1 Introduction
Locomotion represents a complex mechanism that is determined by combinations of structural and functional properties (morphology, muscle structure, and limb kinematic patterns), and is tightly associated with morphology and different aspects of ecology [1–3]. Anurans pose a unique way of locomotion, which required some changes from basic tetrapod's bauplan in anatomical traits [4–6]. This involves an elongation of hindlimbs, a shortening of the presacral vertebral column, a reduction in the tail, the presence of elongated ilia, and the urostyle [7]. Although developmental and genetic programs (‘Bauplan machinery’) constrains the range of morphologies available to anurans, they are flexible enough to allow different body morphologies to evolve in the frame of very diverse lifestyles of anurans (burrowing, swimming, walking, hopping, jumping, climbing and gliding) [5,8,9].
Over the past few decades, studies of anuran locomotion showed that different aspects of postcranial morphology (mainly limb proportions and pelvic traits) correlate to a varying degree with locomotor modes [8–11]. Particularly noteworthy is the role of hindlimb length as a predictor of locomotor mode of anuran species [9]. Anurans with relatively long hindlimbs tend to be relatively good jumpers [4,10,11]. Longer hindlimbs are associated with larger muscle and energy storage [12], and higher jumping force [13]. Opposite to jumpers, walkers have shorter hindlimbs, which provides them movement in a series of short steps and great capacity of sustained locomotion through the forest floor [14,15]. Sacral traits are also related with locomotion, due to their unique biomechanical consequences for controlling the posture of the trunk and head relative to the pelvis and legs during jumping. Small diapophiseal expansions in jumpers allow them to increase their rate of trunk elevation relative to the rates of energy release from the hindlimbs, enabling them to jump farther. On the other hand, an expanded sacrum in walkers allows lateral rotation of the pelvis during walking and burrowing [16]. In contrast to hindlimbs and sacral shape, forelimbs were thought to be less correlated to locomotor modes [9,17], but this point has been less studied in that context. The main roles of forelimbs are to ensure balance during jump control landing [18] and branch grasping [19]. However, forelimbs have a variety of different roles beside locomotion (such as prey capture and manipulation during feeding [20], amplexus [21] and skin-shedding [22]), so we cannot expect clear correlation patterns with different locomotor modes.
The use of ratios of morphometric traits (i.e. of body proportions) has a long tradition and is integrated into taxonomical, phylogenetic and morphological studies. It provides an efficient way of looking at the shape of organisms, which, expressed by ratios, is invariant for a particular measure of the size [23,24]. Due to the fact that the complex interplay of numerous morphological traits determines the locomotion ability of anurans, the analyses of ratios will reveal useful information about the structural and functional properties of the locomotor system, and, as such, they can show differences among locomotor modes. Previous studies showed clear relations between morphological trait ratios and locomotor performances in anurans. For example, the total limb length to body size ratio proved to be important ratio for describing locomotor modes, with shorter hindlimbs and longer forelimbs in walkers compared to jumpers [9,12]. Within hindlimbs, the tibiofibular-to-femur ratio is marked as the key ratio, with higher values in jumpers (> 1), increasing the out-lever arm, and therefore, the jumping force. In contrast, lower tibiofibula to femur ratios (< 1) enhance the walking and burrowing capacities required by walker species [10,25,26].
In the present study, we investigated how patterns of variation in morphological traits and their ratios (fore-, hindlimbs, and sacral vertebrae) are related to two different types of locomotion, jumpers and walkers. First, we described patterns of variation of basic structural parts relevant to locomotion: total length of forelimbs, total length of hindlimbs, sacral vertebrae traits (sacral width and diapophyseal expansion). In this way, we will be able to compare our results with literature data. Second, as was noted by Enriquez-Urzelai et al. [25], studies about anuran morphology associated with locomotion, usually considers fore-, and hindlimbs as a single functional unit. But, biomechanically, every part (proximal, medial, and distal) has specific functional output, so relationships between them may differ between jumpers and walkers. For that reason, as a novel approach, we analysed patterns of variability in fore- and hindlimb proximal (humerus/femur), medial (radioulna/tibiofibula) and distal elements (carpals, metacarpals/tarsals, metatarsals, and phalanges) to highlight how within- and between-limb relations are linked to two locomotor modes (jumpers and walkers).
