1 Introduction
The clingfishes of the family Gobiesocidae are predominantly marine fishes distributed worldwide in tropical and temperate seas, with only eight species of the genus Gobiesox Lacepède 1800 living in freshwater streams of the tropics. They occur on hard substrata, usually on rocky bottom or in coral reefs, mostly in shallow waters, but some live on the continental or insular shelf down to 337 m (Kopua nuimata Hardy 1984 from New Zealand; see Hardy [1]; Hutchins [2]). Clingfishes are small in body size (usually less than 50 mm in total length), characterized by an adhesive disc formed by the pelvic fins, the head depressed, the skin naked, one dorsal and anal fin each, and several specialized osteological characters. The family was revised by Briggs [3], who distinguished 30 genera and 85 species, with some divided in several subspecies. Springer & Fraser [4] examined the osteology of the species in the enigmatic perciform family Cheilobranchidae, and assigned its members (arranged in a single genus Alabes Cloquet 1816) to the Gobiesocidae. The following genera and species were described subsequently to the revisions of Briggs [3] and Springer & Fraser [4]: Lecanogaster, L. chrysea and Opeatogenys cadenati by Briggs [5], Aspasmodes and A. briggsi by Smith [6], Gobiesox marijeanae by Briggs [7], Gobiesox fluviatilis and G. mexicanus by Briggs & Miller [8], Lepadichthys bolini by Briggs [9], Gobiesox lucayanus by Briggs [10], Lepadichthys ctenion and L. erythraeus by Briggs & Link [11], Diplecogaster bimaculata euxinica by Murgoci [12], Apletodon knysnaensis by Smith [13] (synonym of A. pellegrini according to Briggs [14]), Lepadichthys lineatus by Briggs [15], Lissonanchus and L. lusheri by Smith [16], Tomicodon prodomus by Briggs [17], Derilissus and D. nanus by Briggs [18], Lepadichthys caritus by Briggs [19], Arcos decoris, Rimicola brevis and Tomicodon bidens by Briggs [20], Tomicodon rhabdotus by Smith-Vaniz [21], Gymnoscyphus and G. ascitus by Böhlke & Robins [22], Derilissus kremnobates and D. vittiger by Fraser [23], Derilissus altifrons by Smith-Vaniz [24], Discotrema and D. crinophilum by Briggs [25] as D. crinophila), Modicus, M. minimus and M. tangaroa by Hardy [26], Cochleoceps bassensis by Hutchins [27], Propherallodus, P. briggsi, Pherallodichthys and P. meshimaensis by Shiogaki & Dotsu [28], Kopua and K. nuimata by Hardy [29], Aspasmogaster occidentalis by Hutchins [30], Tomicodon abuelorum by Szelistowski [31], Kopua kuiteri by Hutchins [2], Cochleoceps viridis, C. bicolor and C. orientalis by Hutchins [32], Posidonichthys and P. hutchinsi by Briggs [33], Gobiesox juniperoserrai by Espinosa et al. [34], Apletodon incognitus by Hofrichter & Patzner [35], Lepadichthys springeri by Briggs [36], Tomicodon reitzae by Briggs [37], Rimicola cabrilloi by Briggs [38], Tomicodon briggsi, T. clarkei, T. cryptus, T. lavettsmithi and T. leurodiscus by Williams & Tyler [39], Alabes elongata, A. gibbosa, A. obtusirostris, A. occidentalis and A. scotti by Hutchins & Morrison [40], Acyrtus pauciradiatus by Sampaio et al. [41], Lepadicyathus and L. mendeleevi by Prokofiev [42], Alabes bathys and A. springeri by Hutchins [43], Apletodon wirtzi by Fricke [44], Discotrema monogrammum and D. zonatum by Craig & Randall [45], Briggsia and B. hastingsi by Craig & Randall [46], Apletodon barbatus by Fricke et al. [47], Aspasmichthys alorensis and Lepadichthys akiko by Allen & Erdmann [48], Kopua japonica by Moore et al. [49], Derilissus lombardii by Sparks & Gruber [50], Acyrtus lanthanum by Conway et al. [51], Unguitrema and U. nigrum by Fricke [52], Diplecogaster tonstricula by Fricke et al. [53], Kopua vermiculata by Shinohara & Katayama [54], and Lepadichthys bilineatus by Craig, Bogorodsky & Randall in Craig et al. [55].
At present, 166 species belonging to 48 genera of gobiesocids are recognized; they are currently classified into nine subfamilies (Briggs [3]; Conway et al. [56]): Aspasminae Briggs 1955, Cheilobranchinae Günther 1870, Chorisochisminae Briggs 1955, Diademichtyinae Whitley 1950, Diplocrepinae Briggs 1955, Gobiesocinae Bleeker 1859, Haplocyclicinae Briggs 1955, Lepadogastrinae Canestrini 1871–1872, Trachelochisminae Briggs 1955. A list of a generic classification within these subfamilies was provided by Conway et al. [56].
During the PAPUA NIUGINI Biodiversity Expedition in 2012, specimens of an unusual, laterally asymmetrical clingfish were collected in deep water off the north-eastern coast of Papua New Guinea. They were compared with the other known gobiesocid species and other taxa in gobiesocid-related fish families, and were found to represent an undescribed subfamily, genus and species of gobiesocid fishes, which is described in the present paper. In addition, the genus Lepadicyathus Prokofiev 2005, which is currently classified in the Aspasminae, is revised; the only known species, Lepadicyathus mendeleevi Prokofiev 2005, is redescribed.
2 Methods and materials
Our descriptive methods follow Briggs [3] and Fricke et al. [57]. Sex determination based on the presence or the absence of urogenital papillae is confirmed by internal examination (gonads). The abbreviation “SL” refers to the standard length (measured from the tip of the snout to the middle of the caudal fin base), “TL” to the total length (measured from the tip of the snout to the end of the caudal fin). The adhesive disc is divided into three different areas: region A is the anterior portion, region B is the posterior one, and region C is the centre of the disc (as illustrated by Briggs [3]). In the description of new taxa, data of the holotype are given first, followed by data of the paratypes in parentheses. Fin rays are counted using the method of Fricke (1983), where spines are expressed as Roman numerals, unbranched soft rays are expressed as lower-case Roman numerals, and branched rays as Arabic numerals. Authors and dates of family-group taxa follow Laan et al. [58]. The updated key to subfamilies of Gobiesocidae is based on Briggs [3]. To elucidate the relationships of the unusual clingfish discovered in this study and to assign it to its proper higher hierarchic taxonomic rank, its multi-locus DNA sequences were generated and compared to those obtained from the representative taxa in the Gobiesocidae and other gobiesocid-related families determined by previous studies investigating acanthomorph phylogeny (Chen et al. [59,60]; Dettaï & Lecointre [61]; Near et al. [62]). The list of taxa used for the molecular analysis in this study was provided in Table 1. The collection of new molecular data was carried out by the following procedures. Small pieces of muscle or fin tissue were excised from the specimens, preserved in 95% ethanol, and stored at −20 °C in the Marine Biodiversity and Phylogenomics Laboratory at the Institute of Oceanography, National Taiwan University (NTU), Taipei. Genomic DNA was extracted using an automated DNA-extractor 199 (LabTurbo 48 Compact System with LGD 480–220 kits: Taigene 200 Bioscience Corporation, Taipei) following the manufacturer's 201 protocol. The polymerase chain reaction (PCR) was used to amplify the following targeting gene fragments: recombination activating gene 1 (RAG1), rhodopsin (RH), early growth response 2B (EGR2B), cytochrome oxidase subunit I (COI), 12S and 16S ribosomal RNAs (12S and 16S). The first three are encoded in the nuclear genome and the remaining three are part of the mitochondrial genome. These genes were selected for their ability to provide sound phylogenetic information for inter- and intra-familiar relationships in several previous phylogenetic studies focusing on the percomorph fishes (e.g., Chen et al. [60]; Lo et al. [63]). Primer sequences in this study are listed in Table 2. Temperature cycling profiles for amplification consisted of an initial denaturation stage (95 °C, 5 min) followed by 35 cycles, each with a denaturation step (95 °C, 40 s), an annealing step (53 °C for RAG1 and EGR2B; 55 °C for the other genes, 40 s) and an elongation step (72 °C, 90 s for RAG1 and 60 s for the other genes), before a final extension stage (72 °C, 7 min). PCR products were purified using the AMPure magnetic bead clean-up protocol (Agencourt Bioscience Corp., USA). Purified PCR products were sequenced by Sanger sequencing using dye-labelled terminators. Sequence determinations from Sanger reaction products were generated on ABI 3730 analyzers (Applied Biosystems, USA) at Genomics BioSci & Tech (Taipei) and at the Center of Biotechnology (National Taiwan University). For each locus and specimen, the chromatograms of the sequences were checked and edited manually using the program CodonCode Aligner 3.7.2.2 (Codoncode Corporation, Dedham, MA, USA). Chromatograms were examined for evidence of sequencing artefacts or problematic base calls. Verified complementary chromatograms were then assembled to build consensus sequences. All sequences from the orthologous gene were aligned using the automatic multiple-alignment program Muscle (Edgar [64]). The resulting multiple sequence alignments were adjusted by eye using the inferred amino acid translation as a guide for inferred gap placement in protein coding genes and the loop/stem structure of 12S and 16S ribosomal RNAs to identify loop regions where site homology was difficult to ascertain with confidence. Alignments were edited using Se-Al v2.0a11 (Rambaut [65]). Short regions of the alignments from EGR2B, 12S and 16S in which homology assessment is uncertain were excluded from the further steps of phylogenetic analysis. Phylogenetic analysis was conducted based on the combined data matrix complied from the aligned DNA sequences from the six genes with a partitioned maximum likelihood (ML) method as implemented in the parallel version of RAxML 7.4.2 (Stamatakis [66]). A mixed-model analysis was used that allows the independent estimation of individual models of nucleotide substitution for each partition. Three partitions (by codon) were assigned for cytochrome b and rhodopsin. Fourteen partitions (by gene and by codon position of protein coding gene) were assigned. Because RAxML only provides GTR-related (Yang [67]) models of rate heterogeneity for nucleotide data (Stamatakis [66]), the nucleotide substitution model GTR + Γ+I was employed for the analysis. Optimal ML tree search was conducted with 100 separate runs using the default algorithm of the program for each run and the final tree with the best ML score was selected among the 100 ML trees of these runs. Finally, nodal support was assessed with bootstrapping (BS) (Felsenstein [68]) with the maximum likelihood (ML) criterion, based on 500 pseudo-replicates. The inferred phylogenetic tree was rooted by two non-“Ovalentaria” taxa: Hippoglossus stenolepis (Pleuronectidae) and Parastromateus niger (Carangidae). The specimens cited in the present paper are deposited in the following collections: AMS, The Australian Museum, Sydney, Australia; MNHN, “Muséum national d’histoire naturelle”, Paris, France; NTUM, National Taiwan University Museums, Taipei, Taiwan; ZIN, Laboratory of Ichthyology, Zoological Institute, Russian Academy of Sciences, St. Petersburg, Russia. The abbreviations of repositories of additional materials follow Fricke & Eschmeyer [69].
