1 Introduction
The ‘domus’ in ancient Rome was a patrician family house. Those located in the countryside were known as ‘villa rustica’ (rustic villa). The area surrounding the harbour of Portoferraio (Elba Island), site of a subsequent Roman villa (Fig. 1), had been subject to intense extraction of iron ore [1,2]. The oldest traces of primary iron smelting date back to the 6th century BC [3]. Excavations brought to light the remains of walls defining five rectangular areas, belonging to the Roman villa [4]. Ancient texts [5] and interpretation of inscriptions on dolia and amphorae [6,7] provided information about the owners of the building, their activities, and the historical period. The building was constructed at the beginning of the first century BC by Marco Valerio Messalla (Valerii Messallae, 64 BC, 16 AD), supreme Roman magistrate [8]. The property was inherited by Aurelio Cotta Massimo Messalino [9], cited by Pliny [10] as a disseminator of methods of vine, olive, and fruit cultivation [11]. The villa was apparently destroyed by fire in the first century [4].

Coordinates and map locations of archaeological Roman villa at San Giovanni, Elba Island, Italy.
Sets of layers may contain plant remains and the study of carbonised plant material (anthracology) can shed light on human activities and customs [12], while palynological analysis [13] may be useful for clues to climatic conditions. Non-pollen objects identified in pollen microscope slides [14] are excellent indicators of oxygen-poor, stagnant water, deforested land and human pressure on ecosystems [15]. Ultrastructural microscope observations are useful for studying palynomorph alterations [16]. If micro-fossil [17] or carpological remains [18] are buried in a wet anaerobic environment, their morphological integrity and DNA content may be preserved. In this regard, apple seeds have a thin brittle embryo-protective integument. The fruit of the wild apple (Malus sylvestris (L.) Mill.) is 3–4 cm in diameter. The endocarp of the fruit is composed of five locules enclosed in five carpels, each of which normally contains two seeds that remain in pairs in the mesocarp during maturation. Mature apple seeds show angular morphology and morphological discontinuities [19,20], even if they were formed in the same fruit. Nuclear genetic marker studies have produced hypotheses about the origin of the apple [21]. The progenitors are wild species native to central Asia, the trans-Caucasian region [22,23], and Europe [24,25]. SSR (Simple Sequence Repeat) markers have been used to study genetic diversity in wild [26] and domesticated apples [27]. In an archaeological context, the DNA remaining in palaeobotany samples is often highly degraded, and the nuclear SSRs seem to be performing markers, even when DNA has been subject to extreme chemical conditions [28].
The archaeological excavation of the Roman villa was conducted by figures with different skills and roles. With regard to palaeobotanical aspects, many questions emerged during excavation. We had to check written history regarding the site, building structure, the flora of the period, and the conservation, type, origin, and use of the carpological remains in the amphorae. In order to check a hypothesis drawn from historical sources regarding the circumstances that led to the abandon of the Roman villa, we conducted palaeobotanical studies on sediments, vegetation, and human impact at the site. Traditional approaches, such as carpology, were combined with ultrastructural observation and molecular study. The morphology of archaeological apple seeds was observed for differences in size and shape with respect to modern wild and domesticated reference collections of Malus. At the same time, SSR molecular markers were used to compare the genotype of archaeological, wild and modern domesticated seeds.
2 Methods
2.1 Stratigraphy tracking
In the first rectangular area of the excavation (Fig. 2), excluding contemporary alluvial humus of the surface layer, three sets of depositional layers were identified. In descending order, they were: a zone A consisting of three layers (A1, A2 and A3), a zone B corresponding to the floor of the building with a hard dark compact burnt crust at the surface, and a zone C consisting of three other layers (C1, C2 and C3) containing four amphorae and seeds.

Excavation area where the four amphorae were found (broken line), with depth, type and contents of the stratigraphic sections identified under the contemporary surface layer.
2.2 Palynology
Horizontal cores were obtained for palynological analysis with a stainless steel drill. The cores (length 15 × diameter 2.5 cm) were stored in sealed plastic bags at room temperature. Thirty grams of each sample were treated with HCl, HF, and NaOH [13]. After elimination of the organic and mineral parts, the concentration of palynomorphs was increased by flotation in iodide fluids [29]. The final residues were embedded in glycerine, mounted on light microscope slides and viewed with a Zeiss Axiophot 400. Identification of archaeological palynomorphs was conducted by comparison with contemporary collections and Reille atlas [30].
2.3 Anthracology
Sampling of sediment containing charcoal was sampled in circular plots having a radius of about 50 cm. The samples were weighed in the laboratory. Charcoal was separated by hand with microscope tweezers under a Bausch & Lomb SZ-6 plus stereomicroscope, sorted by type, weighed, counted, and percentages calculated. Once the number of taxa was determined, the number of samples plotted in an anthracological graph was set at 81-127 units/sample. Observations on charcoal (cross, tangential and radial sections of samples) were conducted by light microscopy (Zeiss Stemi 2000-C). Reference material carbonised in vitro was used for accurate determination of the archaeological charcoal.
