1 Introduction
Bacteria are ubiquitous and highly diverse. Bacterial population gains different significant properties according to their habitat and surrounding environment. In Gujarat, some areas are well known for natural hot water resources. Well-known hot springs are located in Tuwa, and Unnai. Hot water springs are manifestations of geological activity and represent extreme environment. Extremophiles isolated from extreme environments have a tremendous role in the production of thermopile enzymes that have wide applications in pharmaceutical and other industries [1]. Rhizobacteria growing in the site of hot spring can produce different compounds and enzymes that may have a beneficial role in plant growth promotion and can be used as an alternative chemical for plant growth promotion [2].
In this study, the bacteria isolated from different hot springs located in the province of Gujarat, India, were screened for their (i) plant growth promoting (PGP) properties, (ii) biocontrol properties against different plant pathogens, (iii) extracellular enzymes production, (iv) extreme properties such as salt tolerant and thermo-tolerant, and (v) identification of multipotential strains by 16S rDNA sequencing.
2 Materials and methods
2.1 Collection of samples and isolation of bacteria
Rhizosphere and nonrhizosphere soil samples from natural hot water springs were collected aseptically from Unnai (20°85′33″N; 73°33′42″E), and Tuwa (22°47′58″N; 73°27′37″E). The soil samples were collected in sterile polythene bags and transferred to a laboratory in cool boxes. The samples were kept in a cold room until use. One gram of the soil sample was suspended in 100 ml distilled water and then incubated in an orbital shaker with shaking at 200 rpm for 30 min. The mixtures were allowed to settle and serial dilutions up to 10−5 were prepared using sterile distilled water. Isolation of bacteria from this mixture was carried out with serial dilution technique in nutrient agar (HiMedia, Mumbai, India). Purification of bacteria was carried out by repeated streaking and checked for purity. The mixture was maintained at 4 °C until use.
2.2 Indole acetic acid production
Indole acetic acid (IAA) production by the isolates was detected qualitatively by following Sawar and Kremer [3]. Fresh cultures were streaked onto LB medium amended with l-tryptophan (5 μg/ml) and the plates were overlaid with sterile Whatman No. 1 filter paper, incubated at 28 ± 2 °C for 72 h. After incubation, the filter paper was soaked with 2–3 drops of O-phosphoric acid and Salkowski's reagent (1 ml of 0.5 M FeCl3 in 50 ml of 35% HClO4) was added. Production of IAA was immediately identified by formation of red colour on the filter paper.
2.3 Phosphate solubilization
All bacterial isolates were screened for inorganic phosphate solubilization [4]. A loopful of fresh bacterial culture was streaked onto Pikovaskaya's medium amended with inorganic phosphate and the plates were incubated at 28 ± 2 °C for 3–4 days. The formation of a clear halo around the bacterial colony indicated solubilization of mineral phosphate.
2.4 Antagonistic properties
The antagonistic ability was determined by the dual-culture technique, as described by Dennis and Webster [5]. Antagonistic activity of the bacterial isolates against tomato pathogens such as Fusarium fuski, Sclerotium rolfsii, Sclerotinia sclerotiorum, Rhizoctonia batticaloa, and Rhizoctonia solani was tested by dual culturing on PDA plates (HiMedia); 6-mm agar disks containing grown mycelia of any of the five phytopathogenic fungi were placed approximately 3.5 cm (distance) apart on a potato dextrose agar (PDA) plate. Four bacterial isolates were streaked between the agar disks. Inhibition of fungal mycelium around the bacterial colony was scored for 3–4 days by measuring the radial growth of the pathogen. The PDA plate inoculated only with pathogen was taken as control. Percent inhibition of pathogen growth was calculated by the following formula: I = C − T/C × 100, where, I is the percent inhibition of growth, C the mycelial growth in the control plate, and T the mycelial growth in the test plate. All strains were tested in triplicates and tests were carried out twice for each isolate.
2.5 Production of extracellular enzymes
Isolates were analyzed for three enzymes (i.e., protease, cellulase and lipase) by the plate method. Proteolytic activities of the cultures were qualitatively screened in a medium containing skimmed milk (HiMedia). Zones of precipitation of paracasein around the colonies appearing over the next 48 h were taken as evidence of proteolytic activity [6]. For cellulase activity, a mineral–salt agar plate containing 0.4% (NH4)2SO4, 0.6% NaCl, 0.1% K2HPO4, 0.01% MgSO4, 0.01% CaCl2 with 0.5% carboxymethyl cellulose, and 2% agar (HiMedia) were surface-inoculated. An iodine solution was used to detect cellulase activity [7]. The clear zone formation around the growing colony was considered as positive. The lipase activity of the bacterial isolates was determined according to the diffusion agar methods, i.e. the nutrient agar medium was supplemented with CaCl2·H2O 0.01%. Tween 80 sterilized for 20 min at 120 °C was added to the molten agar medium at 45 °C to give a final concentration of 1% [8]. The medium was shaken until Tween 80 dissolved completely and was then poured into Petri plates. The test is considered as positive if an opaque halo around the colonies occurs.