2 Materials and methods
The study sample consisted of 217 adult individuals (125 males and 92 females) belonging to nine anuran species (Table 1), originating from the Balkan Peninsula, in order to minimize the influence of local environmental conditions on life-history traits, which can influence phenotypic change [25]. The sex of the frogs was determined based on gonads. Although the most anuran species are characterized by marked sexual dimorphism, a preliminary analysis (two-way ANOVA, factors: species, sex) showed that the differences between species were more pronounced than between sexes (structure: species: F32,728 = 183.75, P < 0.001, sex: F4,197 = 15.97, P < 0.001; limb parts: species: F40,949 = 115.76, P < 0.001, sex: F5,217 = 19.11, P < 0.001). Therefore, we pooled sexes to increase the number of individuals and to maximize differences between locomotor modes as the main question of this paper. All individuals were deposited at the osteological collection of the Institute for Biological Research “Siniša Stanković”, Belgrade, Serbia.
Sample size of species and locomotor modes. N, sample size; loc, locality.
| Jumpers | Walkers | ||
| Species | n | Species | n |
| Hyla arborea | 30 | Bombina variegata | 30 |
| loc: Vir Pazar | loc: Prohor Pčinjski | ||
| Pelophylax esculentus complex | 25 | Bufo viridis | 18 |
| loc: Zaječar | loc: Fruška Gora | ||
| Rana graeca | 18 | Pelobates fuscus | 28 |
| loc: River Gornja Trešnjica | loc: Deliblatska peščara, Hrastovača | ||
| Rana temporaria | 21 | Pelobates syriacus | 22 |
| loc: Šar planina | loc: Deliblatska peščara, Đurica | ||
| Rana dalmatina | 25 | ||
| loc: Vražja bara |
The analysed anuran species were associated with two locomotor groups based on the published literature [8,9,11,25]: walkers and jumpers (Table 1). Out of nine analysed species, five of them (P. fuscus, P. syriacus, B. variegata, R. graeca, P. esculentus complex) were studied for the first time in a framework of limb morphology and locomotor modes [8,9,25].
2.1 Data
The samples were preserved in 75% ethanol and prepared as cleared whole mounts that were differentially stained, according to Dingerkus and Uhler [27], using alizarin red for bone, and alcian blue for cartilage. All skeleton measurements were taken on the right-hand side of the frog with a digital calliper (precision: 0.01 mm). The lengths of forelimb elements: humerus (H), radioulna (R), distal part of forelimb (DF, distance from radiocarpal joint to the tip of third finger), hindlimb elements: femur (F), tibiofibula (T), distal part of hindlimb (DH, distance from tibiotarsal joint to the tip of fourth finger) and snout-vent-length (SVL, distance from top of the head to the posterior edge of the cloaca) was measured (Fig. 1). The forelimb (FL) and hindlimb (HL) lengths were calculated as the sum of their segments (FL represents the sum of humerus, radioulna and distal forelimb elements; HL represents the sum of femur, tibiofibula and distal hindlimb elements). The pelvic traits were presented as sacral width (SW) and sacral diapophyseal expansion (DE).
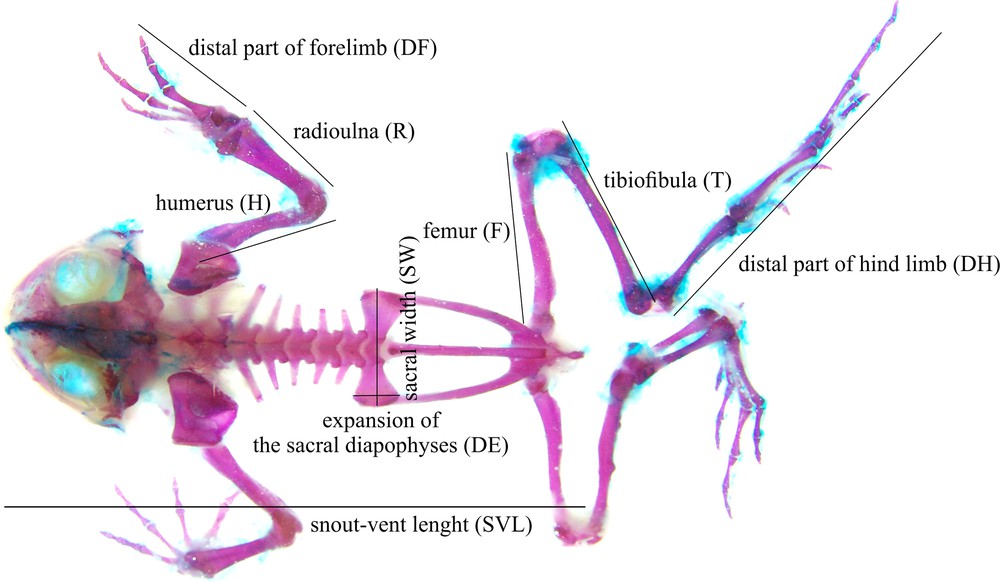
Limb and sacral measurements used in this study; forelimb elements: humerus (H), radioulna (R), distal part of forelimb (DF), hindlimb elements: femur (F), tibiofibula (T), distal part of hindlimb (DH) and snout-vent-length (SVL). Forelimb length (FL) and hindlimb length (HL) were calculated as the sum of their segments.