Taxa, genes, and Genbank accession numbers for the gene sequences of representative species.
| Taxon | GenBank accession no. | |||||||
| Tissue ID | Voucher | RAG1 | Rhodopsin | EGR2B | COI | 12S | 16S | |
| Pleuronectidae a | ||||||||
| Hippoglossus stenolepis Schmidt 1904 | WJC148 | NA | KF311999 | KF312141 | KF312079 | AM749128 | AM749128 | AM749128 |
| Carangidae a | ||||||||
| Parastromateus niger (Bloch 1795) | WJC11 | NA | EF095654 | EF095616 | KC442131 | KC442079 | EF095562 | EF095590 |
| Embiotocidae | ||||||||
| Ditrema temminckii Bleeker 1853 | WJC171 | NA | KY126055 | KY126046 | KY126033 | AP009129 | AP009129 | AP009129 |
| Mugilidae | ||||||||
| Liza aurata (Risso 1810) | WJC18 | NA | KF017112 | KF017144 | KF017049 | JQ060457 | EU715447 | GQ252691 |
| Pomacentridae | ||||||||
| Dascyllus aruanus (Linnaeus 1758) | WJC377 | NA | EF095674 | EF095632 | KY126034 | KJ968020 | AF081228 | AF119402 |
| Cichlidae | ||||||||
| Astronotus ocellatus (Agassiz 1831) | WJC168 | NA | EF095671 | EF095629 | JN231060 | KC442077 | EF095575 | EF095603 |
| Ambassidae | ||||||||
| Parambassis wolffii (Bleeker 1850) | WJC49 | NA | EF095647 | EF095612 | KY126035 | EF095558 | EF095586 | |
| Bedotiidae | ||||||||
| Bedotia geayi Pellegrin 1907 | WJC399 | NA | EF095640 | AY141267 | KC442117 | AY290799 | AY141339 | AY141409 |
| Adrianichthyidae | ||||||||
| Oryzias latipes (Temminck & Schlegel 1846) | NA | NA | EF095641 | AB001606 | AP004421 | EF095555 | EF095583 | |
| Clinidae | ||||||||
| Heterostichus rostratus Girard 1854 | WJC118 | SIO 01-179 | HQ168866 | HQ168988 | KY126036 | HQ168631 | HQ158801 | AY822076 |
| Labrisomidae | ||||||||
| Labrisomus nuchipinnis (Quoy & Gaimard 1824) | WJC86 | NA | KY126056 | KY126047 | KY126037 | KF930015 | AY098808 | AY098848 |
| Gobiesocidae | ||||||||
| Aspasminae | ||||||||
| Aspasma minima (Döderlein 1887) | EU637943 | NC_008130 | NC_008130 | NC_008130 | ||||
| Cheilobranchinae | ||||||||
| Alabes hoesei Springer & Fraser 1976 | WJC5173 | I.45630-050 | KY126057 | KY126038 | KY126065 | KY126072 | KY126080 | |
| Alabes scotti Hutchins & Morrison 2004 | WJC5174 | I.46305-001 | KY126058 | KY126048 | KY126039 | KY126066 | KY126073 | KY126081 |
| Diademichthyinae | ||||||||
| Lepadichthys lineatus Briggs 1966 | WJC5008 | 14-1309 Dili | KY126059 | KY126049 | KY126040 | KY126067 | KY126074 | KY126082 |
| Gobiesocinae | ||||||||
| Acyrtus lanthanum Conway, Baldwin & White 2014 | WJC5006 | 01-033-Arsp | KY126060 | KY126050 | KY126041 | KY126068 | KY126075 | KY126083 |
| Gobiesox strumosus Cope 1870 | WJC65 | NA | KY126061 | KY126051 | KY126042 | KY126069 | KY126076 | KY126084 |
| Acyrtops beryllinus (Hildebrand & Ginsburg 1927) | WJC82 | NA | KY126062 | KY126052 | KY126043 | KY126070 | KY126077 | KY126085 |
| Tomicodon humeralis (Gilbert 1890) | SIO 02-1-1 | HQ168876 | HQ169001 | HQ168639 | ||||
| Lepadogastrinae | ||||||||
| Apletodon dentatus (Facciolà 1887) | WJC305 | NA | KY126063 | KY126053 | KY126044 | KJ616456 | KY126078 | KY126086 |
| Lepadogaster lepadogaster (Bonnaterre 1788) | AY141273 | KF369136 | AY141347 | AY141417 | ||||
| Protogobiesocinae | ||||||||
| Protogobiesox asymmetricus n. sp. | PNG1169 | NTUM10633 | KY126064 | KY126054 | KY126045 | KY126071 | KY126079 | KY126087 |
a Outgroups used for rooting the inferred phylogenetic tree.
Primers used in this study.
| Locus/Primera | Primer sequences (5′-3′) | Source | |
| RGA1 | R1 2533F | CTGAGCTGCAGTCAGTACCATAAGATGT | López et al., 2004 |
| R1 4078R | TGAGCCTCCATGAACTTCTGAAGRTAYTT | López et al., 2004 | |
| R1 4061R | AATACTTGGAGGTGTAGAGCCAGT | Chen et al., 2007 | |
| R1 4090R | CTGAGTCCTTGTGAGCTTCCATRAAYTT | López et al., 2004 | |
| EGR2B | E2B 252F | CGCAACCAGACTTTCACCTAY | Chen et al., 2013 |
| E2B 261F | TTCACCTAYATGGGNAAGTTCTCMAT | Chen et al., 2013 | |
| E2B 270F | ATGGGRAAGTTCTCCATCGAC | Chen et al., 2013 | |
| E2B 278F | AGTTTTCCATCGACTCSCAGTA | Chen et al., 2008 | |
| E2B 287F | TTGACTCSCAGTATCCAGGTAAC | Chen et al., 2008 | |
| E2B 1078R | AATTTGCGNCCGCAGSAGTC | Chen et al., 2013 | |
| E2B 1078R-bis | GAACTTACGNCCGCAGAARTC | Chen et al., 2013 | |
| E2B 1108R | TTTTGTGTGTCTCTTTCTYTCGTC | Chen et al., 2008 | |
| E2B 1112R | ATTTTNGTGTGTCGYTTYCTC | Chen et al., 2013 | |
| E2B 1117R | AGGTGGATTTTGGTGTGTCTYTT | Chen et al., 2008 | |
| E2B 1121R | CCTCAGGTGGATTTTAGTGTGTC | Chen et al., 2013 | |
| Rhosopsin | RH 1F | ATGAACGGCACAGARGGAC | Chen et al., 2003 |
| RH 193F | CNTATGAATAYCCTCAGTACTACC | Chen et al. | |
| RH 1039R | TGCTTGTTCATGCAGATGTAGA | Chen et al. | |
| COI | CoxI_FishF1 | TCAACCAACCACAAAGACATTGGCAC | Ward et al., 2005 |
| CoxI_FishF2 | TCGACTAATCATAAAGATATCGGCAC | Ward et al., 2005 | |
| CoxI_FishR1 | TAGACTTCTGGGTGGCCAAAGAATCA | Ward et al., 2005 | |
| CoxI_FishR2 | ACTTCAGGGTGACCGAAGAATCAGAA | Ward et al., 2005 | |
| 12S | L1090 | AAAGCACGGCACTGAAGATGC | Palumbi et al., 1991 |
| H1478 | TTTCATGTTTCCTTGCGGTAC | Palumbi et al., 1991 | |
| 16S | arL | CGCCTGTTTAACAAAAACAT | Palumbi et al., 1991 |
| brH | CCGGTCTGAACTCAGATCAGT | Palumbi et al., 1991 |
a Reverse primers in italics
2.1 Comparative material for morphological examination
Acyrtops amplicirrus: CAS-SU 18101 (2 paratypes), St. Thomas, Virgin Islands. Acyrtus artius: CAS-SU 23254 (holotype), Curaçao, Netherlands West Indies; CAS-SU 47942 (1 paratype), Curaçao, Netherlands West Indies. Alabes dorsalis: SMNS 1694 (4), Murray River estuary, South Australia; SMNS 21395 (1), Cape Dromedary, New South Wales, Australia. Apletodon barbatus: SMNS 26427 (holotype), São Tiago Island, Cape Verde Islands; MNHN 2009-1592 (1 paratype), same data as the holotype; SMNS 24604 (1), Sal Island, Cape Verde Islands; SMNS 24605 (1), Sal Island, Cape Verde Islands; SMNS 26428 (19 paratypes), same data as the holotype; USNM 396967 (1 paratype), same data as the holotype. A. dentatus: CCML uncat. (2), Alegranza Island, Canary Islands; CCML uncat. (2), Lanzarote Island, Canary Islands; SMNS 12664 (1), Genoa, Italy. A. incognitus: NMW 93029 (holotype), Banyuls-sur-Mer, France; CCML uncat. (2), Gran Canaria, Canary Islands; CCML uncat. (1), La Graciosa, Canary Islands; SMNS 21814 (1), Faial, Azores Islands. A. pellegrini: SAIAB 10255 (1), Knysna, South Africa; SAIAB 10852 (11), Knysna, South Africa; SAIAB 14599 (7), South Africa; SAIAB 43756 (5), False Bay, South Africa; USNM 198169 (1 paratype), Knysna, South Africa; USNM 270272 (1), Algoa Bay, South Africa. A. wirtzi: SMNS 24130 (holotype), Bombom Island, São Tomé and Principe; MNHN 2005-0170 (1 paratype), same data as the holotype; SMNS 24446 (5 paratypes), same data as the holotype; SMNS 24132 (2 paratypes), same data as the holotype; SMNS 25472 (5), Limbe, Cameroon; USNM 381374 (1 paratype), same data as the holotype. Arcos nudus: SMNS 11301 (4), Rio San Juan, Dominican Republic; SMNS 11401 (12), Rio Baonico, Dominican Republic; SMNS 11408 (3), Rio Nazaito, Dominican Republic. Aspasma minima: NMW 76995 (2 syntypes), Sagami Bay, Japan. Aspasmichthys ciconiae: CAS-SU 7136 (1 syntype), Inland Sea, Japan; USNM 50760 (1 syntype), Inland Sea, Japan. Aspasmogaster costata: SMNS 14754 (9), Red Head, New South Wales, Australia. Chorisochismus dentex: USNM 119223 (1), Algoa Bay, South Africa. Cochleoceps spatula: SMNS 12519 (1), Cervantes Island, Western Australia. Conidens laticephalus: SMNS 24701 (10), Northeast Cape, Taiwan. C. samoensis: SMNS 21982 (1), Province Sud, Grande Terre, New Caledonia; SMNS 22836 (3), Province Sud, Grande Terre, New Caledonia. Creocele cardinalis: SMNS 14806 (1), Tasmania, Australia. Dellichthys morelandi: SMNS 13754 (5), North Island, New Zealand; SMNS 13762 (1), North Island, New Zealand; SMNS 13780 (4), North Island, New Zealand; SMNS 13827 (1), North Island, New Zealand; SMNS 14142 (4), North Island, New Zealand. Derilissus altifrons: ANSP 112690 (holotype), Dominica. D. vittiger: ANSP 109626 (holotype), Venezuela. Diademichthys lineatus: SMNS 18156 (2), Fiji, Viti Levu; SMNS 21975 (3), New Caledonia, Récif Goëland; SMNS 21977 (1), New Caledonia, Nouméa; SMNS 21979 (2), New Caledonia, Baie Maá; SMNS 21980 (2), New Caledonia, Bancs Nord; SMNS 22995 (1), New Caledonia, Baie de Pritzbue; SMNS 23522 (1), New Caledonia, Nouméa; SMNS 23997 (1), New Caledonia, Nouméa; SMNS 25414 (1), New Caledonia, Nouméa; SMNS 