2.4 Carpology
In zone C3 (Fig. 2), four broken amphorae (Fig. 3) containing carpological specimens were discovered. In Stratigraphic Units (US) 100/1, conventionally called C3a, we collected about 320 seeds. US 100 (C3b) contained 16 seeds, US 99/1 (C3c) 110 seeds and US 99 (C3d) 45 seeds. The seeds were observed under a Zeiss Stemi 2000-C stereomicroscope with laminar flow hood, collected with tweezers and stored in sterile 50-ml Falcon flasks at room temperature. For morphological observations, archaeological seeds were compared with wild fresh reference collections, namely: ancient native autochthonous wild apples collected on 8th September 2015 from a live tree growing at 43°18′56″N, 11°17′32″E [Malus sylvestris1], seeds from a live tree at 42°59′20″N, 10°53′22″E (29/08/2006) [M. sylvestris2] and seeds from a live tree at 43°05′35″N, 10°59′00″E (20/09/2011) [M. sylvestris3]. Seeds from domestic trees [M. domestica1, M. domestica2, M. domestica3] were added as controls. The morphometric study of size by stereomicroscope considered only seed weight, major and minor axis, perimeter and area. Measurements were made by digital micrography on endocarp images imported from files and processed with the public domain ImageJ programme (CyberMetrics) [31]. To track morphological variations, some fresh seeds were hydrated for 72 h at 20 °C in a moist chamber and then dried at room temperature.

Broken amphorae in the excavation setting (zone C) where about 500 seeds were found.
2.5 DNA isolation and PCR conditions
DNA was extracted from apple seeds that under the microscope showed evidence of hermetically closed integuments after surface sterilization with sodium hypochlorite 2% for 2 min. The seeds were then placed under a sterile UV hood for 72 h. The archaeological seeds extracted were from samples C3a, C3b, C3c and C3d; seeds of reference collections of wild apples (M. sylvestris1, M. sylvestris2, M. sylvestris3) and commercial domestic apples (M. domestica1, M. domestica2, M. domestica3) described in section 2.4 were used as controls. All extractions were processed individually using single-use sterile equipment. We selected 20 seeds from each sample and processed them in duplicate under different laminar flow hoods in two distinct laboratories of the Department (Via Mattioli 4 and Via Aldo Moro 2, Siena). The seeds of each of the above samples were then homogenized in liquid nitrogen in a sterile ceramic mortar and about 90 mg of frozen homogenate was used for DNA extraction with Dneasy Plant kits (Qiagen). DNA quality was checked by visual inspection of transilluminated 1% agarose gels in 1X TAE (40 mM Tris-OH, 20 mM acetic acid, pH 7.8), stained with 1 μg/mL ethidium bromide. Total DNA was quantified by comparing ethidium bromide incorporation in unknown samples and 250 ng of Lambda/HindIII DNA standards. Pooled DNAs from each sample were tested with microsatellite markers (SSRs). The nucleotide sequences and annealing temperatures of the eight-primer pairs used for DNA amplification are shown in Table 1. Except for O2B1 [32], all primers were from HIDRAS (High-quality Disease Resistant Apples for Sustainable Agriculture, http://www.hidras.unimi.it/HiDRAS-SSRdb/pages/index.php) indicated for genetic analysis of apple. PCR was conducted in triplicate for each sample, processing the archaeological DNA samples ahead of DNA extracted from modern Malus accessions, to avoid cross contamination. The PCR reaction mix (12.5 μl) contained genomic DNA (2.5 ng/μl), 2.5 μl of 5X PCR Buffer (Promega), 1 μl of 2.5 mM dNTPs (Fermentas), 0.25 μl of 10 μM of each primer and 0.125 μl of 5 U/μl GoTaq DNA polymerase (Promega). Amplification was performed using an epGradient thermocycler (Eppendorf) according to [33]. For each SSR locus, a PCR negative control was run adding sterile water instead of DNA to the reaction mixtures.
Sequences of SSR primers.
| Locus | Primer sequence (5′–3′) | PCR annealing temp | Alleles size range |
| CH01d03 | CCACTTGGCAATGACTCCTC ACCTTACCGCCAATGTGAAG | 60 | 136–160 |
| CH01f03b | GAGAAGCAAATGCAAAACCC CTCCCCGGCTCCTATTCTAC | 60 | 139–183 |
| CH02a08 | GAGGAGCTGAAGCAGCAGAG ATGCCAACAAAAGCATAGCC | 60 | 133–177 |
| CH02c02a | CTTCAAGTTCAGCATCAAGACAA TAGGGCACACTTGCTGGTC | 60 | 129–176 |
| GD142 | GGCACCCAAGCCCCTAA GGAACCTACGACAGCAAAGTTACA | 60 | 123–158 |
| GD12 | TTGAGGTGTTTCTCCCATTGGA CTAACGAAGCCGCCATTTCTTT | 60 | 141–191 |
| GD96 | CGGCGGAAAGCAATCACCT GCCAGCCCTCTATGGTTCCAGA | 60 | 152–197 |
| 02B1 | CCGTGATGACAAAGTGCATGA ATGAGTTTGATGCCCTTGGA | 60 | 216–242 |
2.6 Genotype analysis
Allele sizing was done by capillary electrophoresis, based on laser scanning of fluorescence-marked DNA fragments using Genescan 400 HD ROX (Life Technologies) as an internal sizing standard. Genotyping was done with an ABI3130 (Life Technologies) DNA analysis system and the fluorescent fragments were scanned for allele detection with GeneMarker software (Softgenetics, USA). After collecting genotypes, a similarity dendrogram was produced by NTSYS ver. 2.0 on six Malus accessions for genetic comparison and on the archaeological samples. Genetic relationships among the 10 accessions in this study were investigated using unweighted pair-group cluster analysis (UPGMA) and BAND coefficient as parameter for determining genetic distances between the accessions.
