1 Introduction
Phlebotomus stantoni (Newstead 1914) and Sergentomyia hodgsoni (Sinton 1933) are sandflies that belong to subfamily Phlebotominae (Diptera: Psychodidae). Sandflies are vectors of various pathogens, among which Leishmania sp.; therefore, their correct taxonomic identification is important for epidemiological investigations. Phlebotomus Rondani & Berte and Sergentomyia Franca & Parrot are Leishmania protozoa vectors in the Old World [1], while Lutzomyia spp. transmit New World leishmaniasis [1,2].
In Thailand, P. stantoni and S. hodgsoni are commonly found in cave dwellers [3–7]. These two species are sympatric in some places, especially on isolated islands in Chumphon province in the southern part of Thailand [8]. In particular, Phlebotomus and Sergentomyia specimens were found in swiftlet caves on Lang Ga Jiew Island of Chumphon province, and S. hodgsoni was the predominant sandfly species on isolated islands. The identification of sandfly species belonging to different genera is not difficult using morphological examination. Our objective was not to test for the morphometric recognition of different genera, but to assess the quality of species recognition within each genus in spite of possible geographic changes.
To set up the control strategies against leishmaniasis transmission, essentially an entomological surveillance program, it may be important to improve our knowledge about the distribution of vectors, or potential vectors. To reach such objective and to ensure regular updates require the use of low-cost, fast and accurate tools to identify sandflies. There have been very few studies on species variation of sandflies from different geographical locations, especially none in Thailand, using geometric morphometrics (GM). In this study, we investigated intraspecific and interspecific wing size and shape variation of P. stantoni and S. hodgsoni in different geographical locations, including between the mainland and isolated islands.
The information derived from this study could help to evaluate the morphometric approach as a routine characterizing tool for future epidemiological studies of sandflies in Thailand.
2 Materials and methods
2.1 Sandfly collection
Using Center for Disease Control (CDC) light traps, sandflies were collected in 2011 in seven different geographical locations across Thailand (Fig. 1). Isolated islands and mainland location sites were selected in this study. Three isolated islands, including Lang Ga Jiew (LGJ), Ka (KA), and Mapraw (MAP) Islands, were selected for sandfly collection; these three islands are located in the Gulf of Thailand, in Chumphon province, southern Thailand. LGJ Island is a small offshore island that covers an area of 0.063 km2 and is located approximately 8 km away from the shore. MAP and KA islands are located approximately 1 km away from the shore. Four other mainland location sites were selected: a small sample from an urban location in Nonthaburi province (NTB), which is located in central Thailand, and other from limestone caves of Kanchanaburi province (KCB, western Thailand), Ratchaburi province (RCB, central Thailand) and mountainous areas of Kamphaeng Phet province (KPP, northern Thailand).
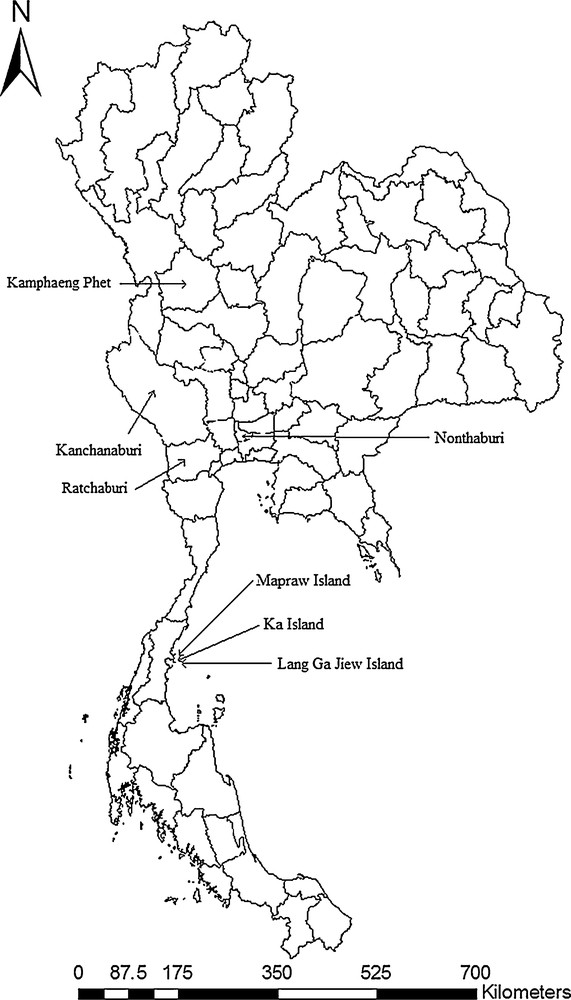
Map of sandfly collection sites in this study.
2.2 Sandfly preparation
Collected female sandflies were stored in 95% ethyl alcohol and mounted in Hoyer medium on glass microscopic slides. Morphological identification was based mainly on the keys of Lewis [9,10]. Subsequently, P. stantoni and S. hodgsoni were photographed using a Nikon DS-Ri1 SIGHT digital camera connected to stereo-microscope Nikon AZ 100 M (Nikon Corp., Tokyo, Japan) with a size scale provided on the picture. A total of 157 right wing pictures from P. stantoni and S. hodgsoni across different locations were processed for landmark-based GM analysis (Table 1).