26545 (1), New Caledonia, Baie de Goro. Diplecogaster bimaculata: HUJ 20577 (7), Balearic Islands, S Mallorca; HUJ 20593 (1), Balearic Islands, NE Mallorca; HUJ 20602 (2), Balearic Islands, NW Mallorca; HUJ 20623 (1), Balearic Islands, SSE Mallorca; SMNS 12541 (1), Pyrenées Orientales, France; SMNS 13177 (1), Giglio Island, Italy; SMNS 14049 (2), Giglio Island, Italy; SMNS 19061 (2), Karavas Alsavcak Bay, Northern Cyprus; SMNS 19204 (2), Giglio Island, Italy; SMNS 20163 (8), Caniço de Baixo, Madeira; SMNS 20347 (1), Tabarca, Tunisia; SMNS 21202 (2), Porto Novo, Madeira. D. ctenocrypta: ZMUC P9037 (holotype), Gran Canaria, Canary Islands. D. pectoralis: SMNS 11916 (4), Faial Island, Azores Islands. D. tonstricula: ZSM 40089 (holotype), Dakar, Senegal; CCML uncat. (2 paratypes), Fuerteventura, Canary Islands; ZSM uncat. (5 paratypes), Dakar, Senegal. Diplocrepis puniceus: SMNS 10012 (2), North Island, New Zealand; SMNS 13755 (2), North Island, New Zealand; SMNS 13769 (1), North Island, New Zealand; SMNS 13782 (1), North Island, New Zealand; SMNS 13828 (8), North Island, New Zealand; SMNS 13832 (4), North Island, New Zealand; SMNS 13922 (42), South Island, New Zealand; SMNS 13936 (32), South Island, New Zealand; SMNS 13945 (24), South Island, New Zealand; SMNS 14003 (1), South Island, New Zealand; SMNS 14043 (1), South Island, New Zealand; SMNS 14069 (1), South Island, New Zealand. Discotrema crinophilum: SMNS 16677 (1), Philippines, Balicasag Island; SMNS 16678 (1), Philippines, Balicasag Island; SMNS 17896 (1), Society Islands, Moorea (new record); SMNS 21981 (1), New Caledonia, Nouméa. D. monogrammum: BPBM 39040 (holotype), Papua New Guinea, New Britain; BPBM 36504 (2), Indonesia, Flores. D. zonatum: BPBM 38972 (holotype), Fiji, Charybdis Reef. Gastrocyathus gracilis: SMNS 10011 (1), North Island, New Zealand; SMNS 14124 (1), North Island, New Zealand. Gastroscyphus hectoris: SMNS 13763 (1), North Island, New Zealand; SMNS 13770 (1), North Island, New Zealand; SMNS 13829 (3), North Island, New Zealand; SMNS 13834 (27), North Island, New Zealand; SMNS 13854 (1), North Island, New Zealand; SMNS 13862 (1), North Island, New Zealand; SMNS 13923 (26), South Island, New Zealand; SMNS 13924 (76), South Island, New Zealand; SMNS 13938 (6), South Island, New Zealand; SMNS 13947(9), South Island, New Zealand; SMNS 13948 (17), South Island, New Zealand; SMNS 13949 (30), South Island, New Zealand; SMNS 14008 (5), South Island, New Zealand; SMNS 14018 (1), South Island, New Zealand. Gobiesox strumosus: SMNS 13612 (1), Rio de Janeiro, Brazil. Gouania willdenowi: SMNS 421 (4), Hvar Island, Croatia; SMNS 576 (2), Hvar Island, Croatia; SMNS 13175 (1), Giglio Island, Italy; SMNS 13573 (1), Costa Brava, Spain; SMNS 13574 (1), Crete Island, Greece; SMNS 16808 (6), Sicily, Italy; SMNS 22064 (6), Cres Island, Croatia. Gymnoscyphus ascitus: ANSP 113587 (holotype), St. Vincent. Haplocylix littoreus: CAS-SU 47678 (2), North Island, New Zealand. Kopua nuimata: SMNS 10013 (1), North Island, New Zealand; SMNS 13737 (6), North Island, New Zealand; SMNS 13781 (1), North Island, New Zealand; SMNS 13826 (22), North Island, New Zealand; SMNS 13833 (2), North Island, New Zealand; SMNS 13943 (4), South Island, New Zealand; SMNS 13996 (20), South Island, New Zealand; SMNS 14030 (1), South Island, New Zealand; SMNS 14042 (2), Stewart Island, New Zealand; SMNS 14070 (1), South Island, New Zealand; SMNS 14127 (1), South Island, New Zealand; SMNS 14151 (2), South Island, New Zealand. Lecanogaster chrysea: BMNH 1958.7.3.1 (holotype), Lingo, Ghana. Lepadichthys caritus: BPBM 34140 (1), Indonesia, Flores. L. coccinotaenia: BPBM 21711 (1), South Africa, KwaZulu-Natal. L. erythraeus: SMNS 22550 (1), Red Sea, Gulf of Aqaba, Egypt, 14 km north of Nuweiba. L. frenatus: SMNS 21978 (1), New Caledonia, off Nouméa. L. lineatus: SMNS 22573 (4), Red Sea, Gulf of Aqaba, Egypt, 14 km north of Nuweiba. L. minor: SMNS 15130 (1), Fiji, Viti Levu; SMNS 17822 (2), Cook Islands, Aitutaki; SMNS 20909 (2), La Réunion, Les Filaos; SMNS 21020 (2), La Réunion, Les Filaos; SMNS 21161 (1), La Réunion, Les Filaos; SMNS 21178 (2), La Réunion, Saint-Leu; SMNS 22980 (1), Loyalty Islands, Lifou; SMNS 26966 (3), New Caledonia, Nouméa. Lepadichthys sp.: SMNS 21976 (1), New Caledonia, Baie Maá; SMNS 23962 (15), New Caledonia, Ile Nou; SMNS 26967 (4), New Caledonia, Nouméa; SMNS 26968 (1), New Caledonia, Nouméa. Lepadogaster candolii: SMNS 579 (1), Hvar Island, Croatia; SMNS 993 (1), Trieste, Italy; SMNS 2662 (6), Naples, Italy; SMNS 8996 (1), Senj, Croatia; SMNS 9416 (1), Cres Island, Croatia; SMNS 13176 (1), Giglio Island, Italy; SMNS 13575 (5), Saronic Gulf, Greece; SMNS 13576 (1), Elba Island, Italy; SMNS 13677 (1), Peloponnes, Greece; SMNS 13578 (3), Crete Island, Greece; SMNS 13579 (3), Muros, Galicia, Spain; SMNS 13580 (1), Saronic Gulf, Greece; SMNS 13581 (1), Costa Brava, Spain; SMNS 13582 (2), Elba Island, Italy; SMNS 13613 (1), Biograd, Croatia; SMNS 13614 (1), Sicily, Italy; SMNS 13615 (1), Sicily, Italy; SMNS 13616 (2), Corfu Island, Greece; SMNS 13620 (2), Corfu Island, Greece; SMNS 14700 (6), Reis Magos, Madeira; SMNS 14721 (2), Cres Island, Croatia; SMNS 16020 (1), Caniçal, Madeira; SMNS 16069 (1), Siciliy, Italy; SMNS 25479 (2), Caniço de Baixo, Madeira. L. lepadogaster: SMNS 1079 (1), Azores; SMNS 13556 (1), Biograd, Croatia; SMNS 13557 (1), Golfo di Trieste, Italy; SMNS 13558 (1), Bari, Italy; SMNS 13559 (1), Karlobag, Croatia; SMNS 13560 (2), Corfu Island, Greece; SMNS 13561 (5), Elba Island, Italy; SMNS 13562 (1), Collioure, Pyrenées-Orientales, France; SMNS 13563 (1), Crete Island, Greece; SMNS 13564 (1), Cap Negro, N Tétouan, Morocco; SMNS 13565 (2), Elba Island, Italy; SMNS 13566 (2), Sea of Marmara, Turkey; SMNS 13567 (1), İstanbul Province, Turkey, Black Sea; SMNS 13568 (1), Granada Province, Spain; SMNS 13569 (1), Banyuls-sur-Mer, France; SMNS 13570 (2), Gran Canaria, Canary Islands; SMNS 13617 (5), Costa Brava, Spain; SMNS 13618 (1), Corfu Island, Greece; SMNS 14699 (4), Reis Magos, Madeira; SMNS 16726 (6), Fuerteventura, Canary Islands; SMNS 19070 (1), Karavaş Alsavçak Bay, Northern Cyprus; SMNS 22609 (8), La Palma, Canary Islands; SMNS 25316 (1), Bodrum, Turkey; SMNS 25481 (2), Caniço de Baixo, Madeira. L. purpurea: SMNS 10295 (1), Lanzarote, Canary Islands; SMNS 12549 (1), Collioure, France; SMNS 13571 (1), Biograd, Croatia; SMNS 13572 (2), Saronic Gulf, Greece; SMNS 15286 (2), Cap Béar, Pyrenées Orientales, France; SMNS 15315 (2), La Palma, Canary Islands; SMNS 15328 (1), La Palma, Canary Islands; SMNS 16727 (4), Fuerteventura, Canary Islands; SMNS 16766 (2), Fuerteventura, Canary Islands; SMNS 22610 (4), La Palma, Canary Islands; SMNS 22623 (1), La Palma, Canary Islands; SMNS 23501 (5), El Hierro, Canary Islands. Liobranchia stria: USNM 149910 (holotype), Saipan, Marianas Islands. Modicus minimus: BMNH 1982.6.17.55 (1 paratype), North Island, New Zealand. Opeatogenys cadenati: MNHN 1959-0059 (holotype), Senegal; MNHN 1959-0060 (2 paratypes), Senegal. Parvicrepis parvipinnis: AMS I.7708 (5 syntypes), Sydney, New South Wales, Australia. Pherallodichthys meshimaensis: NSMT-P 46752 (holotype), Kyushu, Japan. Pherallodiscus funebris: CAS-SU 20 (9 paralectotypes), Gulf of California, Mexico. Pherallodus indicus: CAS-SU 47683 (1), Raroia, Tuamotu Archipelago. P. smithi: CAS-SU 31349 (holotype), KwaZulu-Natal, South Africa. Posidonichthys hutchinsi: AMS I.17615-002 (holotype), Fiddler's Bay, South Australia. Propherallodus briggsi: NSMT-P 46749 (holotype), Kyushu, Japan. Rimicola muscarum: CAS-SU 3030 (holotype), Monterey Bay, California, USA. Sicyases sanguineus: SMNS 390 (2), Chile. Tomicodon humeralis: CAS-SU 70 (5 syntypes), Gulf of California, Mexico. Trachelochismus melobesia: SMNS 13926 (1), South Island, New Zealand; SMNS 13940 (1), South Island, New Zealand. T. pinnulatus: SMNS 13925 (1), South Island, New Zealand; SMNS 13937 (1), South Island, New Zealand; SMNS 13939 (2), South Island, New Zealand; SMNS 13946 (4), South Island, New Zealand; SMNS 13950 (6), South Island, New Zealand; SMNS 13951 (34), South Island, New Zealand. Unguitrema nigrum: NTUM 10603 (holotype), Madang, Papua New Guinea; MNHN uncat. (1 paratype), same data as the holotype.