3 Results and discussion
3.1 Excavation context
The three layers A1, A2 and A3 (Zone A, Fig. 2) had similar soil textures with heterogeneous compact agglomerates of clay, fine sand and contained slightly different palaeobotanical remains (Table 2). The first layer (A1) contained palynomorphs, followed by palynomorphs and charcoal (A2) and a layer consisting of large fragments of charcoal (A3). The imprints of canes in burnt earth and the large quantity of iron nails found between A2 and A3 confirmed a Roman construction technique known as opus craticium [34], while palynomorphs found in A1 and A2 confirmed less human impact in those zones. The sterile samples B, probably exposed to strong heat, consisted of a compact crust of dark grey burned earth enclosing charcoal pieces. The fire hypothesis was sustained by the finding of inorganic matter containing stony plaster agglomerates. Calcium sulphate (CaSO4·2H2O) can be calcined. At high temperatures, it dehydrates (CaSO4) and subsequent rehydration gives it a stony consistency (hydraulic properties) [35]. This layer isolated anything below it, while proximity to the sea maintained the palaeobotanical remains in a waterlogged environment. Zone C showed compact sediment of clay and sand (C1) with little pollen and no charcoal. C2 showed coarse heterogeneous sediment composed of clay, sand, amphorae, larger pieces of charcoal and larger pieces of unburned wood. C3 samples with about 500 well-conserved apple seeds near the bottom of the amphorae under a wet compact layer of mud (fine sand and clay) and organic encrustations.
Description of paleobotanical content in stratigraphy of archaeological excavation (see also Fig. 2).
| Depth (cm) | Palaeobotanical content | Stratigraphy | |
| 0–19 | 0 | Contemporary alluvial humus sediment, organogenic and fine sand | |
| 19–38 | A1 | Palynomorphs | Compact agglomerate of clay and fine sand, grey-brown |
| 38–82 | A2 | Palynomorphs and charcoals | Heterogeneous clay, fine sand containing charcoal and wall detritus detritus, brown |
| 82–98 | A3 | Charcoals | Heterogeneous clay, fine sand, charcoal, walls detritus, reeds and plaster, dark brown |
| 98–122 | B | Dust | Compact homogeneous sterile greenish black layer containing fine coal dust and molten iron droplet particles |
| 122–134 | C1 | Palynomorphs | Compressed clay, light brown |
| 134–152 | C2 | Wood | Heterogeneous compressed course fragments wood and amphorae light brown |
| 152–160 | C3 | Seeds | Fine sand and clay and organic encrustations sediments |
3.2 Palynological observations
The pollen material and the spore-bearing and non-pollen objects found [14] were produced by vegetation coeval with the sediment. Palynological analysis of the three layers of zone A showed palynomorphs with little pollen and abundant fungal spores in A1 and A2 (Fig. 4). Non-pollen objects, in particular spores such as bi- or tri-septate Ascospores, Asplenium, Clamidospores, Zygnemataceae and Hidrocharis typical of acid soils poor in oxygen [15], where many factors act (e.g., stagnant moisture and decomposition due to human activities), were identified in pollen slides. Layers of fused agglomerates of ferrous minerals found together with palynomorphs bear witness to earlier metallurgical activity on the island [2,3]. Layers A3 and B, the latter a dark compact crust, were sterile and without palynomorphs. Only in C1 of zone C was some arboreal pollen of Quercus ilex, while among human-related herbaceous plants, some pollen of Urtica was found. Finally, the pollen preparations for light microscopy showed abundant microscopic particles of charcoal, 10–15 μm in diameter, that were interpreted as indicators of areas prone to fires [18].