Number of female Phlebotomus stantoni and Sergentomyia hodgsoni wing pictures used for geometric morphometric analysis.
| Species | Locations | Symbols | Area description | Number of wing pictures |
| P. stantoni | Lang Ga Jiew Island, Chumphon province | LGJ | Limestone swiftlet cave on an isolated island | 21 |
| P. stantoni | Nonthaburi province | NTB | Urban area in central Thailand | 6 |
| P. stantoni | Kamphaeng Phet province | KPP | Mountainous areas in northern Thailand | 17 |
| S. hodgsoni | Ka Island, Chumphon province | KA | Limestone swiftlet cave on an isolated island | 7 |
| S. hodgsoni | Lang Ga Jiew Island, Chumphon province | LGJ | Limestone swiftlet cave on an isolated island | 57 |
| S. hodgsoni | Mapraw Island, Chumphon province | MAP | Limestone swiftlet cave on an isolated island | 13 |
| S. hodgsoni | Kanchanaburi province | KCB | Limestone cave in western Thailand | 14 |
| S. hodgsoni | Ratchaburi province | RCB | Limestone cave in central Thailand | 22 |
| Total | 157 |
2.3 Landmark-based GM analysis
Twelve landmarks were digitized (Fig. 2). When connected after a Procrustes superimposition onto the consensus configuration, they appear as polygons allowing an easier visual comparison of mean wing shape between sandfly species and populations. The Procrustes superposition was conducted according to the Generalized Procrustes Superimposition (GPA) procedure [11,12], generating both size (centroid size [CS]) and shape (partial warps [PW]) variables.
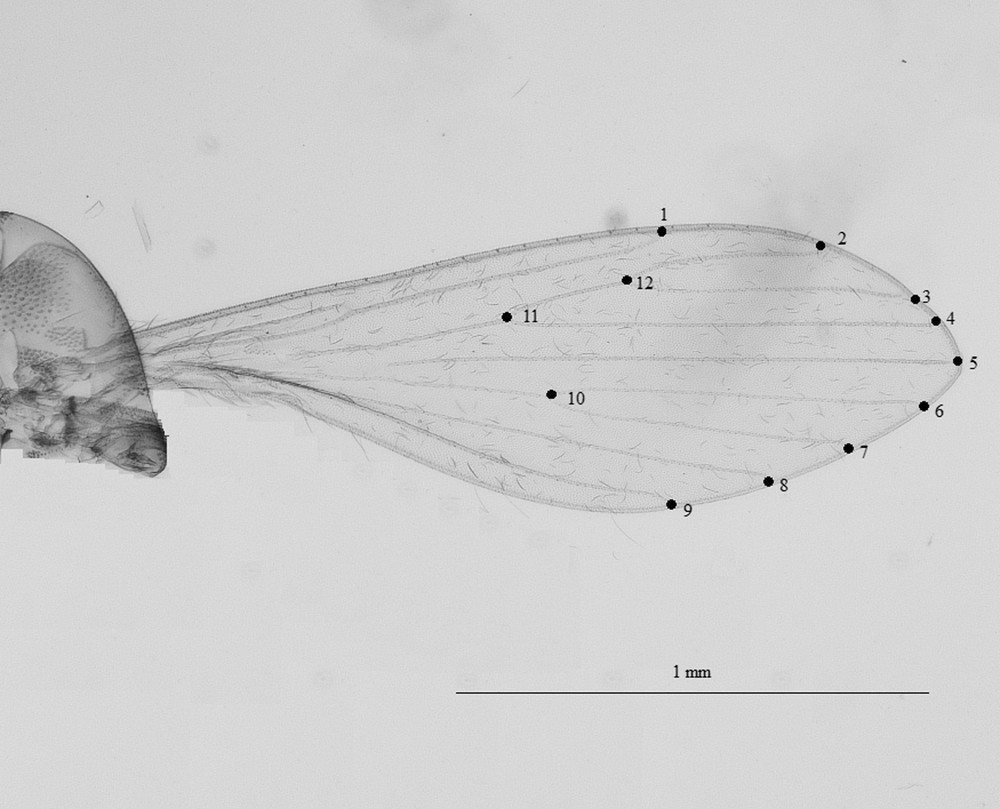
Position of 12 landmarks digitized for female Phlebotomus stantoni and Sergentomyia hodgsoni.
CS is defined as the square root of the sum of the squared distances between the center of the configuration of landmarks and each individual landmark [13]. Size comparisons were performed using a non-parametric permutation test (1000 cycles), and comparisons were illustrated by quantile boxes.
The shape variables (partial warps scores [PW]) were processed by standard multivariate analysis to statistically compare groups. The principal components (PCA) of the PW (i.e., the relative warps [RW]) were computed to visualize the morphospace for sandfly species from different locations. The discriminant analysis (DA) used as input variables the RW, or a few first of them according to the sample sizes, and was illustrated by the corresponding factor maps. For these DA, the two localities with very low sample sizes were removed: NTB (6 individuals) and KA (7 individuals). Validated reclassification scores were estimated after a DA on the two taxa, after the DA on the geographic populations within each species. In this validated reclassification, each individual was allocated to its closest group (Mahalanobis distance) without being used to help determine a group center [14]. The statistical significance (P ≤ 0.05) of shape variation was analyzed using non-parametric methods (1000 runs) applied to the pairwise Mahalanobis distances. A neighbor-joining tree was generated based on Procrustes distances between sandflies from different locations.