3 Results
3.1 Gobiesocid phylogeny
The combined dataset for inferring gobiesocid phylogeny contains the sequences of RAG1 gene (1476 bp), Rhodopsin gene (819 bp), EGR2B gene (855 bp), COI gene (633 bp), 12S gene (344 bp) and 16S gene (334 bp) from a total of 22 examined taxa that include 11 gobiesocid representatives from five out of their nine currently recognized subfamilies (Table 1). The total of 4461 nucleotides comprise 2184 variable sites (49%) and 736 parsimony-informative sites. The resulting ML tree is shown in Fig. 1.
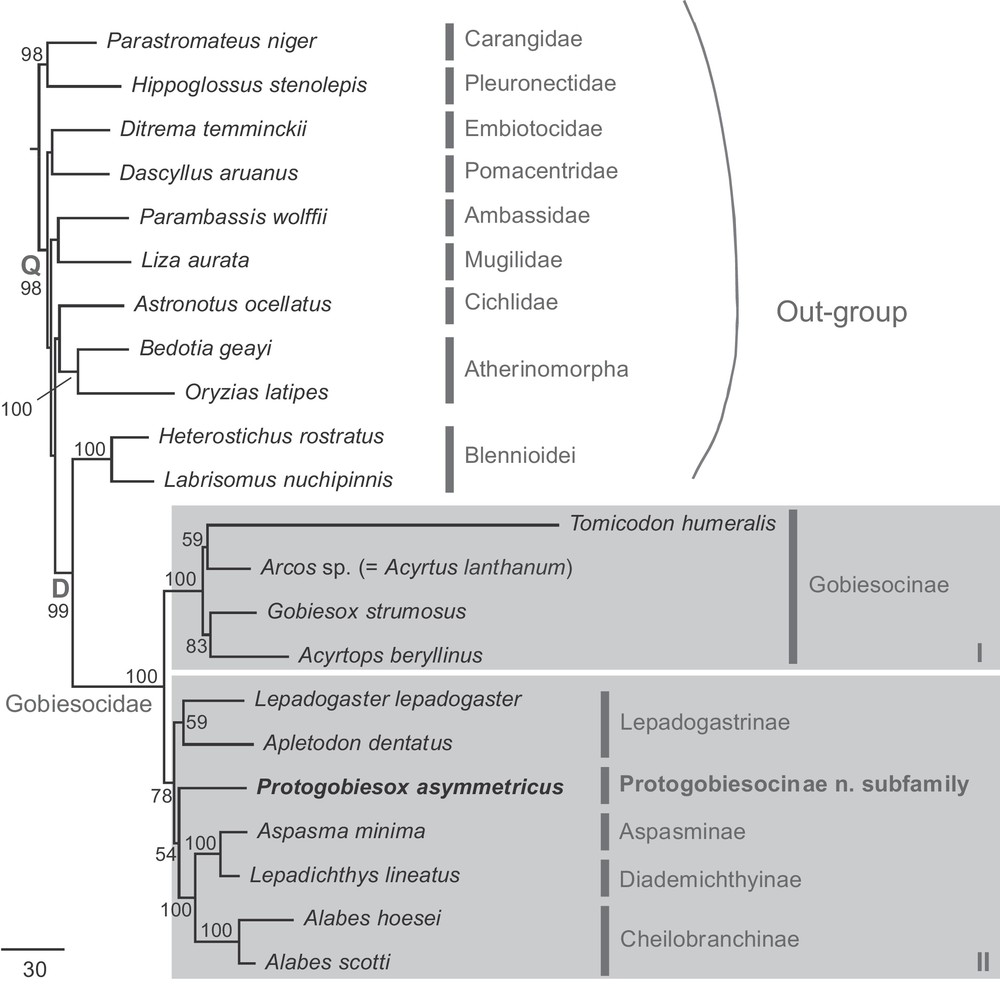
Phylogenetic tree of the Gobiesocidae inferred by the partitioned maximum-likelihood method with the GTR + Γ+I nucleotide substitution model based on the combined dataset. Branch lengths are proportional to the inferred nucleotide substitutions. Numbers at nodes represent bootstrap values in percentage. Values below 50% are not shown. An undetermined specimen of Arcos sp. was further determined to represent a recently described species, Acyrtus lanthanum Conway, Baldwin & White 2014 by COI sequence similarity.
From the inferred tree, the monophyly of the Gobiesocidae as well as its subfamilies Gobiesocinae and Cheilobranchinae is confirmed with 100% bootstrap nodal support. The Lepadogastrinae is monophyletic, but it received only week support. Among the outgroup taxa, the blennioids are the most closely related to the Gobiesocidae and their sister-group relationship is supported by a strong bootstrap value of 99%. Within the family, our result divides the gobiesocid taxa sampled in this study into two sub-groups (I and II), one containing the taxa from the Gobiesocinae and the other with the remaining gobiesocids in four subfamilies plus the newly discovered clingfish from deep water off the north coast of Papua New Guinea (see the description below) (Fig. 1). According to our phylogenetic result, we can induce that the new taxon is a gobiesocid of sub-group II, and it appears to be an independent gobiesocid lineage or subfamily as it is not tightly clustered with any known subfamilies of the Gobiesocidae, at least sampled in this study (Fig. 1).
3.2 Protogobiesocinae new subfamily (Figs. 2–6)
3.2.1 Type genus
Protogobiesox n. gen.
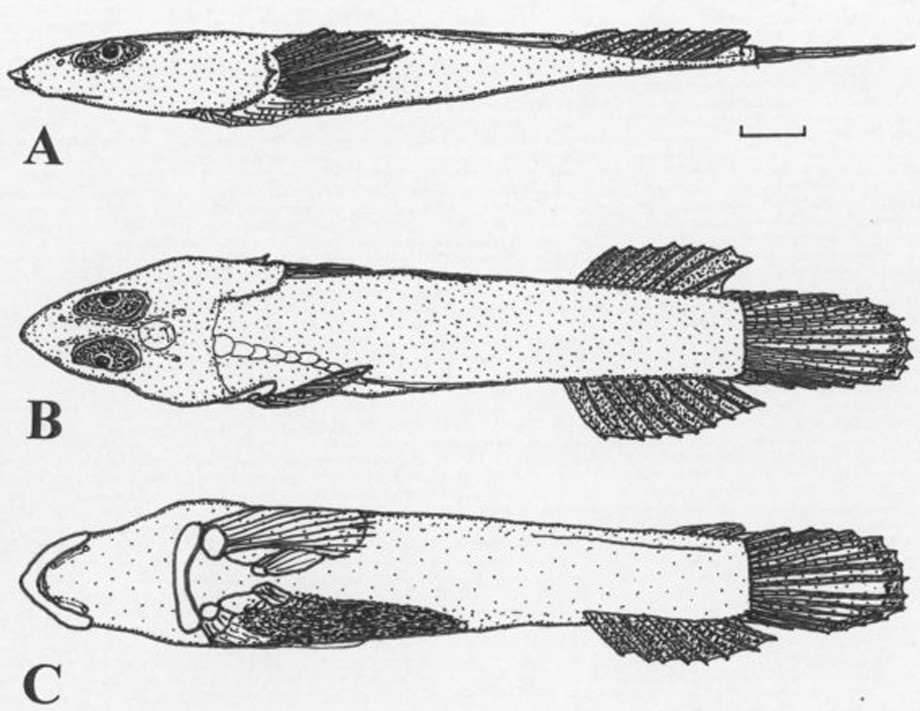
Protogobiesox asymmetricus gen. nov., sp. nov., NTUM 10628, holotype, male, 60.0 mm SL, Papua New Guinea, Madang Province, northeast of Taviltae. A (upper) Lateral view; B (centre) dorsal view; C (lower) ventral view. Bar: 5 mm.