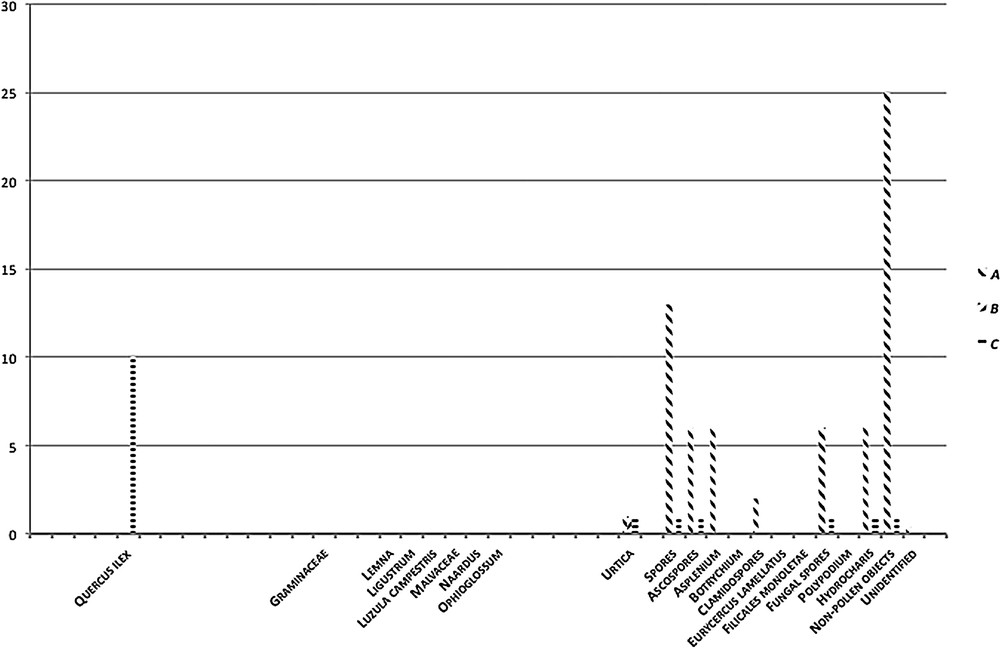
Palynology graph summarising pollen, sporigenous and non-pollen objects found.
3.3 Anthracological analysis
Anthracological analysis (Table 3) showed Mediterranean tree formations characterised by holm oak, deciduous oak, hornbeam, chestnut and Turkey oak belonging to Mesolithic Tertiary flora. In particular, samples A1 did not contain charcoal and samples A2 and A3, besides imprints of cane matting and nails used for opus craticium walls, showed much charcoal from Quercus cerris, Castanea, Q. pubescens, Ostrya, and Q. ilex. The curvature of growth rings indicated sizes compatible with trunks for Castanea and Q. pubescens, and branches for Q. cerris and Q. ilex, which in the case of Ostrya were also large. The presence of fragments of seasoned sapwood with burnt fungal hyphae in Q. ilex indicates that the wood was collected from the ground. We can postulate that the carpenters used beams of chestnut and oak (Castanea, Quercus pubescens) to build the villahouse. The latter has strong curved fibres. Chestnuts and acorns would have been used to feed pigs and in times of famine also to make bread. Slow-burning, high calorie wood such as holm oak (Q. ilex), hornbeam (Ostrya) and Turkey oak (Q. cerris), were used for firewood. Samples B contained about 27% of charcoal dust aggregates formed by combustion and subsequent aggregation due to pressure. Samples C1 did not contain charcoal. Samples C2 contained particles of the amphorae, non-carbonised wood residues with recognisable fragments of Castanea and Q. ilex and larger fragments of Q. cerris and Castanea, as well as a woody material of Q. suber, probably used as a lid for the amphorae. Mixtures of charcoal fragments of Q. cerris, Castanea and Q. pubescens and larger fragments of Q. cerris and Q. pubescens, the latter from beams for carpentry, were also assessed. About 150 pieces of charcoal weighing 130 g from all layers could not be recognised.
Palaeobotanical content of excavated layers.
| Depth (cm) | Layer | Palaeobotanical content | Quercus cerris | Castanea | Quercus pubescens | Ostrya | Quercus ilex | Quercus suber | ||||||
| g | n | g | n | g | n | g | n | g | n | g | n | |||
| 0–19 | 0 | Contemporary humus | ||||||||||||
| 19–38 | A1 | Palynomorphsa | ||||||||||||
| 38–82 | A2 | Palynomorphsa | ||||||||||||
| Charcoal fragmentsb | 19 | 37 | 128 | 79 | 65 | 53 | ||||||||
| Macro-charcoal fragmentsb | 30 | 2 | ||||||||||||
| 82–98 | A3 | Charcoals fragmentsb | 127 | 51 | 50 | 23 | 24 | 48 | 49 | 55 | ||||
| Macro-charcoal fragmentsb | 93 | 3 | 113 | 3 | ||||||||||
| 98–122 | B | Dust | Aggregate 27% charcoal powder | |||||||||||
| 122–134 | C1 | Palynomorphsa | ||||||||||||
| 134–152 | C2 | Wood fragmentsb | 28 | 26 | 27 | 17 | ||||||||
| Macro-wood fragmentsb | 30 | 5 | 38 | 3 | 33 | 2 | ||||||||
| Charcoal fragmentsb | 29 | 30 | 15 | 22 | 11 | 33 | ||||||||
| Macro-charcoal fragmentsb | 30 | 4 | 33 | 6 | ||||||||||
| 152–160 | C3 | Seeds | About 500 Malus sylvestris seeds | |||||||||||
| Possible plant usesc | W | B, H | A, B, H | W | W | C | ||||||||
| Total grams charcoal identified | 235 | 224 | 181 | 158 | 141 | 33 | ||||||||
| Total number of charcoal pieces identified | 127 | 77 | 90 | 81 | 125 | 2 | ||||||||
| Total number and grams of charcoal pieces unidentified | 150 about 130 g |
a See Fig. 3 and palynological observation.
b Charcoal and woods fragments from 0.5 to 2 cm; macro-charcoal and macro-wood pieces from 2.1 to 10 cm.
c Possible uses of plant taxa identified: A: fruit animal food, B: building material, C: handcrafts, H: fruit human food, W: firewood.