The allometric effect on the discrimination between collecting sites was explored by regressing the discriminant factors on centroid size and computing the coefficient of determination.
2.4 Software
CLIC version 97 [15,16], freely available at http://mome-clic.com, was used to conduct the digitization of the images and subsequent multivariate analyses on the coordinate data. The following modules were used: COO for landmarks collection, MOG to generate centroid size and shape variables, as well as to compute Procrustes distances and VAR to compare the size between groups. The Procrustes distances were used to compute the neighbor-joining tree using the PHYLIP neighbor module [17], and the resulting tree was visualized using the NJPLOT software [18]. The module PAD of CLIC was used to perform discriminant analyzes between groups, as well as to compute Mahalanobis distances and the corresponding statistical significance.
3 Results
3.1 Wing size variation
Mean wing CS comparison of P. stantoni and S. hodgsoni from different geographical locations (Table 1) showed size variation (Fig. 3, Table 2). Mean wing CS was significantly (P < 0.05) larger in P. stantoni than in S. hodgsoni (1.22 mm and 0.91 mm, respectively). P. stantoni from mainland in Kamphaeng Phet province had the largest wings (mean, 1.28 mm), significantly different from P. stantoni from Lang Ga Jiew Island and an urban location in Nonthaburi province (P < 0.05). However, the mean wing CS of P. stantoni from caves on Lang Ga Jiew Island and Nonthaburi province were not significantly different (P > 0.05). The wing CS of S. hodgsoni from a mainland cave in Ratchaburi province was the largest (mean, 1.02 mm), whereas S. hodgsoni from Lang Ga Jiew Island had the smallest wing CS (0.89 mm). Almost all populations of S. hodgsoni were significantly different (P < 0.05) from each other. However, there was no significant (P > 0.05) difference of wing size between S. hodgsoni from Ka Island and Lang Ga Jiew Island, Ka Island and Kanchanaburi province, or Mapraw Island and Kanchanaburi province.
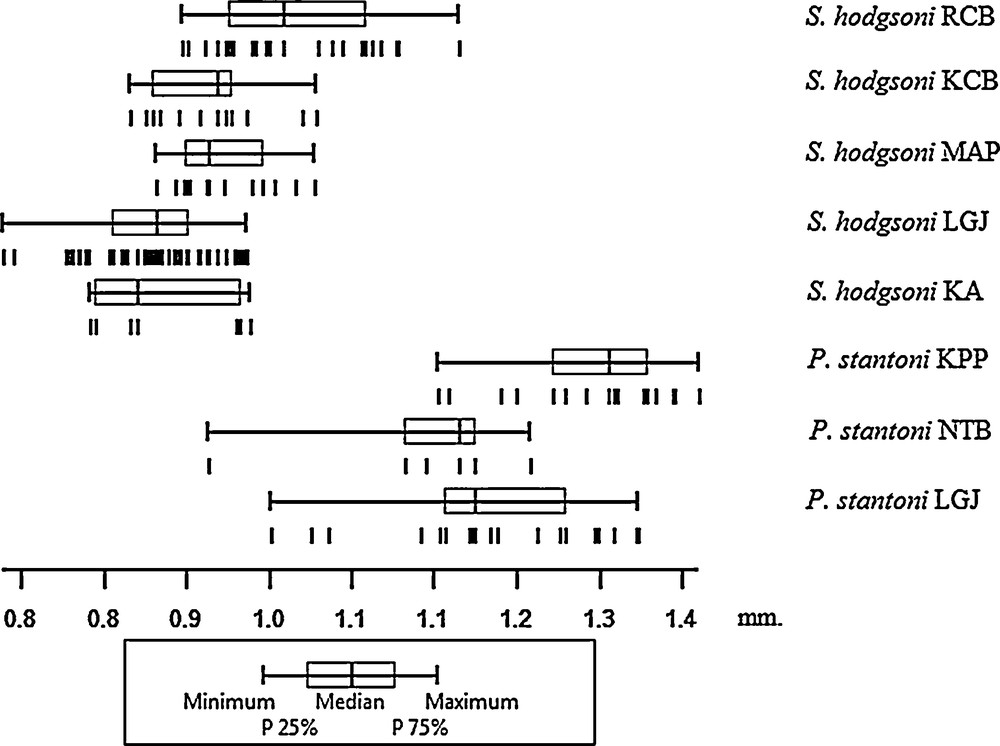
Variation of the wing centroid size (in mm) of female Phlebotomus stantoni and Sergentomyia hodgsoni from different localities. Vertical bars represent individuals. P 25%, percentiles 25%, P 75%, percentiles 75%. Boxes are quantile boxes between P 25% and P 75%, with indication of the median position.