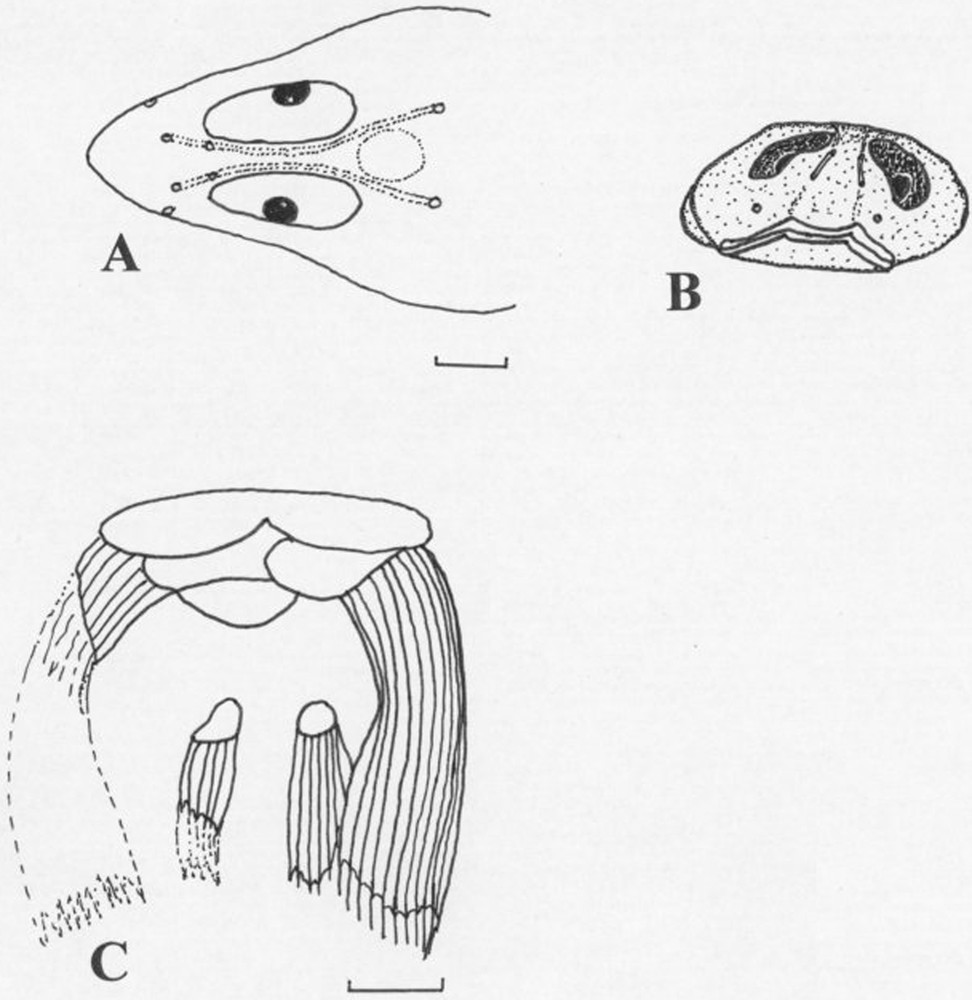
Protogobiesox asymmetricus gen. nov., sp. nov., NTUM 10628, holotype, male, 60.0 mm SL, Papua New Guinea, Madang Province, northeast of Taviltae. A (upper left) Head, dorsal view, indicating lateral line canals and pores. B (upper right) head, frontal view, showing skull, eye and snout asymmetry. C (lower) pelvic fin disc. Bars: 3 mm.

Protogobiesox asymmetricus gen. nov., sp. nov., NTUM 10628, holotype, male, 60.0 mm SL, Papua New Guinea, Madang Province, northeast of Taviltae. Photograph immediately after collection. Vertebral column bent towards the left-hand side.

Protogobiesox asymmetricus gen. nov., sp. nov., MNHN XXXX-XXXX, paratype, female, 59.0 mm SL, Papua New Guinea, Madang Province, northeast of Taviltae. Photograph immediately after collection. Vertebral column bent towards the right-hand side.
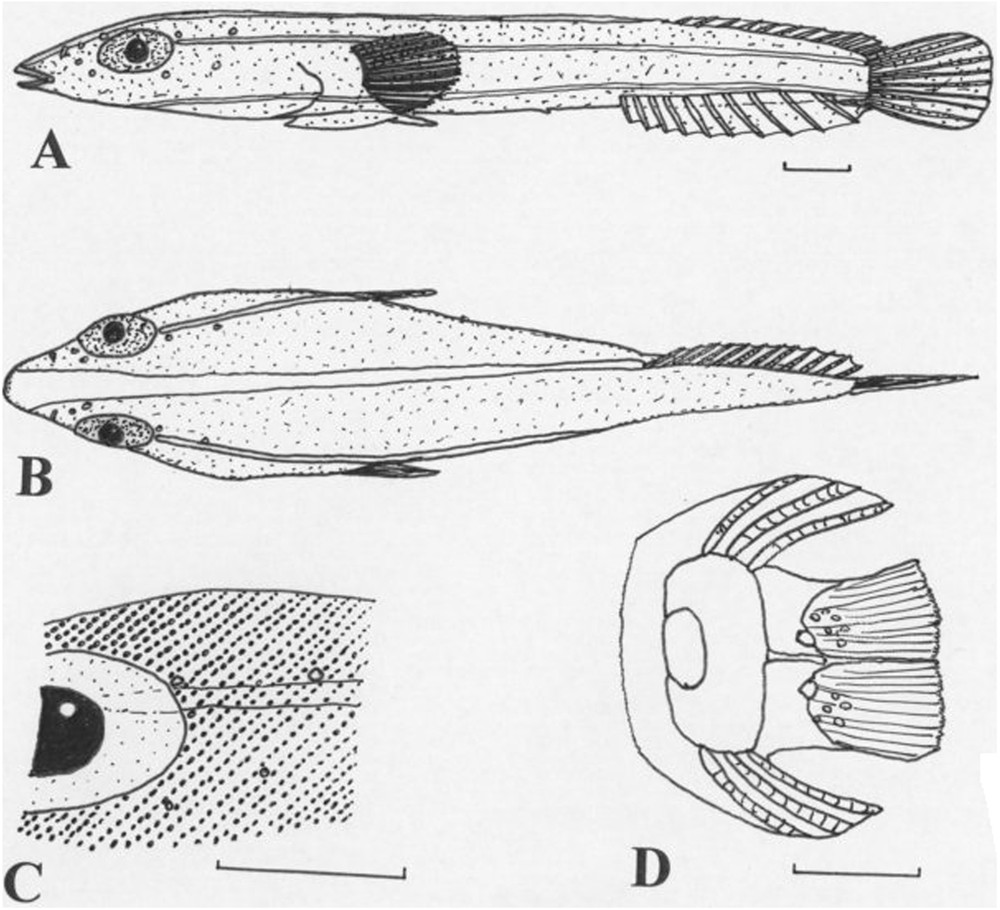
Lepadicyathus mendeleevi Prokofiev, 2005, ZIN 53413, holotype, female, 13.6 mm SL, Papua New Guinea, Madang Province, near Bongu. A (upper) Lateral view. B (centre) dorsal view. C (lower left) detail of postorbital region showing striae. D (lower right) pelvic disc. Bars: 1 mm.
3.2.2 Diagnosis
A subfamily of gobiesocid fishes with a partial lateral asymmetry; head slightly asymmetrical when seen from the front; vertebral column laterally bent, at an angle of 40–92°; anus situated on the right-hand side of the body in males, and on the left-hand side in females; position of guts slightly asymmetrical to strongly asymmetrical; gills 3; pelvic fin disc rudimentary, in two parts, at most with a few rudimentary papillae basally in region B. Pelvic bones present, pelvic fin forming a double disc; scapula, pectoral radial and rays present; total vertebrae 33–35.
3.2.3 Description
Dorsal-fin rays ix–xii; anal-fin rays viii–x; pectoral-fin rays xvii–xxiv; caudal-fin rays (iv-v),x-xv,(iv-v). Gills 3; gill rakers on 3rd arch 0–3.
Head lateral line system with 2 pores in the nasal canal, 1–2 pores in the postorbital canal, 0–2 pores in the lacrimal canal, 0–2 pores in the preopercular canal, and 0–2 pores in the mandibular canal. Vertebrae 33–35 (15 + 18–20). Pleural ribs 12–13 pairs.
Head broad, depressed. Head and skull at least slightly asymmetrical in frontal view. Gill membranes attached to isthmus. Pelvic bones present. Body bent to the left-hand side (males) or right-hand side (females); vertebral column bent at angle of 40–92°, bending starts between skull and level of anus. Anus situated on right-hand side of the body (males) or the left-hand side of the body (females); male with a short urogenital papilla. Skin naked; surface of head and body may bear striae.
Dorsal fin origin on the left-hand side of the body (males), and on the right-hand side (females); anal fin origin on the right-hand side of the body (males) or the left-hand side of the body (females). Dorsal and anal-fin bases attached to caudal-fin base by membranes. Pelvic fin forming a double disc; scapula, pectoral radial and rays present. Disc membrane inserting at the base of the 12th–16th pectoral-fin ray. Disc rudimentary, incomplete, completely or mostly without adhesive papillae.
3.2.4 Etymology
The name of the new subfamily is based on the type genus, Protogobiesox; its meaning is “ancestral gobiesocid,” referring to the pelvic fins resembling the putative ancestral form of gobiesocids.
3.2.5 Distribution
The two described species are distributed off the north coast of the main island of Papua New Guinea, western Pacific Ocean (Fig. 7).
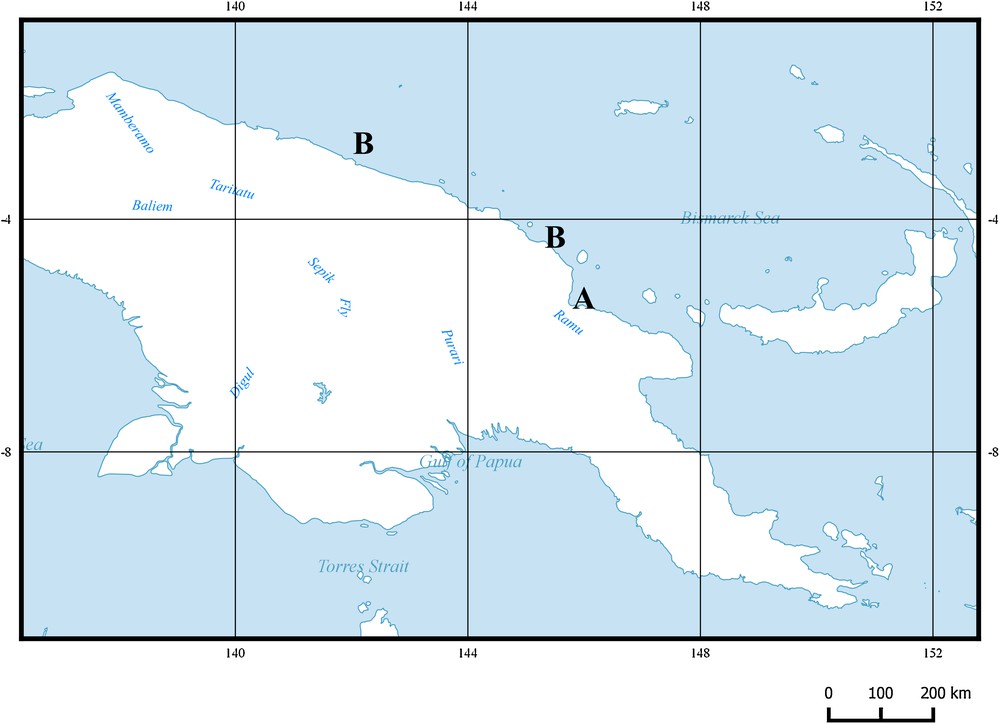
Geographical distribution of the species of the subfamily Protogobiesocinae n. subfam. in Papua New Guinea. A. Lepadicyathus medeleevi. B. Protogobiesox asymmetricus gen. nov., sp. nov.