In order to determine differences among locomotor modes, we analysed the relations between:
basic structural parts (snout-vent-length, total forelimb length, total hindlimb length and sacral traits: sacral width [SW], and sacral diapophyseal expansion [DE]);
fore- and hindlimb elements (humerus [H], radioulna [R], distal part of forelimb [DF], femur [F], tibiofibula [T], distal part of hindlimb [DH]).
As the separation of the locomotor modes could be influenced by the phylogeny of the groups, we sampled species in that manner that species from same locomotor mode are from different phylogenetic lineages. Also, we used PDAP – Phenotypic Diversity Analysis Program [28,29] – to determine if morphometric traits showed phylogenetic signals (i.e. whether more closely related were more similar). The test of the null hypothesis of no phylogenetic signal in the data is based upon a comparison of the fit of the observed phylogeny (using the variance in the independent contrast values) with a 95% confidence interval of fit values calculated by randomization tests (i.e. confidence interval fit values are the variance in the independent contrast values for 1000 iterations of random reassignment of tip values). As analyses showed the absence of phylogenetic signal, we did not transform data set prior to analyses of ratios. Further analyses were performed on log-transformed and centred data as noted by Baur and Leuenberger [24].
We applied the recently developed method of Baur and Leuenberger [24], which allows the interpretation of principal components analysis (PCA) and linear discriminant analysis in terms of ratios or body proportions. This approach permits a separation between differences in size and shape. The first step involves defining an isometric size axis (isosize) as the geometric mean of the original measurements, which includes only differences in scaling. The second step consists in calculating isometry free shape variables by projecting the measurements orthogonal to the isosize. After this, principal components analysis (PCA) taking exclusively into account differences in proportions was carried out on the covariance matrix of the shape parameters. Several tools were created to explore the structure of data variations. The PCA ratio spectrum contains information about dominant ratios and their interrelationships. The position of variables along the PCA ratio spectrum indicates how much each of them contributes to the variation in relation to the others (ratios between variables positioned at the opposite ends of the spectrum have high explanatory power, while the ratios between variables lying adjacent to each other in the spectrum explain very little noticeable variation). To assess the amount of allometry in the data, we first plotted the isosize against each one of the shape PCs to see how strongly shape correlates with size, and second, we calculated the allometry ratio spectrum as the statistical derivation of Jolicoeur's allometric size vector using the method of least squares [23]. To evaluate the differences between locomotor groups, the data were subjected to a linear discriminant analysis (LDA ratio extractor), which allows the extraction of the ratios that are most informative/optimal for distinguishing between them. The measure δ was calculated in order to determine how much of the variation between locomotor groups is due to shape differences, and how much is due to size differences. Values of δ are close imply separation due to size, while values close to zero indicate a higher impact of the shape. The discriminating power of ratios obtained from LDA is presented as a measure of the standard distance. The calculations were performed using software R [30] with a code provided by Baur and Leuenberger [24]. Further analyses as one-way ANOVA, correlation coefficient, and discriminant analyses were performed with Statistica 10 [31].
3 Results
All morphometric traits (structure and fore- and hindlimb elements) varied considerably across the phylogeny, which implies the absence of the phylogenetic signal in data (P < 0.05). Traditional statistical tests were used in further analyses.