Mean wing centroid size of female Phlebotomus stantoni and Sergentomyia hodgsoni.
| Species | Locations | N | Mean (Min–Max) (mm.) | S.D. | S.E. |
| P. stantoni | LGJ | 21 | 1.19 (0.99–1.32) | 0.09 | 0.02 |
| P. stantoni | NTB | 6 | 1.12 (0.94–1.23) | 0.10 | 0.04 |
| P. stantoni | KPP | 17 | 1.28 (1.15–1.38) | 0.07 | 0.02 |
| S. hodgsoni | KA | 7 | 0.91 (0.84–0.98) | 0.06 | 0.02 |
| S. hodgsoni | LGJ | 57 | 0.89 (0.76–0.98) | 0.05 | 0.007 |
| S. hodgsoni | MAP | 13 | 0.96 (0.90–1.04) | 0.04 | 0.01 |
| S. hodgsoni | KCB | 14 | 0.94 (0.83–0.93) | 0.05 | 0.01 |
| S. hodgsoni | RCB | 22 | 1.02 (0.92–1.16) | 0.07 | 0.01 |
3.2 Wing shape variation
After superimposition of the mean landmark configurations onto the consensus one, P. stantoni and S. hodgsoni wing shapes were clearly different (Fig. 4). Polygons of mean wing shape of within species samples are shown in Fig. 5 (P. stantoni) and Fig. 6 (S. hodgsoni). Consistent intraspecific variation was visible in both sandfly species.
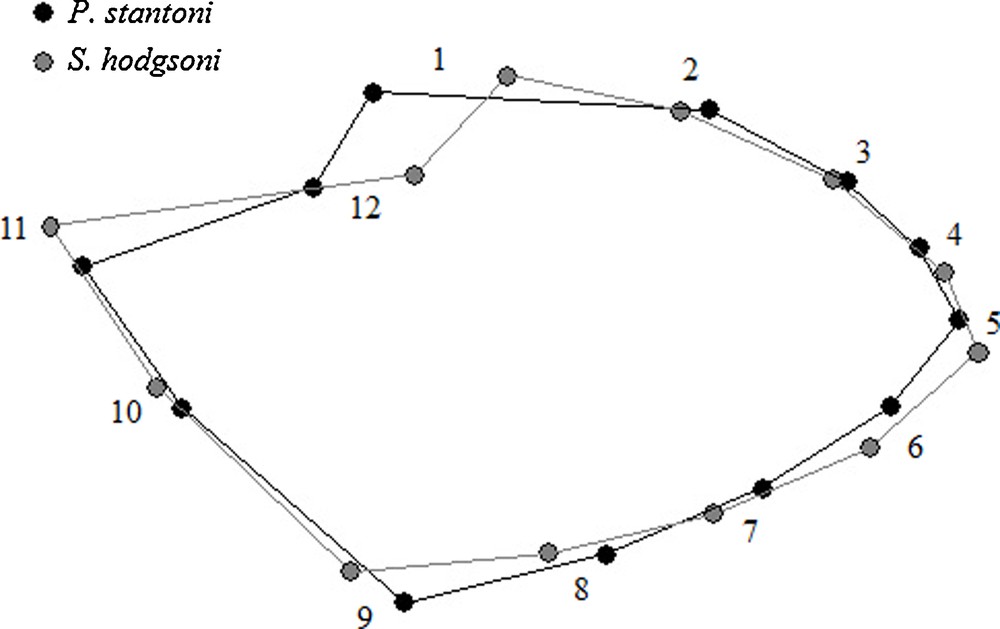
Polygons as connected mean landmark positions to visualize wing shape differences between female Phlebotomus stantoni (black) and Sergentomyia hodgsoni (gray) after Procrustes superposition. No amplification applied.
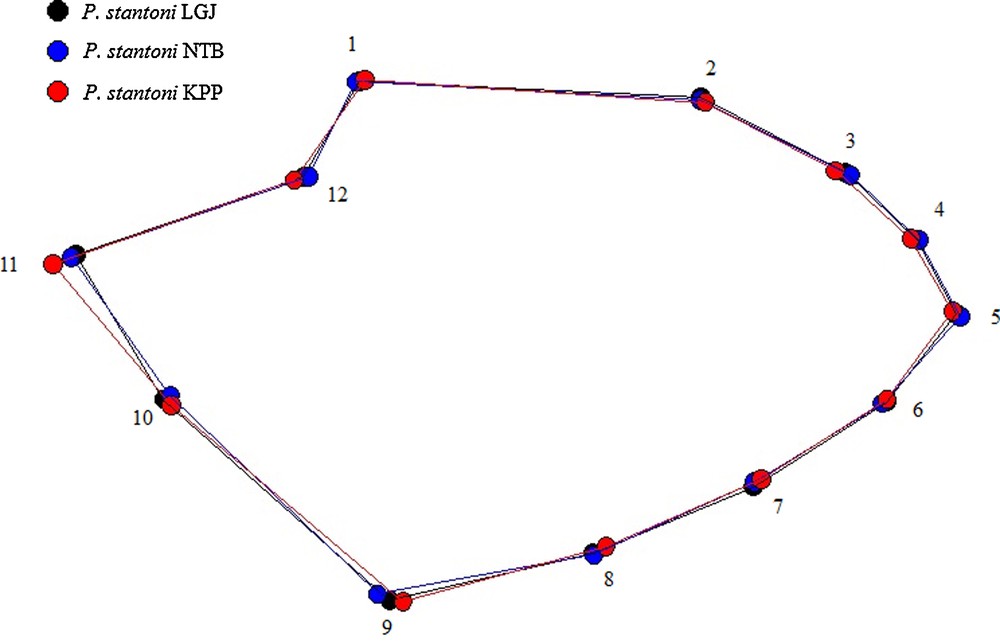
Polygons as connected mean landmark positions to visualize the wing shape differences in female Phlebotomus stantoni from various geographical locations, as obtained after Procrustes superposition. No amplification applied.