3.4 Carpological analysis
The integument of apple seeds is thin (about 9 μm) and 80% of archaeological seeds were cracked and empty (Fig. 5A–C) while 20% had integuments sealed around a well-preserved mummified embryo (Fig. 5D–F). Stereomicroscope observations processed with ImageJ programme [31] revealed differences between archaeological, wild and domestic apple seeds (Fig. 6A) and also between seeds from the same fruit. We did not calculate the average by homologous or pseudohomologous profile investigations as in [36]. Indeed, as previously described [19,20], seed morphology confirmed discontinuous characters except for the wild fresh reference collection M. sylvestris1 (Fig. 6B) and archaeological samples C3a (Fig. 6C) from one amphora that showed morphological affinity (Table 4). In addition, morphological observation showed that all archaeological seeds had a rounded shape. After fresh seed hydration tests, angular seeds showed rounded epidermal structure similar to archaeological seeds. It is plausible that the archaeological seeds underwent hydration when fresh and were maintained in an excellent state of preservation in waterlogged sediment. The amphorae could have contained cider obtained from the fermentation of apples, explaining why the seeds were rounded beyond recognition, but this hypothesis was not confirmed by analysis of organic encrustations in the amphorae.
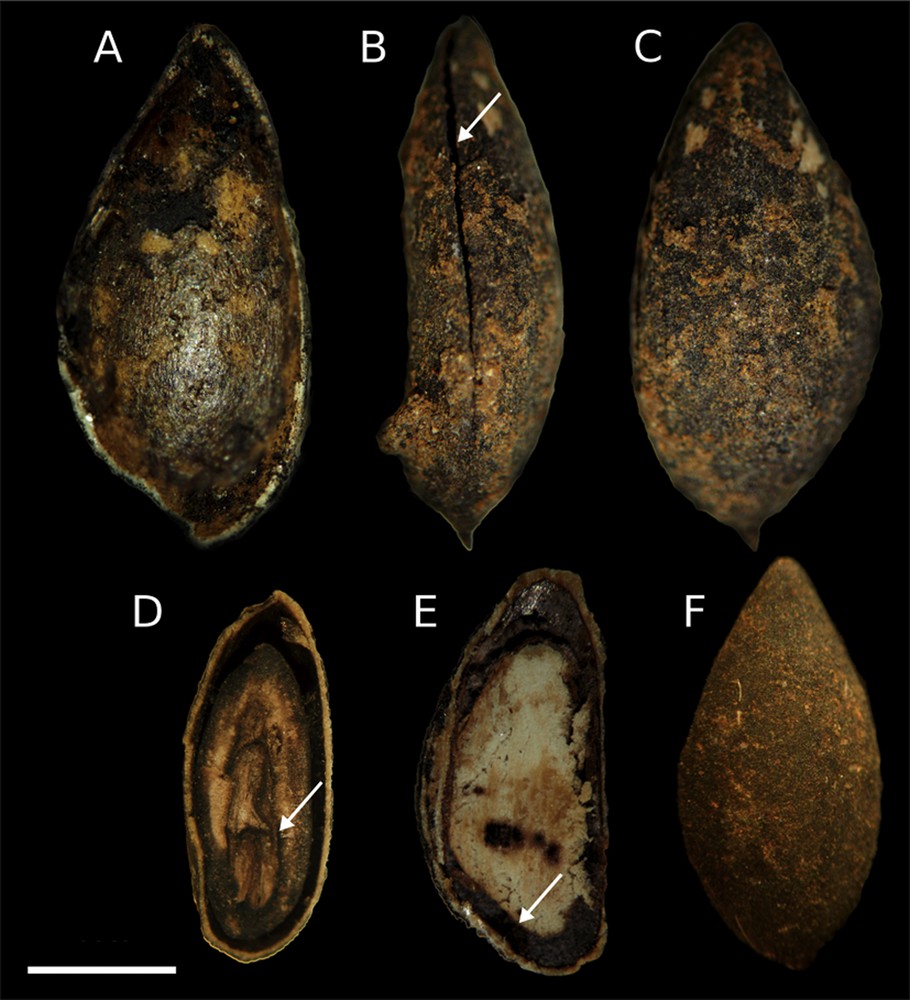
Some seeds have the teguments coat damaged and they are empty: A, inside surface of the coat in frontal view; B, seed coat broke in sagittal view; C, external surface of the coat in frontal view. Some seeds are intact and the inside tissues are conserved: D, embryo and cotyledons in sagittal view; E, cotyledons in frontal view; F, external surface of the coat in frontal view (bar = 2 cm).

A: wild fresh reference collection seeds (1), (2), (3) domestic fresh (4) and archaeological seeds (5), (6), (7), (8); B: micrograph of control seeds of Malus sylvestris1 reference collection (bar = 0.7 cm) with structural tegument angles; C: archaeological seeds with rounded tegument angles (bar = 0.7 cm).