3.2.6 Comparisons
Within the family Gobiesocidae, this subfamily is unique in its lateral asymmetry. It further differs from the subfamily Cheilobranchinae in the presence of pelvic bones and a disc (pelvic bones and disc greatly reduced or absent in Cheilobranchinae), the presence of a scapula, pectoral radials and pectoral-fin rays (all these absent in the Cheilobranchinae, and the total vertebrae number 33–35 (60–78 in Cheilobranchinae), and from the other subfamilies in the presence of three gills (three and one half in the Chorisochisminae, Haplocyclicinae, Lepadogastrinae, Trachelochisminae), the gill membranes which are attached to the isthmus (free from isthmus in Diplocrepinae, Gobiesocinae, Haplocyclicinae, Trachelochisminae), the double disc (disc single in Chorisochisminae, Diademichthyinae, Gobiesocinae, Haplocyclicinae), and the disc region B lacking papillae or with very few rudimental papillae in 1–2 rows (with 6–10 rows of flattened papillae in the Aspasminae). A key to the subfamilies of the Gobiesocinae is presented below, as well as a key to the species of the Protogobiesocinae n. subfam.
3.2.7 Key to the subfamilies of the family Gobiesocidae
| 1a. Pelvic bones present, pelvic fin forming a single or double disc; scapula, pectoral radial and rays present; total vertebrae 25–54 | 2 |
| 1b. Pelvic bones and pelvic fin greatly reduced or absent; scapula, pectoral radial and rays absent; total vertebrae 60–78 | Cheilobranchinae |
| 2a. Gills three and one-half | 3 |
| 2b. Gills three | 6 |
| 3a. Gill membranes free from isthmus | 4 |
| 3b. Gill membranes attached to isthmus | 5 |
| 4a. Disc double (Indo-Pacific to Japan) | Trachelochisminae |
| 4b. Disc single (New Zealand) | Haplocyclicinae |
| 5a. Disc double (Eastern Atlantic and South Africa) | Lepadogastrinae |
| 5b. Disc single (South Africa) | Chorisochisminae |
| 6a. Gill membranes free from isthmus | 7 |
| 6b. Gill membranes attached to isthmus | 8 |
| 7a. Disc double (South Africa to Indo-Pacific; New Zealand) | Diplocrepinae |
| 7b. Disc single (America plus South Africa) | Gobiesocinae |
| 8a. Disc double (Indo-Pacific) | 9 |
| 8b. Disc single (South Africa to Indo-Pacific) | Diademichthyinae |
| 9a. Body laterally symmetrical, vertebral column not bent; disc region B with 6–10 rows of flattened papillae (Indo-Pacific) | Aspasminae |
| 9b. Body laterally asymmetrical, vertebral column bent at an angle of 40–92°; disc region B lacking papillae or with very few rudimental papillae in 1–2 rows (Western Pacific) | Protogobiesocinae n. subfam |
3.2.8 Key to the species of the subfamily Protogobiesocinae n. subfam
| 1a. Dorsal fin with 9–10 rays; anal fin with 8 rays; caudal fin with 15 principal rays; vertebral column strongly bent at an angle of 85–92°; bending starts behind skull; skin naked, without striae | Protogobiesox asymmetricus n. gen., n. sp. |
| 1b. Dorsal fin with 11–12 rays; anal fin with 10 rays; caudal fin with 10 principal rays; vertebral column bent at an angle of 40–45°; bending starts just before level of anus; skin naked, with numerous, closely set, oblique striae consisting of minute tubercles | Lepadicyathus mendeleevi |
3.3 Protogobiesox new genus (Figs. 2–5)
3.3.1 Type species
Protogobiesox asymmetricus n. gen., n. sp.
3.3.2 Diagnosis
A genus of protogobiesocine fishes with a partial lateral asymmetry; head slightly asymmetrical when seen from the front; vertebral column strongly bent towards the left in males, towards the right in females, at an angle of 85–92°; bending starts behind the skull; anus situated on the right-hand side of the body in males, on the left-hand side in females; position of guts strongly asymmetrical; gills 3; pelvic fin disc rudimentary, in two parts, without adhesive papillae. Dorsal fin with 9–10 rays; anal fin with 8 rays; caudal fin with 15 principal rays. Skin naked, without striae.
3.3.3 Description
Dorsal-fin rays x (ix–x); anal-fin rays viii (viii); pectoral-fin rays xxiv (xvii–xxiv); caudal-fin rays (v),xv,(iv) [(iv–v),xv,(iv)]. Gills 3 (3); gill rakers on 3rd arch 3 (3).
Detailed description see below under Protogobiesox asymmetricus n. gen. n. sp.
3.3.4 Etymology
The name of the new genus means “ancestral gobiesocid;” it refers to the pelvic fins resembling the putative ancestral form of gobiesocids. The gender is masculine.
3.3.5 Remarks
This genus is monotypic, with Protogobiesox asymmetricus n. gen., n. sp. as the only known species.
3.4 Protogobiesox asymmetricus n. sp. (Figs. 2–5)
Asymmetrical deep-water clingfish.
3.4.1 Type material
Holotype. NTUM 10628, male, 60.0 mm SL, Papua New Guinea, Madang Province, northeast of Taviltae, 4°31'S 145°31’E, 380-382 m depth, St. CP4035, sunken logs, some covered with sponges, W.-J. Chen, R/V Alis, 17 Dec. 2012.
Paratypes. Four specimens: MNHN 2016-03333, 1 female, 59.0 mm SL, same data as the holotype; NTUM 10633, 1 male, 53.6 mm SL, same data as holotype; NTUM 10629, 1 male, 56.0 mm SL, Papua New Guinea, Sandaun Province, northwest of Aitape, 3°01'S 142°16’E, 400–560 m in depth, St. CP4056, sand bottom with sunken logs, W.-J. Chen, R/V Alis, 20 Dec. 2012; NTUM 10674, 1 male, 51.8 mm SL, Papua New Guinea, West Sepik Province, 13.5 km northwest of Aitape, 3°03'S 142°18’E, 370–374 m depth, St. CP 4055, Wei-Jen Chen, R/V Alis, 20 Dec. 2012.
3.4.2 Diagnosis
Dorsal fin with 9–10 rays, anal fin with 8 rays, pectoral fin with 17–24 rays, principal caudal-fin rays 15, three gills, third arch with 3 gill rakers. Vertebral column with a total of 34–35 vertebrae, with asymmetrical lateral bending starting behind the skull, bent at an angle of 85°–92°. Skull asymmetrical in frontal view. Skin naked, surface of head and body without striae. Disc without adhesive papillae; posterior margin may be partially fringed; region B small, left- and right-hand sides separate, larger on the right-hand side than on left-hand side.
3.4.3 Description
Dorsal-fin rays x (ix–x); anal-fin rays viii (viii); pectoral-fin rays xxiv (xvii–xxiv); caudal-fin rays (v),xv,(iv) [(iv–v),xv,(iv)]. Gills 3 (3); gill rakers on 3rd arch 3 (3). Measurements of the holotype and paratypes, see Table 3.
Counts and measurements of the holotype and paratypes of Protogrammus asymmetricus n. gen. n. sp.
| Holotype NTUM 10628 Male |
Paratype NTUM 10674 Male |
Paratype NTUM 10629 Male |
Paratype NTUM 10633 Male |
Paratype MNHN 2016-03333 Female |
|
| Standard length | 60.0 | 51.8 | 56.0 | 53.6 | 59.0 |
| Head length | 20.7 | 16.6 | 18.5 | 18.0 | 19.3 |
| Body depth | 6.4 | 4.4 | 5.4 | 6.3 | 6.0 |
| Body width | 11.5 | 9.5 | 10.8 | 12.6 | 11.4 |
| Orbit diameter | 5.3 | 6.3 | 5.1 | 4.9 | 5.8 |
| Preorbital length | 4.4 | 2.1 | 4.1 | 3.9 | 4.1 |
| Interorbital distance | 1.4 | 0.7 | 1.5 | 0.6 | 0.9 |
| Upper jaw length | 8.1 | 4.8 | 7.3 | 6.7 | 7.7 |
| Lower jaw length | 6.4 | 4.1 | 6.0 | 5.5 | 7.3 |
| Preanus length | 36.7 | 32.9 | 33.3 | 31.5 | 35.9 |
| Urogenital papilla | 0.3 | 0.2 | 0.3 | 0.2 | – |
| PC length | 5.3 | 5.5 | 4.6 | 4.7 | 6.1 |
| PC depth | 6.5 | 4.6 | 5.2 | 4.9 | 6.0 |
| PC width | 1.4 | 1.6 | 1.1 | 2.0 | 1.4 |
| Predorsal length | 43.9 | 35.9 | 42.1 | 38.4 | 42.4 |
| Preanal length | 45.6 | 40.0 | 41.4 | 40.4 | 46.2 |
| Distance disc to anus | 14.6 | ? | 10.4 | 10.9 | 9.9 |
| Distance anus to anal-fin origin | 9.2 | 8.1 | 7.1 | 7.7 | 6.9 |
| Prepectoral length | 20.6 | 17.0 | 20.7 | 20.7 | 22.0 |
| Predisc length | 13.2 | 11.0 | 12.4 | 11.9 | 14.2 |
| 1st dorsal ray | 5.3 | 3.2 | 5.1 | 4.8 | 4.1 |
| Last dorsal ray | 6.0 | 5.5 | 4.0 | 4.6 | 5.3 |
| Dorsal-fin base | 14.6 | 7.9 | 11.0 | 8.9 | 12.2 |
| 1st anal ray | 4.5 | 2.5 | 3.9 | 2.7 | 2.9 |
| Last but one anal ray | 6.5 | 3.1 | 5.8 | 4.3 | 4.6 |
| Last anal ray | 5.1 | 2.4 | 4.7 | 3.9 | 4.1 |
| Anal-fin base length | 9.5 | 6.8 | 9.5 | 8.3 | 9.5 |
| Pectoral-fin length | 10.4 | 9.7 | 10.7 | 9.8 | 10.6 |
| Pectoral-fin base | 3.6 | 3.6 | 3.9 | 3.9 | 4.3 |
| Disc length | 16.0 | ? | 11.4 | 11.0 | 13.9 |
| Caudal-fin length | 12.8 | 10.6 | 11.4 | 10.2 | 12.4 |
Both upper and lower jaws with several series of undifferentiated conical teeth, no canines or incisors.
Head lateral line system with 2 pores in the nasal canal, 1 pore in the postorbital canal, lacrimal and preopercular canals missing, and 2 pores in the mandibular canal (Figs. 2 and 3).
Vertebrae 35 (34–35) [15 + 20 (15 + 19–20)]; asymmetrical bending of the vertebral column starting from the 1st (1st) vertebra. Pleural ribs 13 (13) pairs.