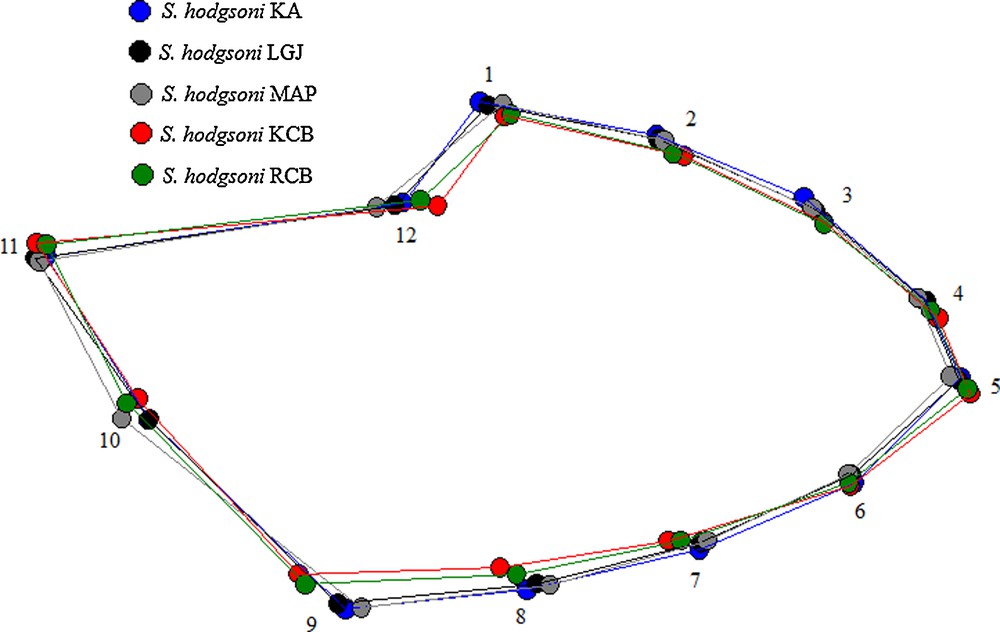
Polygons as connected mean landmark positions to visualize wing shape differences of female Sergentomyia hodgsoni from various geographical locations, as obtained after Procrustes superposition. No amplification applied.
In agreement with the polygon superimpositions, the RW–based morphospace showed no overlapping of wing shapes between P. stantoni and S. hodgsoni, as well as between some geographic localities within S. hodgsoni (Fig. 7). Within each species, significant geographic differences (P < 0.05) could be found (Table 3). P. stantoni from Lang Ga Jiew Island and Kamphaeng Phet province were significantly different (P < 0.05), as were P. stantoni from Nonthaburi province and Kamphaeng Phet province (P < 0.05).
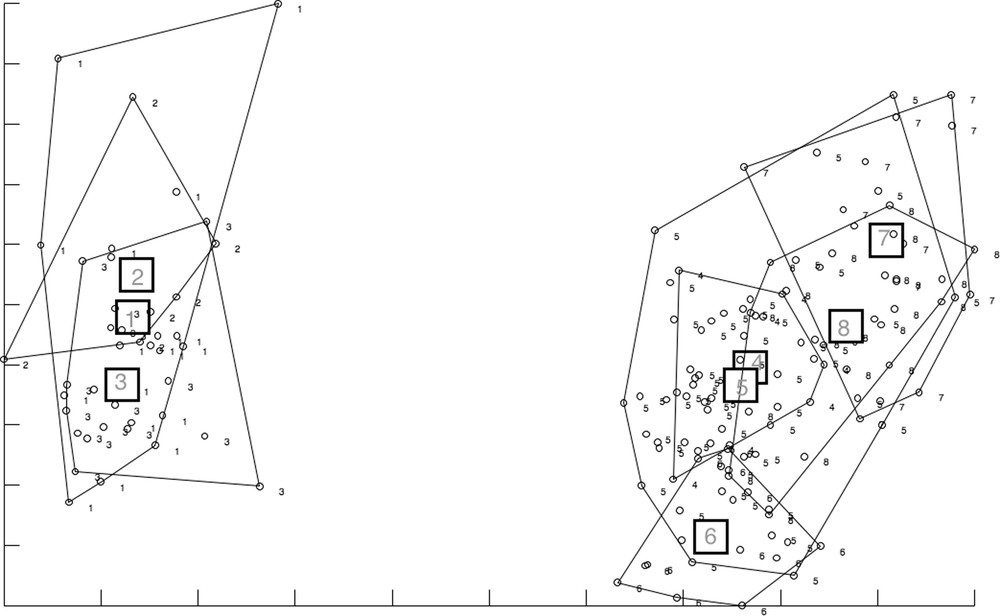
Wing shape morphospace based on RW variables of female Phlebotomus stantoni (1, 2, 3) and Sergentomyia hodgsoni (4, 5, 6, 7, 8) samples, represented by convex hulls. The horizontal axis is the first relative warp (RW1), and the vertical axis is the second relative warp (RW2). 1, P. stantoni LGJ; 2, P. stantoni NTB; 3, P. stantoni KPP; 4, S. hodgsoni KA; 5, S. hodgsoni LGJ; 6, S. hodgsoni MAP; 7, S. hodgsoni KCB; 8, S. hodgsoni RCB.