Morphometric size measurements (weight, axes, area and perimeter 20 seeds average) of archaeological (C3x), Sylvestris (M. sylvestrisx) and domesticated (M. domesticax) Malus seeds.
| Weight g × 20 seeds |
Major axis mm |
Minor axis mm |
Area mm2 × l00 |
Perimeter mm |
|
| C3a | 0.29–0.35 | 5 | 2.70–2.75 | 10.8–11.3 | 13.4–13.45 |
| C3b | 0.25–0.40 | 3.7–5.5 | 2.13–2.82 | 6.8–12.6 | 10.3–15.5 |
| C3c | 0.25–0.40 | 3.7–5.5 | 2.13–2.82 | 6.8–12.6 | 10.3–15.5 |
| C3d | 0.25–0.40 | 3.7–5.5 | 2.13–2.82 | 6.8–12.6 | 10.3–15.5 |
| M. sylvestrisl | 0.50 | 5 | 2.70–2.75 | 10.8–11.3 | 13.4–13.45 |
| M. sylvestris2 | 0.55 | 4.45–6.5 | 2.25–2.75 | 9.3–11.3 | 12.3–15.45 |
| M. sylvestris3 | 0.60 | 4.45–6.5 | 2.25–2.75 | 9.3–11.3 | 12.3–15.45 |
| M. domestical | 1.60 | 7.2 | 3.96 | 21.25 | 19.83 |
| M. domestica2 | 1.60 | 7.25 | 3.95 | 21.35 | 19.9 |
| M. domestica3 | 1.60 | 7.22 | 3.95 | 21.36 | 19.95 |
3.5 DNA analysis
To simplify and standardize the genetic analysis procedure, we selected 20 seeds from the modern and archaeological samples, seeds from the latter with well-preserved mummified embryos being chosen. Total high molecular weight DNA was successfully extracted in modest quantities (1–3 ng/μl) from the four archaeological specimens. An agarose gel with the total genomic DNA extracted from archaeological samples (C3a in lane 1, C3b in lane 2, C3c in lane 3, C3d in lane 4) and modern M. sylvestris1 (lane 5) is shown in Fig. 7. No amplification occurred in negative controls (data not shown). The electropherogram (Fig. 8) shows an example of the PCR products obtained at locus GD12 from the same reference collections of Malus sylvestris1 (8.1) and the archaeological sample C3a (8.2). The range of amplification and identification at the SSRs amplified alleles (binning) were comparable in archaeological and contemporary samples (e.g., at locus GD12: 147.3 and 155.6 M. sylvestris1; 143.8 and 155.2 C3a). Amplification of ancient DNA C3a is shown by peak amplitude: degraded DNA formed alleles of lower amplitude than controls, but still legible, being within the instrument's significance threshold (RFU ≥ 100) and was useful for constructing a similarity dendrogram to compare with contemporary Malus accessions (Fig. 9). All eight-primer pairs generated multiple alleles when amplified in SSR reactions with genomic DNA from each of the six modern Malus accessions. The multiple alleles per locus found were compatible with those reported in previous studies of Malus species [37]. Sample C3a showed a significant genetic correlation with a contemporary Malus sylvestris accession found in Tuscany, as well as similar morphology. SSR band variations were in line with phenotypic divergence and seed weight, as previously demonstrated for other species [38]. The dendrogram shows a weak genetic correlation between most of the archaeological Malus accessions (C3b, C3c, and C3d), which clustered together, with only 17% correlation with contemporary Malus accessions. The samples presumably came from a single receptacle before transfer to the three amphorae. Ancient DNA only partially amplified at SSR loci, presumably due to degradation of DNA. The specificity of the amplified alleles obtained was however encouraging for genotyping from archaeological seeds with well-preserved mummified embryos, and the archaeological seeds inside the amphorae certainly belong to apples.