Head broad, depressed. Head and skull asymmetrical in frontal view (Fig. 3B). Head length 34.5 (32.0–33.6)% SL (2.9–3.1 in SL). Maximum body depth 10.7 (8.5–11.8)% SL (8.5–11.8 in SL). Maximum body width 19.2 (18.3–23.5)% SL (4.2–5.4 in SL). Maximum (horizontal) orbit diameter 8.8 (9.1–12.2)% SL (2.6–3.9 in head length). Snout relatively long, pointed (Fig. 3A). Preorbital length 7.3 (4.0–7.3)% SL (4.5–7.9 in head length). Interorbital distance narrow, 2.3 (1.1–2.7)% SL (12.3–30.0 in head length). Upper jaw length 13.5 (9.3–13.0)% SL. Lower jaw length 10.7 (7.9–12.4)% SL. Gill membranes attached to isthmus. Body bent to the left-hand side (males) or right-hand side (females); vertebral column bent at angle of 87° (85–92°), bending starts behind skull. Anus situated on the right-hand side of the body (male) or the left-hand side of the body (female); closer to anal-fin origin than the disc; male with a short urogenital papilla, its length 0.5 (0.4–0.5)% of SL (62–90 in head length); distance between disc and anus 24.3 (16.8–20.3)% SL, distance between anus and anal-fin origin 15.3 (11.7–15.6)% SL. Pre-anus length 61.2 (58.8–63.5)% SL (1.6–1.7 in SL). Sides of body with an elongate dermal fold on left-hand side below the anal-fin base. Caudal-peduncle length 8.8 (8.2–10.6)% SL (9.4–12.2 in SL). Caudal-peduncle depth 10.8 (8.9–10.2)% SL (9.2–11.3 in SL). Caudal-peduncle width 2.3 (2.0–3.7)% SL (26.8–50.9 in SL). Skin naked; surface of head and body without striae.
Predorsal-fin length 73.2 (69.3–75.2)% SL (1.3–1.4 in SL). Pre-anal-fin length 76.0 (77.2–78.3)% SL (1.3–1.4 in SL). Dorsal fin origin on the left-hand side of the body (male), on the right-hand side of the body (female); anal fin origin on the right-hand side of the body (male), on the left-hand side of the body (female). Dorsal and anal-fin bases attached to caudal-fin base by membranes. Prepectoral-fin length 34.3 (32.8–38.6)% SL (2.6–3.0 in SL). Predisc length 22.0 (21.2–24.1)% SL (4.2–4.7 in SL). Disc length 26.7 (20.4–23.6)% SL (3.8–4.9 in SL). Disc membrane inserting at the base of the 13th (13th) pectoral-fin ray. Disc rudimentary, incomplete, with left- and right-hand parts of regions A and B separate, without adhesive papillae; fin-ray branches at distal margins with short filaments; region B small (Fig. 3C). Caudal-fin length 21.4 (19.0-21.0) % SL (4.7-5.2 in SL).
Colour in life (Figs. 4 and 5). Head and body brown, with an irregular, slightly darker banding, anteriorly reddish, posteriorly yellowish; eyes dark grey. Pectoral-fin rays reddish, caudal fin rose; dorsal and anal-fin rays rose, membranes blackish.
Colour in alcohol. Head and body uniformly yellowish brown, lower sides of head and body pale; eyes dark grey; occiput transparent, brain visible, yellowish white; peritoneum black. Dorsal and anal fins and distal margin of caudal fin dark grey.
3.4.4 Distribution
North coast of Papua New Guinea (Madang Province; Sandaun Province) (Fig. 7). This new species was collected at depths of 381–480 m.
3.4.5 Etymology
The name of the new species, asymmetricus, refers to the unusual asymmetry of head and body.
3.4.6 Comparisons
This new species is distinguished from the related species Lepadicyathus mendeleevi by its 9–10 dorsal-fin rays (11–12 in L. mendeleevi), the 8 anal-fin rays (10 in L. mendeleevi), 15 principal caudal-fin rays (10 in L. mendeleevi), the vertebral column strongly bent at an angle of 85–92° (40–45° in L. mendeleevi), the bending that starts behind the skull (just before the level of the anus in L. mendeleevi), and the naked skin without striae (with numerous, closely-set, oblique striae consisting of minute tubercles in L. mendeleevi). From other gobiesocids, it is distinguished by the characters of the subfamily (see above).
3.4.7 Remarks
This species is highly asymmetrical. While the head is normally orientated, the skull is already slightly asymmetrical when seen from the front (Fig. 3B), with an oblique mouth slit and the left eye situated lower than the right eye in the male holotype; the vertebral column inserts slightly on the left-hand side of the skull (Fig. 2B), and then strongly turns left, meeting the left-hand side of the body on the level of the dorsal fin insertion; it continues running along the left-hand side towards the hypural plate. The anal fin, consequently, inserts on the right-hand side of the body; the caudal fin lies flat on the ground. Asymmetries are also found in the position of the internal organs, which are restricted to the right half of the body (Fig. 2C); the anus is situated on the right-hand side. Asymmetries are observed also in the pectoral and pelvic fin skeletons; the fins and supporting elements on right-hand side and are much smaller than the corresponding elements on the left-hand side (disc asymmetry, see Fig. 3C). In the female paratype, the asymmetries are the other way around, with the vertebral column turning towards the right-hand side of the fish, and the anus and the internal organs shifted towards the left-hand side. These asymmetries are not artificial, as all four specimens of the type series show exactly the same pattern, which was already apparent in the freshly collected fish.
The right pectoral fins, and right lobes of the pelvic disc, were partially removed for molecular studies prior to the morphological examination.
This new species is found in relatively cool water in an area with temporary upwelling along the northern coast of Papua New Guinea (Kuroda [70], Lee et al. [71], Hasegawa et al. [72]). It was found in association with sunken logs; its specializations and especially the lateral asymmetry may be useful to be attached to these logs.
3.5 Lepadicyathus Prokofiev, 2005 (Fig. 6)
3.5.1 Synonymy
Lepadicyathus Prokofiev 2005: Prokofiev [42]: 559 [546] (type species: Lepadicyathus mendeleevi Prokofiev 2005 by original designation (also monotypic).
3.5.2 Diagnosis
A genus of protogobiesocine fishes with a partial lateral asymmetry; head slightly asymmetrical when seen from the front; vertebral column bent towards the left in males, towards the right in females, at an angle of 40–45°; bending starts slightly before level of anus; anus situated towards the right-hand side of the body in males, towards the left-hand side in females; position of guts slightly asymmetrical; gills 3; pelvic fin disc rudimentary, in two parts, with a few rudimentary papillae basally in region B. Dorsal fin with 11–12 rays; anal fin with 10 rays; caudal fin with 10 principal rays. Skin naked, surface of head and body covered with a dense pattern of obliquely arranged striae consisting of minute tubercles.
3.5.3 Description
Dorsal-fin rays xi–xii; anal-fin rays x; pectoral-fin rays xxiv; caudal-fin rays (v),x,(v). No gill rakers on 3rd arch; 1–2 gill rakers on the 1st and 2nd arches.
Premaxillary with a single row of incisor-like teeth, some with a hook-like appendage. Dentary with a single row of smaller incisor-like teeth, fused at the bases.
Head lateral line system with 2 pores in the nasal canal, 2 pores in the postorbital canal, 2 pores in the lacrimal canal, and 2 pores in the preopercular canal, pores in mandibular canal lacking.
Pelvic disc rudimentary, in two parts; anterior margin with small fringes; disc only with a few rudimentary papillae basally in region B.
Skin naked; surface of head and body covered with a dense pattern of obliquely arranged striae consisting of minute tubercles (Fig. 6C).
3.5.4 Remarks
The dermal striae found in Lepadicyathus are an unusual, specialised character not found in other gobiesocids. Prokofiev [42] compared it with Liobranchia stria of Briggs [3], which has deep grooves in the skin, but the state of Liobranchia is quite different from that of Lepadicyathus. Liobranchia has just grooves surrounded by folds, while the skin of Lepadicyathus is even, but covered with striae consisting of minute tubercles. This seems to be an independent specialisation, though we are unaware of the function of these striae. The tubercles may be based on reduced scales, which would support the primitive state of Lepadicyathus.
3.6 Lepadicyathus mendeleevi Prokofiev, 2005 (Fig. 6)
Mendeleev's clingfish.
3.6.1 Synonymy
Lepadicyathus mendeleevi Prokofiev 2005: Prokofiev [42]: 560 (547) (Papua New Guinea, near village of Bongu, Madang Province, 1 m depth. Holotype: ZIN 53413). Prokofiev [73]: 181 (discussion on systematic position).
3.6.2 Material
ZIN 53413 (holotype, female, 13.6 mm SL), Papua New Guinea, Madang Province, near Bongu, 1 m depth, in Millepora coral, R/V Dmitrii Mendeleev, Cruise 18,14 Feb. 1977. ZIN 53413a (1 paratype, male, 13.9 mm SL), same data as the holotype.
3.6.3 Diagnosis
Dorsal fin with 11–12 rays, anal fin with 10 rays, pectoral fin with 24 rays, principal caudal-fin rays 10, three gills, third arch without gill rakers. Vertebral column with a total of 33 vertebrae, with asymmetrical bending staring just before the level of the anus, bent at an angle of 40–45°. Skull not asymmetrical in frontal view. Skin naked, surface of head and body, with numerous, closely set, oblique striae consisting of minute tubercles. Disc without adhesive papillae, except a few basally in region B, arranged in two rows; posterior margin not fringed; region B small, left and right-hand sides connected by a membrane.
3.6.4 Description
Dorsal-fin rays xii (xi); anal-fin rays x (x); pectoral-fin rays xxiv (xxiv); caudal-fin rays (v),x,(v) [(v),x,(v)]. Gills 3 (3); no gill rakers on 3rd arch; 1-2 gill rakers on 1st and 2nd arches. Measurements of the holotype and paratypes: see Table 4.
Counts and measurements of the holotype and paratype of Lepadicyathus mendeleevi Prokofiev, 2005.
| Holotype ZIN 53413 Female |
Paratype ZIN 53413a Male |
|
| Standard length | 13.6 | 13.9 |
| Head length | 4.9 | 4.4 |
| Body depth | 1.5 | 1.5 |
| Body width | 2.9 | 2.8 |
| Orbit diameter | 1.4 | 1.7 |
| Preorbital length | 1.2 | 1.5 |
| Interorbital distance | 1.0 | 1.0 |
| Upper jaw length | 0.6 | 0.6 |
| Lower jaw length | 0.4 | 0.4 |
| Preanus length | 8.4 | 10.3 |
| Urogenital papilla | 0.05 | – |
| PC length | 0.5 | 0.6 |
| PC depth | 1.1 | 1.2 |
| PC width | 0.7 | 0.8 |
| Predorsal length | 10.1 | 10.4 |
| Preanal length | 9.7 | 11.3 |
| Distance disc to anus | 1.2 | 2.4 |
| Distance anus to anal-fin origin | 1.2 | 2.45 |
| Prepectoral length | 5.5 | 5.8 |
| Predisc length | 4.4 | 4.1 |
| 1st dorsal ray | 1.1 | 1.2 |
| Last dorsal ray | ca. 0.8 | 1.0 |
| Dorsal-fin base | 3.0 | 4.4 |
| 1st anal ray | 1.0 | 1.1 |
| Last but one anal ray | 1.4 | 1.5 |
| Last anal ray | 0.9 | 1.0 |
| Anal-fin base length | 2.4 | 2.9 |
| Pectoral-fin length | 1.5 | 1.3 |
| Pectoral-fin base | 1.0 | 1.4 |
| Disc length | 2.5 | 2.6 |
| Caudal-fin length | 1.9 | ca. 1.8 |
Premaxillary with a single row of incisor-like teeth, some with a hook-like appendage. Dentary with a single row of smaller incisor-like teeth, fused at bases.
