors in a wide range of species, including zebra finches [38,50–52]. In green anole lizards, the number of VT cells in the preoptic area is positively correlated with the hierarchical position of the individual [74]. The negative correlation observed in our study underlines the fact that the mediation of aggressive behaviors in zebra finches via VT cells in the POM remains to be elucidated.
5 Conclusions
Individual cages with maintained auditory and visual contact represent an extensively used housing condition, which not only restrains birds but also suppresses physical social interactions. In this paper, we showed that individual housing also modifies vocal activity compared to communal housing. This is of particular importance in the zebra finch, a model species for the study of vocal communication. This study indicated also some effects of individual housing—an impoverished environment on both physical and social aspects—on the IEG activity of several forebrain regions of the Social Behavior Network when birds experience loss of neighbors. Albeit moderate, these results underline the importance of controlling social history and immediate living environment of subjects when studying brain substrates of social behaviors and that the choice of housing conditions and sacrifice protocol must gain some attention in behavioral neuroscience protocols.
Acknowledgments
We warmly thank Colette Bouchut and Nicolas Boyer for all the technical support in the ENES laboratory and Olivier Chastel from the CEBC (CNRS, Centre d’études biologiques de Chizé, UPR 1934, Villiers-en-Bois, France) for titration of testosterone in blood samples.
This study was funded by the French “Agence nationale de la recherche” (ANR, projects “Birds’ voice” and “Acoustic Partnership”) and the “Saint-Étienne Métropole”. C.V. was supported by a Young Investigator Sabbatical of the “Université de Saint-Étienne” and by the “Institut universitaire de France”. N.M. was supported by the “Institut universitaire de France” and was funded during part of this study by the Miller Institute for Basic Research in Science, University of California, Berkeley (Visiting Miller Professorship). J.E.E. was supported by the French Ministry of Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.