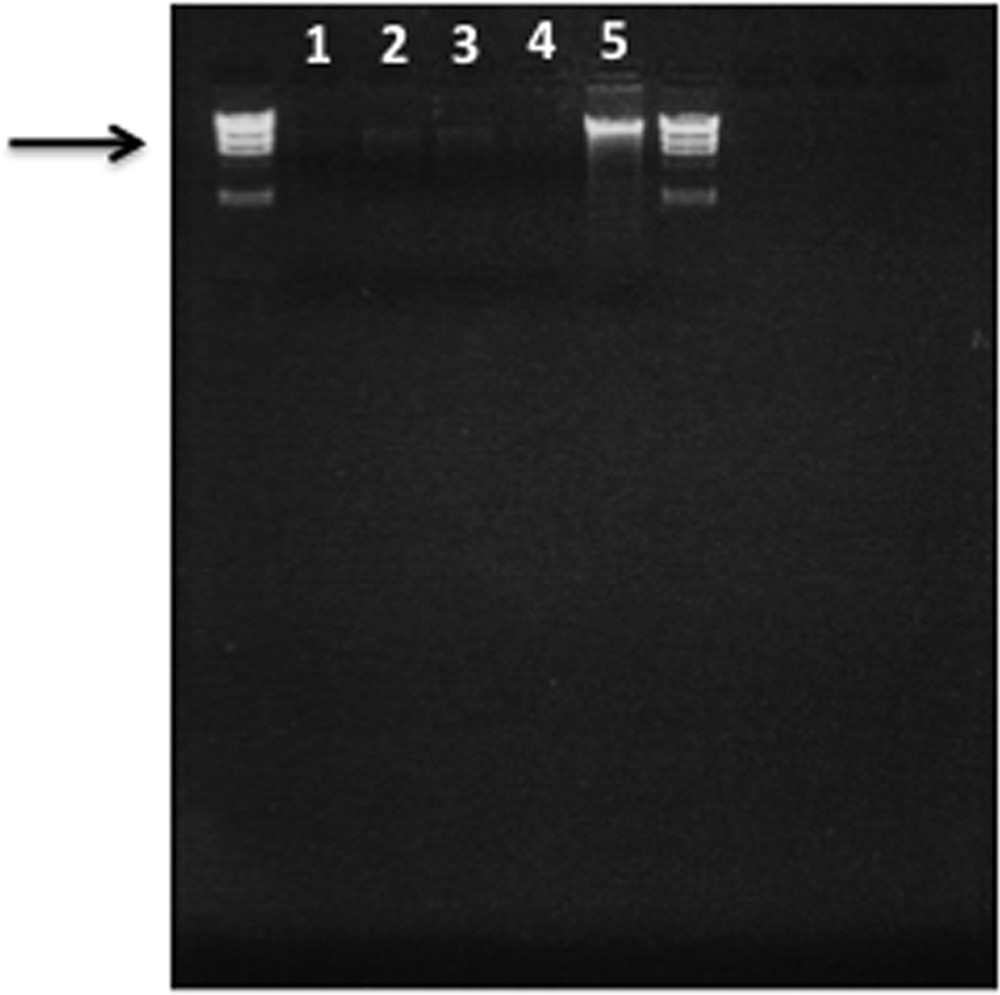
Agarose gel electrophoresis of total genomic DNA from archaeological seeds (C3a, lane 1; C3b, lane 2; C3c, lane 3; C3d, lane 4) and DNA from seeds of a modern Malus accession (Malus sylvestris1, lane 5).
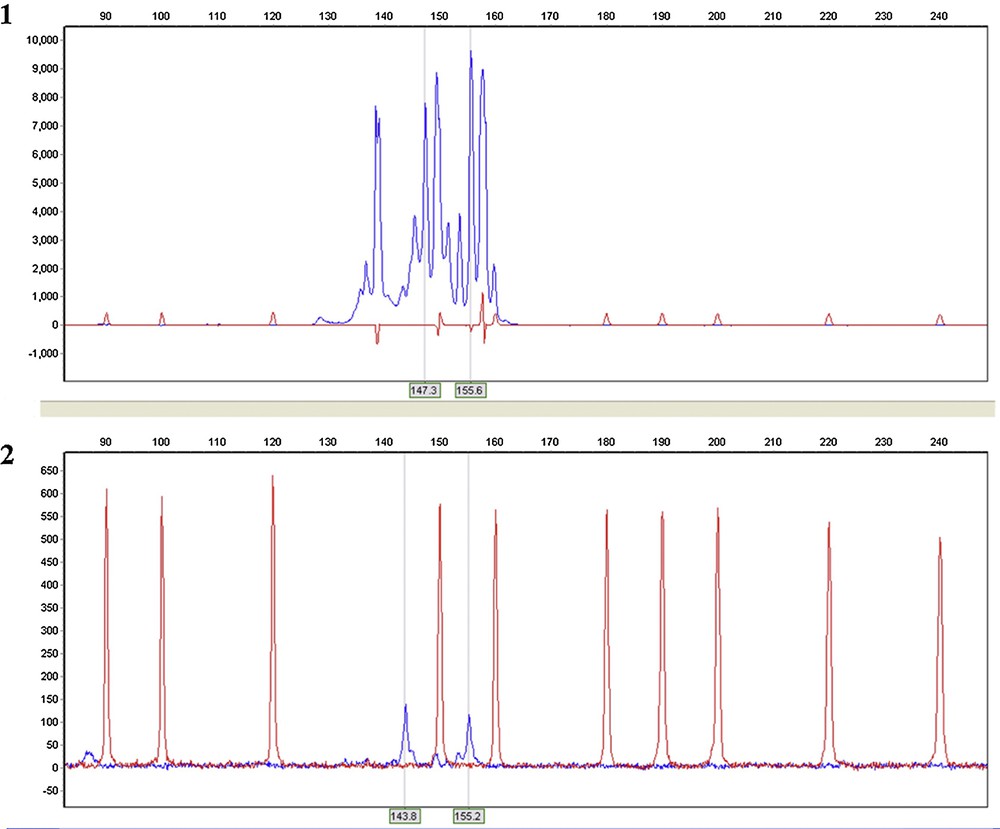
Electropherogram of GD12 SSR loci comparing the fresh control sample Malus sylvestris1 (8.1) and archaeological amplificates C3a (8.2).