Head lateral line system with 2 pores in the nasal canal, 2 pores in the postorbital canal, 2 pores in the lacrimal canal, and 2 pores in the preopercular canal, pores in the mandibular canal lacking.
Vertebrae 33 (15 + 18); asymmetrical bending of vertebral column starting from 11th vertebra. Pleural ribs 12 pairs.
Head moderately broad, depressed. Head and skull slightly asymmetrical in frontal view. Head length 36.0 (31.6)% SL (2.8–3.2 in SL). Maximum body depth 11.9 (10.8)% SL (9.1–9.4 in SL). Maximum body width 21.3 (20.1)% SL (4.7–5.0 in SL). Maximum (horizontal) orbit diameter 10.3 (12.2)% SL (2.6–3.5 in head length). Snout elongate, slightly pointed, tip rounded (Fig. 6B). Preorbital length 8.8 (10.8)% SL (2.9–4.1 in head length), in the male not longer than in the female. Interorbital distance 7.4 (7.2) %SL (4.4–4.9 in head length). Upper jaw length 4.4 (4.3)% SL. Lower jaw length 2.9 (2.9)% SL. Gill membranes attached to the isthmus. Body bent to the left-hand side (male) or the right-hand side (female); vertebral column bent at an angle of 40° (45°), bending starts just before the level of the anus. Anus situated in the middle between the disc and anal-fin origin; both sexes with a short urogenital papilla; distance between disc and anus 8.8 (17.3)% SL, distance between anus and anal-fin origin 8.8 (17.6)% SL. Anus situated only slightly towards the right-hand side of the body (male) or the left-hand side of the body (female). Pre-anus length 61.8 (74.1)% SL (1.4–1.6 in SL). Sides of body without an elongate dermal fold below the base of the anal fin. Caudal-peduncle length 3.7 (4.3)% SL (23.2–27.2 in SL). Caudal-peduncle depth 8.1 (8.6)% SL (11.6–12.4 in SL). Caudal-peduncle width 5.1 (5.8)% of SL (17.3–19.4 in SL).
Predorsal-fin length 74.3 (74.8)% SL (1.3-1.4 in SL). Pre-anal-fin length 71.3 (81.3)% SL (1.2–1.4 in SL). Dorsal fin origin towards the left-hand side of the body (male), the right-hand side of the body (female); anal fin origin near the right-hand side of the body (male), the left-hand side of the body (female). Prepectoral-fin length 40.4 (41.7)% SL (2.4-2.5 in SL). Predisc length 32.3 (29.5)% SL (3.1–3.4 in SL). Disc length 18.4 (18.7)% SL (5.3–5.4 in SL). Disc membrane inserting at the base of the 12th (16th) pectoral-fin ray. Pelvic disc rudimentary, in two parts; anterior margin of region A and posterior margin of region B with small fringes; disc only with a few rudimentary papillae basally in region B. Caudal-fin length 14.0% SL (7.2 in SL). Skin naked; surface of head and body covered with a dense pattern of obliquely arranged striae consisting of minute tubercles (Fig. 6C).
Colour in life.–Unknown.
Colour in alcohol.–The holotype and paratype are reddish brown, the head and anterior dorsal surface carmesine red, with 5 white stripes; one stripe running from the tip of the snout along the dorsal midline to the dorsal-fin base (Fig. 6B), a lateral stripe on each side from posterior margin of eye to the dorsal part of the caudal peduncle, and another lateral stripe on each side from below the dentary to the lower part of the caudal peduncle. Eye dark grey. Occiput not transparent. Fins reddish; caudal fin with a basal brown spot, central part of fin carmesine red.
3.6.5 Distribution
North coast of Papua New Guinea (Madang Province) (Fig. 7). This new species is known only from the type locality. It was collected at a depth of 1 m, inhabiting a Millepora coral.
3.6.6 Remarks
Prokofiev [42] noticed that the paratype of Lepadicyathus mendeleevi was “crumpled”, but thought this to be artificial, and was apparently not aware of the lateral asymmetry. During a re-examination, however, the moderate asymmetry of this species was discovered both in the holotype and the paratype. In the female holotype, an ovary full of moderately large eggs was visible in the X-ray image.
While the head is normally orientated, the skull is already slightly asymmetrical when seen from the front, with a slightly oblique mouth slit and one cheek larger than the other; in the female holotype, the vertebral column is bent towards the right-hand side (Fig. 6B), with the anus placed slightly towards the left-hand side of the body; the vertebral column is bent to the left-hand side in the male paratype, with the anus closer to the right-hand side. A slight asymmetry is also found in the position of the internal organs, which are shifted towards the left-hand side of the body in the female holotype, but towards the right-hand side of the body in the male paratype.
In a note published subsequently to the original description, Prokofiev [73] noted that the absence of papillae on the disc of Lepadicyathus mendeleevi is not unique in Gobiesocidae, but resembles young specimens of the genus Lepadichthys. However, the holotype of L. mendeleevi is certainly to be considered as an adult specimen, as the ovary is full of eggs.
4 Discussion
Chen et al. [60], in a molecular phylogenetic analysis of the Acanthomorpha, demonstrated that the order Perciformes is polyphyletic, and that several of the discovered clades need formal recognition. From the phylogenetic results of Chen et al. [60], the Gobiesocidae formed a separate clade (Clade D) together with the blennioids; this clade was nested within the inferred clade Q (Chen et al. [60]; Dettaï & Lecointre [61]) or Ovalentaria (Wainwright et al. [74]) of the Percomorpha contained Atherinomorpha, Mugilidae, Pomacentridae (“Labroidei”), Cichlidae (“Labroidei”), Embiotocidae (“Labroidei”), Ambassidae, and some other perciform fishes from Pholidichthyidae, Pseudochromidae, Polycentridae, Plesiopidae, Opistognathidae, and Grammatidae (Near et al. [62]). Our phylogenetic result, by including the main taxa from the clade Q, confirms the previous hypothesis (Fig. 1), and suggest the clade including Blennioidei and Gobiesocidae should be formally named in the future. Very recently, Nelson et al. [75] classified the Gobiesociformes as a separate order for the single family Gobiesocidae, which they considered as closely related to the Blenniiformes (= Blennioidei) (see Lin & Hastings [76]). However, the authors these studies did not give the reason for their rearrangement of the taxonomic rank at the ordinal level; the classification should further be settled.
Within the family Gobiesocidae, a total of 9 subfamilies were previously distinguished (Briggs [3], Conway et al. [56]); five of these were included for the present molecular phylogenetic investigation. The new subfamily Protogobiesocinae is morphologically most similar to the subfamily Aspasminae. Species of the Aspasminae are distributed from East Africa east to Mariana Islands, north to Japan, south to southern Australia and New Zealand; they are known from very shallow to moderately deep water. The deepest occurring aspasmine species are Aspasma minima (Döderlein, 1887) from Japan (183–274 m), and Modicus tangaroa Hardy, 1983 from New Zealand (20–149 m). However, our molecular results revealed that the sister-group of the Aspasminae is the Diademichthyinae, a group of the Indo-West Pacific distributed clingfishes specialized for a commensal relationship with certain shallow-reef crinoids. Interestingly, the enigmatic clingfishes from the subfamily Cheilobranchinae turned out to be the sister-group of the Aspasminae/Diademichthyinae clade in the inferred phylogenetic tree (Fig. 1). The species in the new subfamily Protogobiesocinae also possess a reduced or “ancestral” form of their adhesive disc formed by the pelvic fins, similar to the species of Cheilobranchinae. According to our phylogeny, this feature is apparently a derived state and has been evolved twice within the Gobiesocidae
Acknowledgments
The “Our Planet Reviewed” PAPUA NIUGINI Biodiversity Expedition was a joint project of Pro-Natura International (PNI), “Muséum national d’histoire naturelle”, Paris (MNHN), “Institut de recherche pour le développement” (IRD) and the University of Papua New Guinea (UPNG) – the principal investigators were Philippe Bouchet, Claude Payri, and Sarah Samadi. The organizers acknowledge funding from the Total Foundation, the Prince Albert II of Monaco Foundation, the Fondation EDF, the Stavros Niarchos Foundation and Entrepose Contracting, Fonds Pacifique, the Government of New Caledonia, and the kind support from the Divine Word University (DWU). The expedition operated under a permit delivered by the Department of Environment and Conservation of Papua New Guinea.
We appreciate the support by Sarah Samadi (MNHN, Paris) who provided information on station data and photographs of specimens collected during the PAPUA NIUGINI Biodiversity Expedition. We would also like to thank M. McGrouther, D.F. Hoese and J.R. Paxton (AMS, Sydney), D. Didier (ANSP, Philadelphia), O. Crimmen, J. Maclaine, A. Wheeler and P.J.P. Whitehead [†] (BMNH, London), J.E. Randall and A.Y. Suzumoto (BPBM, Honolulu), L.J. Dempster, W.N. Eschmeyer, M. Hearne, T. Iwamoto and P.M. Sonoda (CAS-SU, San Francisco), A. Brito (CCML, La Laguna, Tenerife), M.-L. Bauchot, M. Desoutter, G. Duhamel and P. Pruvost (MNHN, Paris), H. Ahnelt, R. Hacker [†] and P. Kähsbauer [†] (NMW, Vienna), K. Matsuura (NSMT-P, Tokyo), M.E. Anderson and V. Mthombeni (SAIAB, Grahamstown), J.T. Williams (USNM, Washington D.C.), A.V. Balushkin (ZIN, St. Petersburg), M.A. Krag, P.R. Møller and J.C. Nielsen (ZMUC, Copenhagen) and who gave access to specimens in their care, and provided information and/or photographs. We are grateful to M.J. Zhukov (ZIN, St. Petersburg) who provided assistance and prepared x-rays of Lepadicyathus mendeleevi., and to G. Duhamel and P. Pruvost (MNHN, Paris) who provided a catalogue number for a paratype. We wish to thank Kevin Conway, Bruno Chanet, Masaki Miya and The Australian Museum Sydney (via Mark McGrouther) for the loan or gift of tissue samples. WJC appreciates the grant support from the Ministry of Science & Technology, Taiwan (MOST 102-2923-B-002 -001-MY3).



Vous devez vous connecter pour continuer.
S'authentifier