Mahalanobis distances between wing shapes of female Phlebotomus stantoni and Sergentomyia hodgsoni.
| P. stantoni | S. hodgsoni | ||||||||
| LGJ | NTB | KPP | KA | LGJ | MAP | KCB | RCB | ||
| LGJ | 0.00 | KA | 0.00 | ||||||
| NTB | 3.78 | 0.00 | LGJ | 2.37 | 0.00 | ||||
| KPP | 3.73a | 5.65a | 0.00 | MAP | 3.03 | 2.29a | 0.00 | ||
| KCB | 4.46a | 4.18a | 4.91a | 0.00 | |||||
| RCB | 4.72a | 3.67a | 4.32a | 3.04a | 0.00 |
The factor map of the DA clearly showed the large shape divergence between taxa, as well as some non-overlapping groups within both species: P. stantoni was almost completely subdivided into island and mainland samples (Fig. 8), while in S. hodgsoni the trend was similar, showing divergence between mainland (KCB, RCB) and island samples (LGJ, MAP), and, within these areas, between sampling sites (KCB versus RCB, and LGJ versus MAP) (Fig. 9). Statistical significance was frequently found, except with localities NTB and KA, represented by only a few individuals (Table 3). For S. hodgsoni the contribution of size to shape-based discrimination ranged from 0.099 (with DF1) to 0.165 (with DF2), and for P. stantoni (a single DF since two populations were compared), it was 0.117.
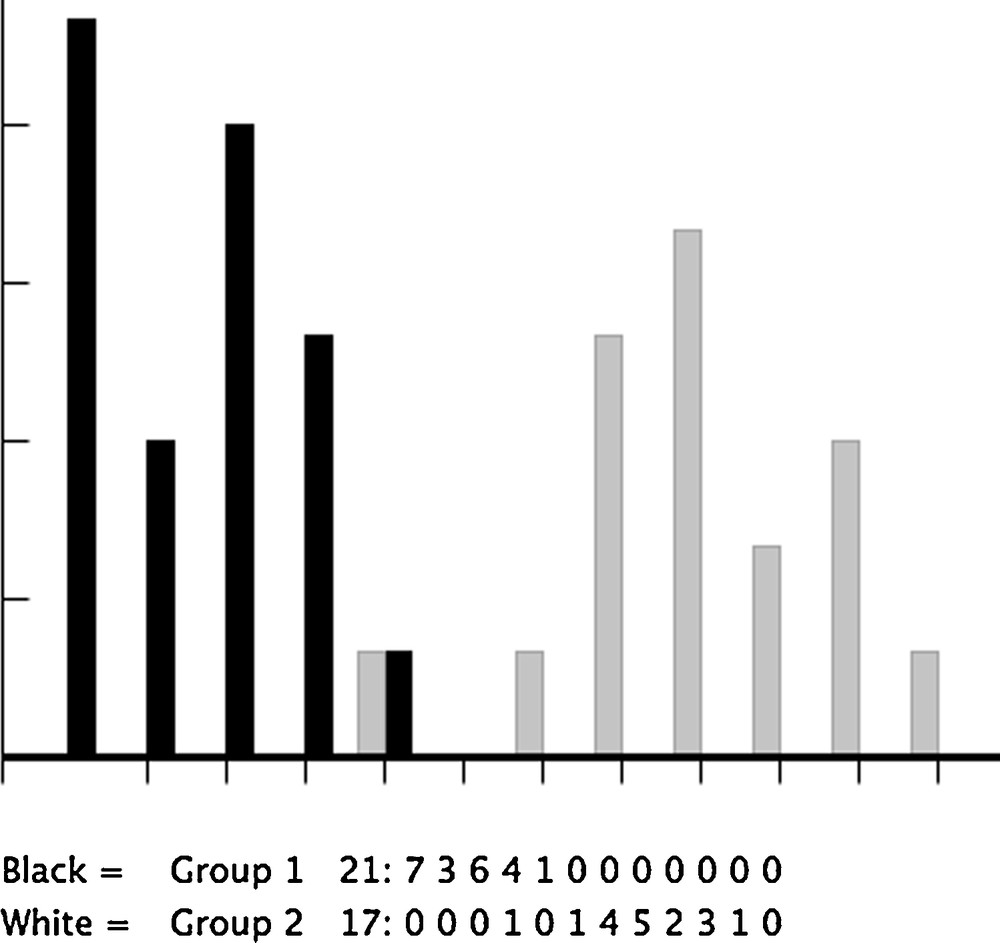
Factor map of the discriminant factor separating LGJ island (black bars) and KPP northern mainland (grey bars) samples of female Phlebotomus stantoni, as derived from shape variables (16 first RW). The NTB sample has been removed from this analysis due to low sample size (6 individuals).