3.1 Structural differences
The descriptive statistics for all analysed structural parts are given in Table 2. A principal component analysis (PCA) was performed in isometry free-shape space. The first two principal components (PC) explained 99.16% of the total variance in the data. Fig. 2A and B showed that the first principal component is congruent with the separation of locomotor modes, jumpers, and walkers. Most of the variation can be explained by the ratio between sacral diapophyseal expansion and hindlimb length (DE/HL), which corresponds to the position of these variables at the opposite ends of the PCA ratio spectrum (Fig. 2C). The second shape PC explained a very small part of the total variance (1.66%) and was mainly correlated with the ratio between sacral width and hindlimb length (SW/HL) (Fig. 2D).
Descriptive statistics of limb variables for the analysed locomotor modes (jumpers and walkers). The displayed values are the mean ± st.error.
| Trait | Jumpers | Walkers | |||||
| Structural parts | SVL | 63.40 | ± | 1.34 | 56.05 | ± | 1.15 |
| HL | 107.31 | ± | 2.44 | 72.70 | ± | 1.71 | |
| FL | 43.19 | ± | 0.82 | 37.35 | ± | 1.07 | |
| SW | 10.53 | ± | 0.23 | 10.00 | ± | 0.30 | |
| DE | 2.30 | ± | 0.05 | 8.42 | ± | 0.29 | |
| Fore- and hindlimb parts | H | 16.78 | ± | 0.38 | 14.82 | ± | 0.47 |
| R | 9.94 | ± | 0.20 | 8.43 | ± | 0.25 | |
| DF | 16.48 | ± | 0.27 | 14.10 | ± | 0.37 | |
| F | 28.92 | ± | 0.61 | 20.54 | ± | 0.50 | |
| T | 32.28 | ± | 0.76 | 18.61 | ± | 0.47 | |
| DH | 46.12 | ± | 1.09 | 33.60 | ± | 0.78 |
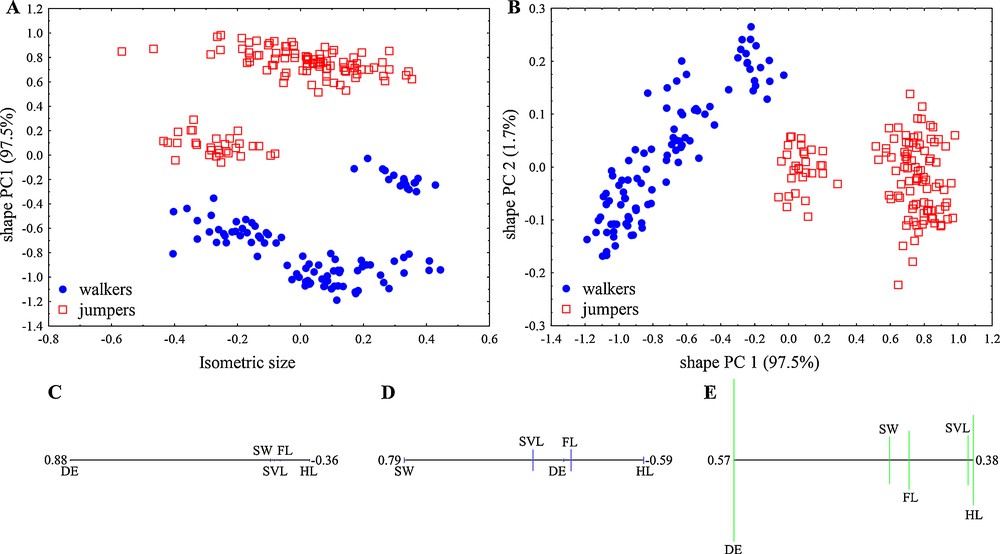
Size and shape analysis of the structural traits in anurans from two locomotor modes (jumpers and walkers). A. Scatterplot of isometric size versus first principal component in shape space. B. Scatterplot of the first- against second-shape PC; in parentheses the variance explained by each shape PC. C. PCA ratio spectrum for first PC shape. D. PCA ratio spectrum for second PC shape. E. Allometry ratio spectrum; vertical bars in the ratio spectra represent 68% bootstrap confidence intervals based on 1000 replicates. SVL: snout-vent-length; HL: hindlimb length; FL: forelimb length; SW: sacral width; DE: diapophyseal expansion.