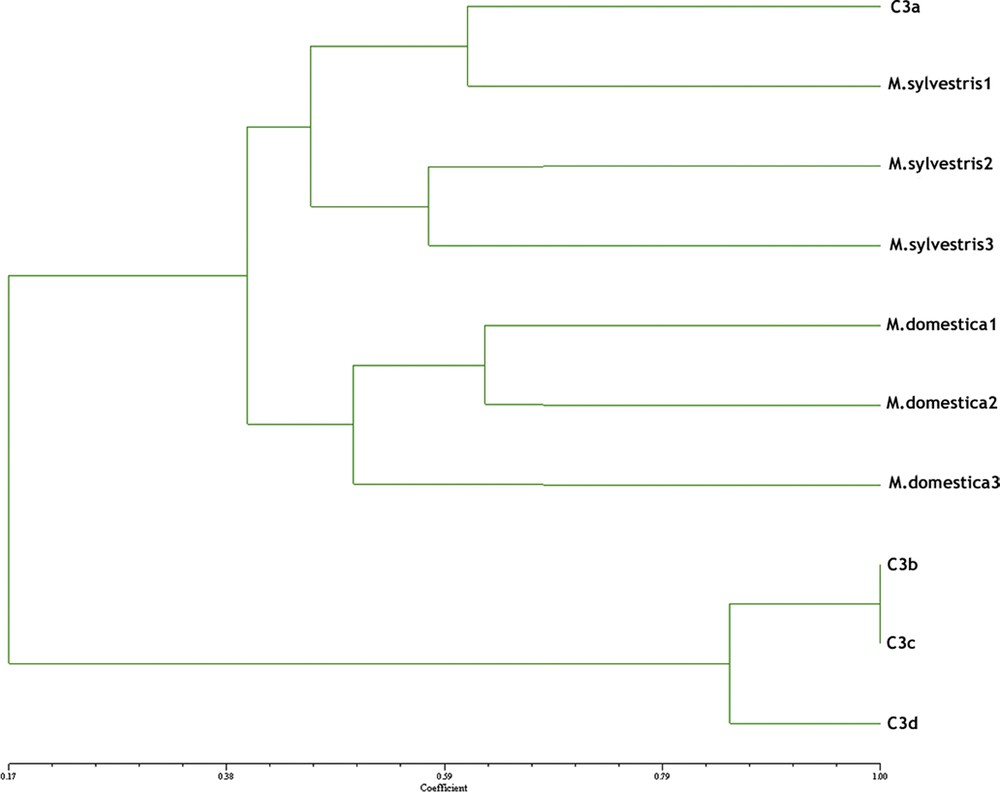
Dendrogram of genetic similarity.
In a DNA-typing laboratory, every precaution must be taken to ensure that the results are accurate and do not cast doubt on the data:
- • before DNA analysis, all seed surfaces where sterilized with sodium hypochlorite in a physically separate working area subject to night sterilization with UV rays. All operations were done using single sterile equipment and individual samples were processed separately in duplicate in two different laminar flow hoods;
- • the reduced peak height of the archaeological samples and the form of their electrophoretic peaks are typical when PCR amplifies degraded template DNA. For instance, similar results are obtained amplifying Vitis vinifera DNA from wine [39] or DNA from processed tomatoes [40];
- • the electropherograms in Fig. 8 showed an allele size range compatible with that of modern Malus accessions, without any background, sustaining the specificity of the PCR amplifications;
- • had there been any contamination with exogenous Malus DNA during DNA extraction or PCR amplification, the electropherogram peaks corresponding to amplified alleles of the Malus genome would have been similar (in height and form) to those obtained for the modern Malus accessions. SSR-based genotyping of archaeological seeds exploits species-specific primer design, making it possible to detect the correct target at species level, even in a complex DNA mixture from multiple biotic sources.
4 Conclusions
The recognition of depositional layers at the archaeological site and study of their contents, as well as the quality of palynomorphs, non-pollen objects and charcoal, were useful to survey the arboreal and herbaceous vegetation and human impact at the site. The findings supported the hypothesis that the building was abandoned after a fire. The archaeological data confirmed that the type of construction was opus craticium, as expected for a rustic villa. We were also able to provide a more detailed description of the effects of fire at the site. The compact sterile layer caused by the fire formed a seal over the underlying amphorae containing the seeds. Morphological observations of the shape and size of the archaeological seeds provided a detailed view of rounded integuments, caused by conservation in a wet environment. Well-preserved mummified embryos containing traces of ancient DNA enabled genotyping with SSR primers specific for Malus, confirming that the four amphorae contained archaeological apple seeds. Three groups of seeds showed morphological discontinuity and genetic distance from other Malus accessions, suggesting that the samples came from a single receptacle probably intended for cider production. The other group of seeds from an amphora showed genetic and morphological correlations with living Malus sylvestris of the reference collections, confirming phenotypic correlations reported in the literature for other species of seeds. The present interdisciplinary data is pertinent to the Roman economy and culture on Elba Island from the 2nd to the 1st century BC.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgements
We thank Ing. Ariano Buracchi for his passionate study and selection of palaeobotanic material.