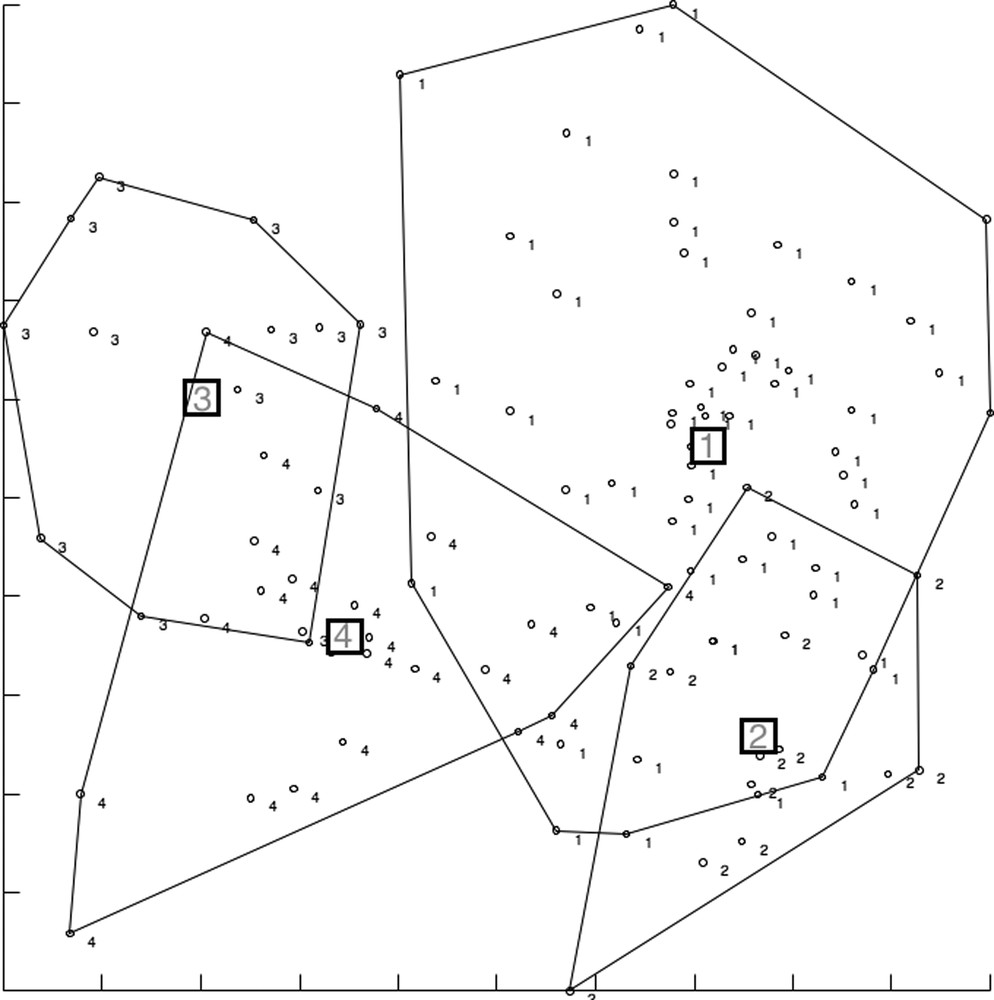
Factor map of the two first discriminant factors (DF) derived from shape variables of Sergentomyia hodgsoni originating from various geographic locations: 1, LGJ island; 2, MAP island; 3, KCB western mainland; 4, RCB central mainland. Each point represents an individual. The horizontal axis is DF1; the vertical axis is DF2. The KA sample has been removed from this analysis due to low sample size (seven individuals).
The validated reclassification scores (Table 4) confirmed the complete separation of the two taxa, providing an average correct assignment of 79% between two samples in P. stantoni (scores ranging from 71 to 88%), and of 65% between four samples in S. hodgsoni (scores ranging from 63 to 71%).
Validated reclassification scores between two species, Phlebotomus stantoni and Sergentomyia hodgsoni, between LGJ and KPP within P. stantoni, and between LGJ, MAP, KCB and RCB within S. hodgsoni.
| Species | Assigned | Observed | Percent (%) |
| P. stantoni | 38 | 38 | 100 |
| S. hodgsoni | 106 | 106 | 100 |
| P. stantoni | |||
| LGJ | 15 | 21 | 71 |
| KPP | 15 | 17 | 88 |
| Total | 30 | 38 | 79 |
| S. hodgsoni | |||
| LGJ | 36 | 57 | 63 |
| MAP | 9 | 13 | 69 |
| KCB | 10 | 14 | 71 |
| RCB | 14 | 22 | 64 |
| Total | 69 | 106 | 65 |
Based on Procrustes distances, the neighbor-joining tree first separated the two genera and then, within species, continental and islands locations (Fig. 10).