Comparisons of calculated isosize as a general size measure showed that the ranges of analysed locomotor modes were more or less overlapped. Nevertheless, there were statistically significant differences in terms of mean isosize between locomotor groups (one-way ANOVA, P < 0.001) with greater values for walkers. Based on isometric size and the first principal shape component, all individuals were correctly classified in a discriminant analysis (Fig. 2A).
According to Baur and Leuenberger [24], in order to evaluate the amount of allometry in the data, we plotted the isosize against each of the shape PCs (Fig. 2A), to see how strongly shape correlates with size. The correlation of the first shape PCs with the isosize (as second shape PCs explained only a small portion of the total variance) was non-significant (r = –0.107, P > 0.05), indicating a small impact of allometry on the total variation in the dataset. Inspection of the allometry ratio spectrum showed that the strongest allometric variation is related to the sacral diapophyseal expansion to the hindlimb length ratio (DE/HL), which is the most important ratio for the separation of the groups on the first shape PC (Fig. 2E).
The optimal ratios that describe differences between locomotor modes were the ratio sacral diapophyseal expansion to hindlimb length (DE/HL) and sacral width to hindlimb length (SW/HL), with differences mainly caused by shape (first ratio δ = 0.096; second ratio δ = 0.197). The standard distance and its discriminating power were 4.97 for the first ratio, while the standard distance of the second best ratio was 2.14. Based on both ratios, 100% of the individuals were correctly classified (Fig. 3) in a discriminant analysis. The first ratio, sacral diapophyseal expansion and hindlimb length (DE/HL) showed a large mean difference between locomotor groups (one-way ANOVA, P < 0.001). Walkers have a higher DE/HL ratio (mean value of 0.118) which results from higher mean values for DE and smaller hindlimb length. Jumpers have longer hindlimbs and smaller sacral vertebra expansion than walkers (see Table 1 for descriptive statistics) and a smaller ratio. The mean of the second ratio, sacral width/hindlimb length (SW/HL) significantly differ between locomotor groups (one way ANOVA, P < 0.001). Walkers were characterized with lower mean values for sacral width and hindlimb length, while jumpers had higher values for the same variables. Based on these two ratios (DE/HL, SW/HL), locomotor groups were well defined: walkers have higher values for sacral expansion, but lower for sacral width and hindlimb length, while jumpers have lower values for sacral diapophiseal expansion, but higher for sacral width and total hindlimb length.
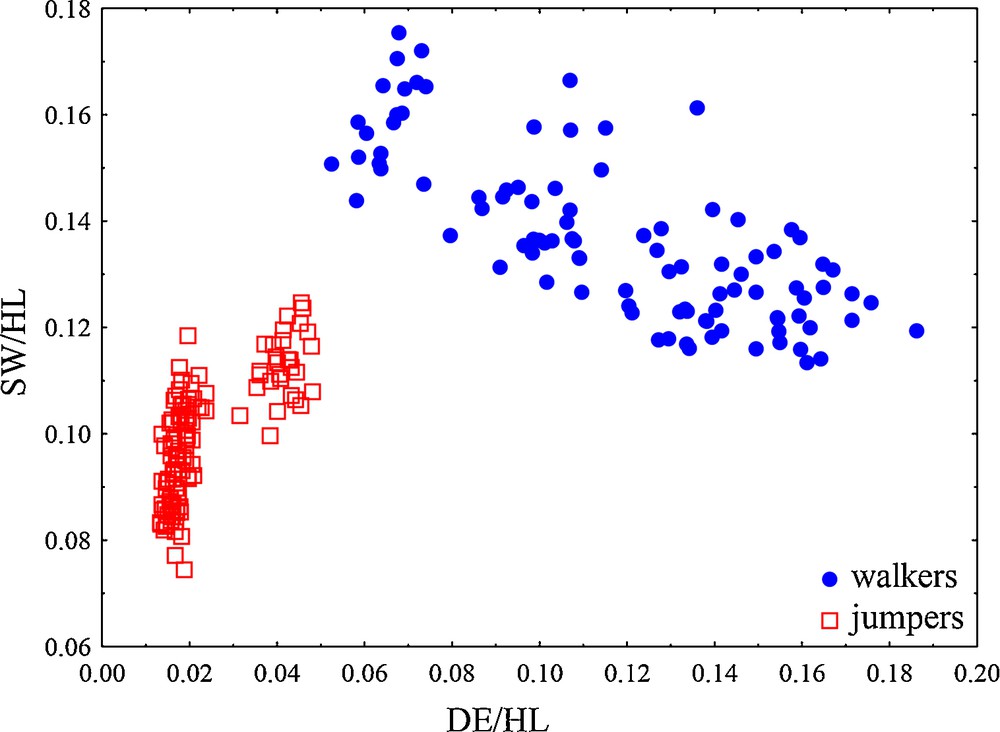
Scatterplots of the two ratios that most differed between two locomotor modes in the analyses of the structural parts (based on LDA ratio extractor). DE/HL: diapophyseal expansion/hindlimb length; SW/HL: sacral width/hindlimb length ratio.
3.2 Differences in proportions in fore- and hindlimbs
The descriptive statistics of forelimb and hindlimb elements are given in Table 2.
The first shape PC explained 65.15% of the variance (Fig. 4A and B), mainly correlated with the tibiofibular-to-distal part of the forelimb length ratio (T/DF). However, the remaining elements of forelimbs (humerus and radioulna) are also of great importance for differentiation between jumpers and walkers in the shape space due to the close positions of all forelimb elements on the same side of the PC ratio spectrum (Fig. 4C). The second shape PC explained 20.91% of the variance and was mainly correlated with the ratio of forelimb elements, the radioulna-to-distal part of forelimbs (R/DF) (Fig. 4D).
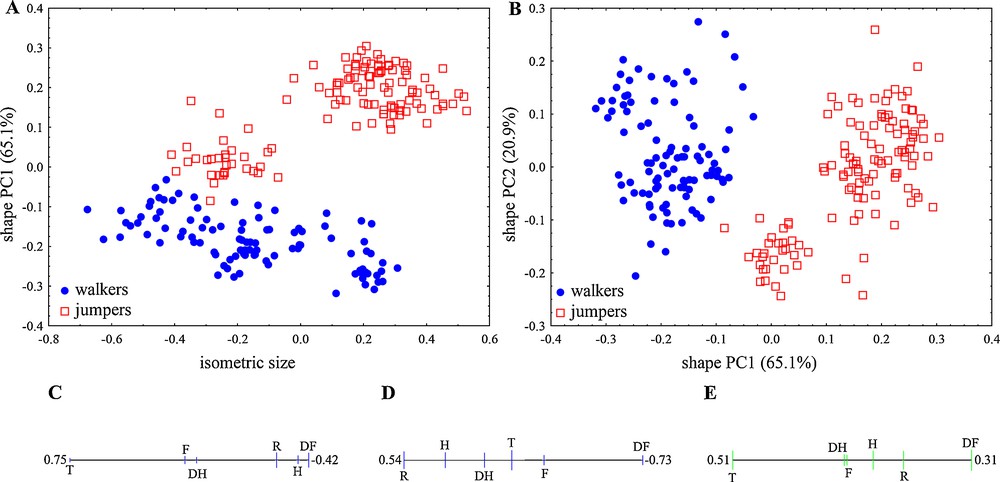
Size and shape analysis of fore-, and hindlimb traits in anurans from two locomotor modes (jumpers and walkers). A. Scatterplot of isometric size versus first principal component in shape space. B. Scatterplot of first against second shape PC; in parentheses the variance explained by each shape PC. C. PCA ratio spectrum for first PC shape. D. PCA ratio spectrum for second PC shape. E. Allometry ratio spectrum; vertical bars in the ratio spectra represent 68% bootstrap confidence intervals based on 1000 replicates. Forelimb elements: humerus (H), radioulna (R), distal part of forelimb (DF). Hindlimb elements: femur (F), tibiofibula (T), distal part of hindlimb (DH).
Comparisons of mean isosize between locomotor groups revealed significant differences (one-way ANOVA, P < 0.001), with higher values for jumpers. Based on the isometric size and the first shape component (Fig. 4A), 95.81% of the individuals were correctly classified in a discriminant analysis (walkers 100%, jumpers 92.91% with the misclassification of individuals within Hyla arborea).