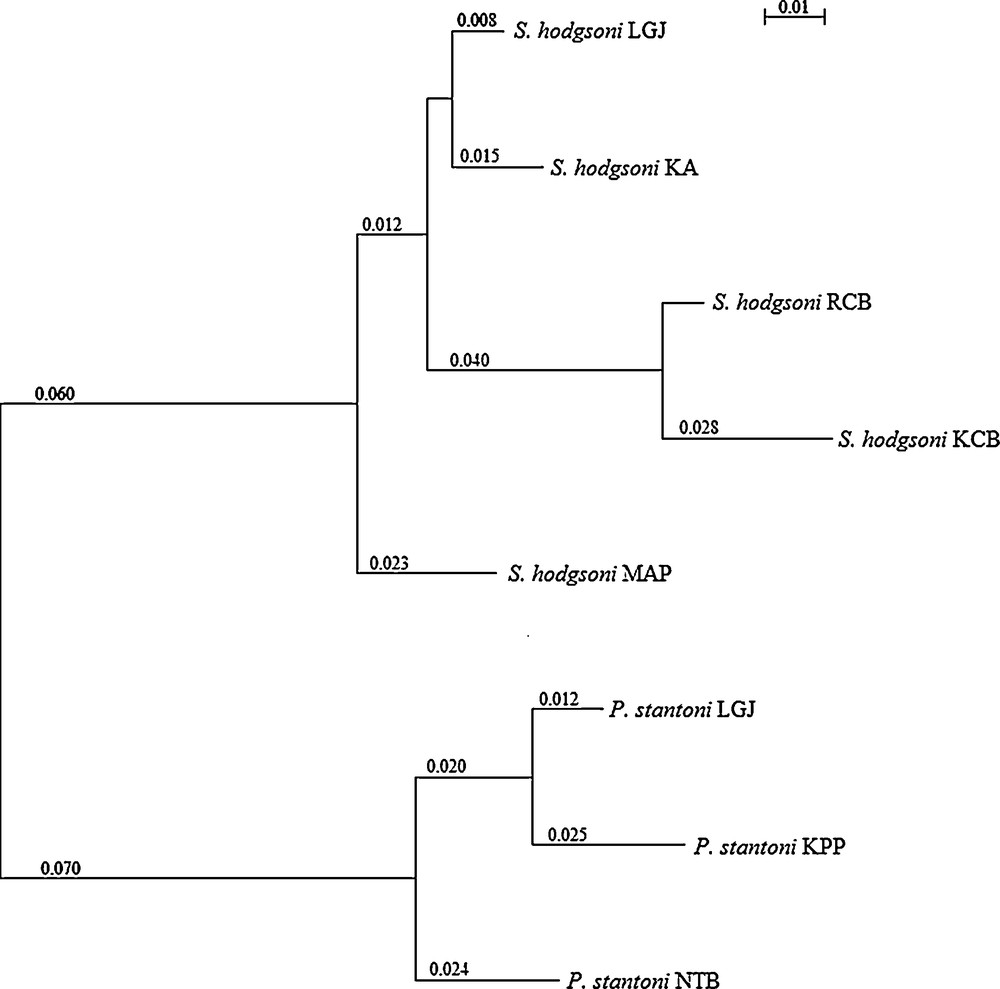
Neighbor-joining tree based on Procrustes distances, with indication of the patristic distances values, for female Phlebotomus stantoni and Sergentomyia hodgsoni from different geographical locations.
4 Discussion
This is the first GM study on sandflies from Thailand, here represented by various mainland and island geographic locations of two taxa: P. stantoni and S. hodgsoni. We selected the simplest and fastest geometric approach, i.e. the landmark-based approach. The wings of sandflies are hairy, which could represent a problem in accurately identifying some landmarks [19]. However, in our samples, manipulating the samples for dissection and mounting was generally enough to clear most of the hairy cover. Our results suggest that with no further changes in the entomological practice of dissecting and mounting sandflies, relevant information was provided with only 12 landmarks. We used the right wing of the specimens disregarding possible asymmetric effects. However, this source of variation has been estimated at a 1 or 2% of the interindividual variation [20], which should not interfere with our comparisons based on one side of the insect.
Size and shape provided taxonomic information of unequal values. The geometry of the wing venation distinguished both taxa without any possible confusion. This was in agreement with the likely high evolutionary divergence between two genera. The same geometric shape could also allow one to distinguish the geographic location within each species. P. stantoni from two geographical locations were almost completely distinct. S. hodgsoni from mainland and island, as well as within these areas, showed on average diverging geometric shapes, although with some overlapping. Discriminating between geographic localities was weakly under the influence of size variation, suggesting true shape variation.
The shape divergence between intraspecific sites has two possible origins: the environmental effects, or the genetic drift due to geographic isolation. There is a growing consensus claiming that, even if the environmental effect is probably acting in geometric shape differentiation, genetic drift is likely to be the primary force affecting it [21–26]. An argument supporting that hypothesis in our sample is that, in spite of significant and many times non-overlapping difference between geographic locations, there was no interference of this shape variation with the interspecific shape variation. Similar observation was already evidenced in a heterogeneous species sample of neotropical sandflies from various altitudes [27], and have been published for various other arthropods [23–26,28–30].
The size of the wings was computed as a global estimation, the centroid size, allowing one to compare it among samples based on a global, single value. Because wing size indicates specimen size [24], we can infer that P. stantoni specimens were generally larger ones than S. hodgsoni. More interestingly, in both taxa, specimens from the mainland were significantly larger than those from island populations. Alternatively, it can be said that in both species, northern populations showed larger sizes than southern ones. Such observation has been already made in sandflies: the New World Lutzomyia ayrozai and L. geniculata showed larger size in higher altitudes [27], the Old World P. papatasi also showed size difference in relation to different altitudes in Turkey [31]; and the same species from the northern Atlas Mountains of Morocco was larger than that from the southern Atlas Mountains [32]. This size variation may be related to environmental factors including temperature, host population, and other ecological or climatic effects [32], and appears to be in agreement with the Bergmann's rule [33]. This “rule” suffers some exceptions: for instance in our P. stantoni sample from an urban area, the specimens were smaller than those from the northern areas and island areas.
As such, the GM approach provides a powerful characterizing feature, the geometric shape, and represents a low-cost and useful tool to identify species and geographic populations. It could become a routine complement to morphological studies for future epidemiological investigation of leishmaniasis vectors in Thailand.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgements
We would like to thank all staffs members of Lang Ga Jiew Island, Chumphon province for sandfly collection.