The allometry ratio spectrum indicated that the tibiofibular-to-distal part ratio of the forelimbs (T/DF) showed the greatest amount of allometry (Fig. 4E). As the correlation of the isosize and the first and second shape PC address the strength of allometry, the values r = 0.556, P < 0.05 and r = 0.269, P < 0.05 indicate a moderate impact of allometry on the present data and the presence of allometric relation between tibiofibula and distal part of forelimbs.
The most optimal ratios for describing locomotor modes were: humerus to tibiofibula (H/T) and tibiofibula to distal part of hindlimbs (T/DH), with differences mainly related to shape changes (δ = 0.160, δ = 0.256, with standard distances 5.83 and 3.23, respectively). The discriminating power of these ratios is inspected with discriminant analysis and the percentage of correctly classified individuals was 98.98% and 100% for walkers and jumpers, respectively (Fig. 5).
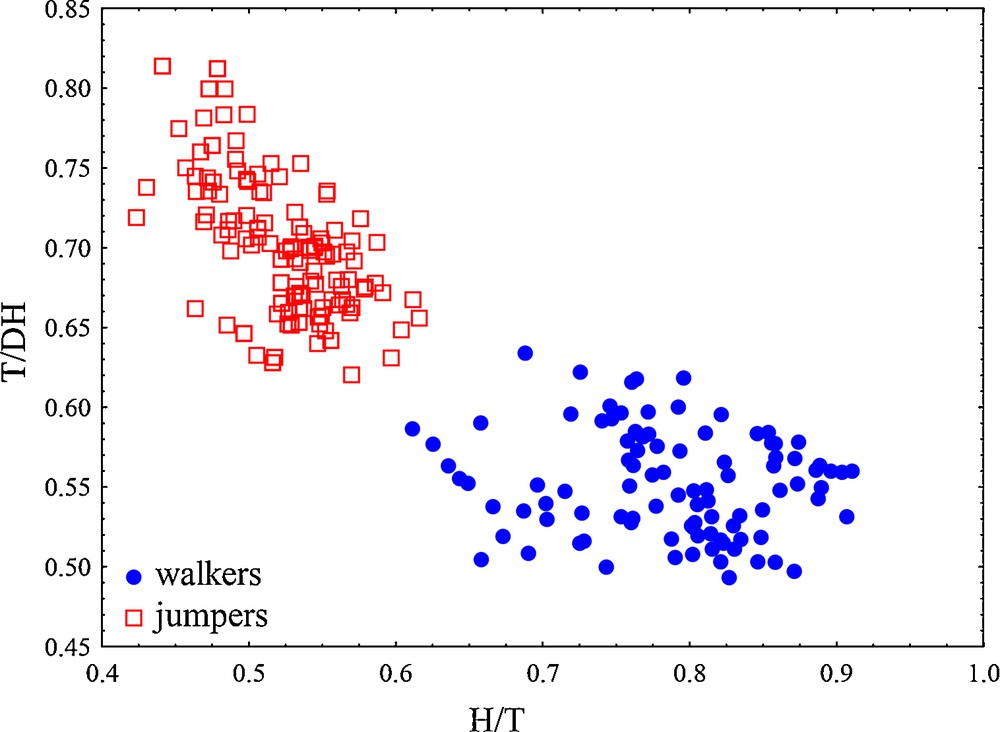
Scatterplots of the two ratios that most differed between two locomotor modes in the analyses of fore- and hindlimb parts (based on LDA ratio extractor). H/T: tibiofibula/humerus ratio; T/DH: tibiofibula/distal part of hindlimbs.
4 Discussion
This study explored the morphological variation of limbs and sacral traits in nine anuran species, in order to relate morphological trait ratios (as integrated system) to locomotor groups. The first part of this study examined inter-connections of basic structural parts of body in the context of two different locomotor modes. Our results were concordant with previous studies, with the most pronounced variations in total hindlimb length and sacral traits [9,13]. The second part of this study examined how within and between limb elements correlations relate to locomotor modes. Our study highlights that the functional specialization in different locomotor modes is attained not only through modifications of hindlimbs that are directly linked to jumping ability/performance (as was noticed in the literature), but probably through modifications of the whole integrated locomotor system (fore-, hindlimbs and girdle elements).
4.1 Relationships between structural parts
The analyses of basic structural differences of anuran body showed the relation of body size and shape to locomotor modes (jumpers and walkers), with a larger impact of body shape than of size. As has been observed [11,15,25], some anuran families show high body size disparity, but low diversity of locomotor modes. Therefore, it has been suggested that body size is evolutionary disconnected from locomotor behaviour. Regarding the shape, our study showed that the ratio of sacral diapophysis to total hindlimb length (DE/HL), as well as the ratio of sacral width to total hindlimb length (SW/HL) contributes the most to the disparity between jumpers and walkers. Jumpers are characterized by long hindlimbs and narrow sacral vertebrae, while walkers have shorter hindlimbs accompanied with expanded sacral vertebrae. Indeed, our results confirmed, as it is already known, that jumper species have longer hindlimbs compared to walkers species, not only to enhance jumping capacity, but longer limbs can be an advantage for locomotion in complex vegetation structure [25]. The important role of hindlimbs and sacral traits is already showed in Jorgensen and Reilly's study [9]. They showed that diapophyseal expansion, sacral width and pelvic length explained nearly 65% of the variation in the multivariate phylomophospace of the skeleton of the frog. Also, they found that walker frogs tended to have greater diapophyseal expansion than jumping frogs. Due to such large impact of sacral traits on variation between locomotor modes, the relative hindlimb length had received a secondary role in their study.
Although our study showed that locomotor groups had specific relation between sacral traits and hindlimb length, the European tree frog (Hyla arborea) is slightly separated in morphospace from the other analysed jumpers. The European tree frog possesses a good jumping ability, but also uses climbing as a way of locomotion, which additionally affects its morphology. It is observed that in these species, the diapophyseal expansion is larger, which allows greater fore-aft translation of the iliac shafts during climbing. Emerson [5] marked that this pelvic morph as fore-aft slider and is present in Hylidae, but also in some species with different locomotor modes (Pelobatidae, Pipidae).
4.2 Relationship between elements of fore- and hindlimbs
As every limb part has different biomechanical properties, and as the relationship between them may determine their functional output, we analysed the interrelations between fore-, and hindlimbs proximal, medial and distal elements regarding to specific locomotor modes, jumpers and walkers. According to previous studies, the tibiofibular-to-femur ratio was used as the key ratio describing locomotor modes [4,12,25]. Our study showed that the impact of tibiofibula length remains high, but the most profound ratios are the ones between the tibiofibula and each part of the forelimb (primarily humerus, but also the distal part of the forelimbs and the radioulna) (see PCA and LDA ratio spectrums). The importance of forelimbs and their relationships with hindlimbs in locomotor performance can be supported by novel studies by Wang et al. [32] and Reilly et al. [33]. They showed that in walkers during short jumps, forelimbs play a role during take-off by raising or levering body weight. Forelimbs in this case can function as lever system similar to one observed in hindlimbs, whereas the length of humerus enhance the force applied to the substrate and elevate the body to a suitable posture to determine the preparation angle during short jump. Also, a more coordinate movement on the substrate is achieved through high functional association between longer humerus and shorter tiobiofibula. In jumpers, forelimbs are unlikely to be important for generating propulsive forces during long jumps [34], but the correlation between shorter humerus and longer tibiofibula could indirectly enhance jumping performance [4,25,35].
Our comparison of morphological variability in different locomotor modes in anurans showed the important influence of forelimbs, hindlimbs and sacral traits in morphological defining of locomotor modes. The most remarkable findings were the different patterns of within and between limb element inter-relations of different locomotor modes. This indicates that locomotor modes should be analysed in a frame of limb part ratios, not just for hindlimbs, but for forelimbs also. These results are a good start point to further studies about the correlations between serially homologous fore- and hindlimb elements in the context of biomechanical demands and stability of locomotion established through certain intra-limb proportions. As fore- and hindlimbs underwent significant reorganization during anuran evolution, the main question would be related to whether the shared developmental programs of serially homologous structures constrain within limb integration (or their specialization) or to some extent interferes with the biomechanical requirements for an efficient locomotion [36].
Acknowledgements
This research was financed by the Ministry of Education, Science and Technological Development of Republic of Serbia (Grant No. 173043).