2.6 Screening of salt resistance
The resistance of the bacterial isolates to salinity was determined by observing the growth on the nutrient agar medium amended with 5% NaCl (w/v). The plates were incubated at room temperature for 48 h and the growth on the NaCl-amended media was recorded.
2.7 Screening of thermotolerance
All isolates were examined for their ability to tolerate heat stress. Bacteria were grown overnight in 100-ml nutrient broth on a shaker at 28 ± 2 °C. Subsamples (10 ml) of bacterial suspensions were transferred to test tubes. The bacterial cultures in each tube were then placed at different temperatures of 37, 45, 55, 60, 65, 70, 80, 90, and 100 °C for 1 h. After incubation, a small volume of the broth was spread on the nutrient agar and observed for growth in time intervals of 2–4 days.
2.8 Plant growth promotion ability of tomato plants under pot culture
The tomato seeds were sterilized with 70% ethanol for 2 min and in 2% sodium hypochlorite for 2 min, followed by washing 10 times in sterile water. Pure cultures of isolates were grown in the nutrient broth at room temperature. Surface-sterilized seeds were immersed in a grown PGP bacterial suspension for 1 h, air-dried, and immediately sown in sterilized plastic pots. The pots contained sterilized soil autoclaved at 15 lbs for 15 min. The pots were arranged in completely randomized factorial design in a greenhouse. The seedlings were grown at 30–32 °C and 58% relative humidity in the greenhouse under a day–night cycle of 13–14 h. Each treatment was performed in triplicates. After 30 days, the plants were carefully removed from the pots and the root surface was cleaned several times with distilled water. Growth parameters such as root length, shoot length, shoot weight, and root weight of the plants were measured.
2.9 Identification of potential isolates
The isolates that showed multipotential properties were identified by 16S rRNA gene sequencing at Gujarat State Biotechnology Mission, Gujarat, India, and the sequences were compared with the reference 16S rRNA gene sequence using the BLAST algorithm [9]. Phylogenetic dendogram was constructed by the neighbour-joining method and tree topologies were evaluated by performing bootstrap analysis of 1000 data sets using Molecular Evolutionary Genetic Analysis.
3 Results and discussion
3.1 Isolation of bacteria
A total of 123 bacteria were collected from rhizosphere and nonrhizosphere in and around hot springs of Tuwa, and Unnai, Gujarat, India. The colony-forming unit (CFU) was found higher in the rhizosphere soil (4.8 × 10−5 to 5.8 × 10−5) compared to that in the nonrhizosphere soil (2.0 × 10−5 to 2.6 × 10−5) in the hot springs region. Higher CFU in the rhizospheric soil than nonrhizospheric soil might be due to different types of substances released from the roots such as carbohydrate, organic acids, vitamins, nucleotides, flavonoids, enzymes, hormones, and volatile compounds [10], which may have stimulated the microbial activities in the root region.
3.2 Antagonistic properties
All the isolates were screened for their antagonistic activities against different plant pathogens such as F. fuski, R. solani, S. rolfsii, S. sclerotiorum, and R. batticaloa. The results showed that 75.6% (93) isolates inhibited F. fuski, 55.3% (68) isolates S. rolfsii, 54.5% (67) isolates R. batticaloa, 53.6% (66) isolates R. solani, and 48.8% (60) isolates inhibited S. sclerotiorum. Saikia et al. [11] also reported that Brevibacillus laterosporus strain BPM3 isolated from a natural hot water spring strongly inhibited the growth of phytopathogenic fungi (Fusarium oxysporum f. sp. ciceri, F. semitectum, Magnaporthe grisea, and Rhizoctonia oryzae).
3.3 Plant growth promoting properties
During the screening for PGP properties, 61.0% (75) isolates showed IAA production qualitatively and 23.6% (19) isolates were found to solubilize inorganic tricalcium phosphate in in vitro condition (Fig. 1). Ten isolates (TNB2, JNA4, JNB2, TNB3, UNA7, URB2, JRA13, URB5, URB7, and URA7) showed both the properties. Extreme environment bacteria having multiple properties are important not only for plant health but also for other biotechnological applications. For example, they can be used directly for biological control of plant pathogens and plant growth promotion [2,12].
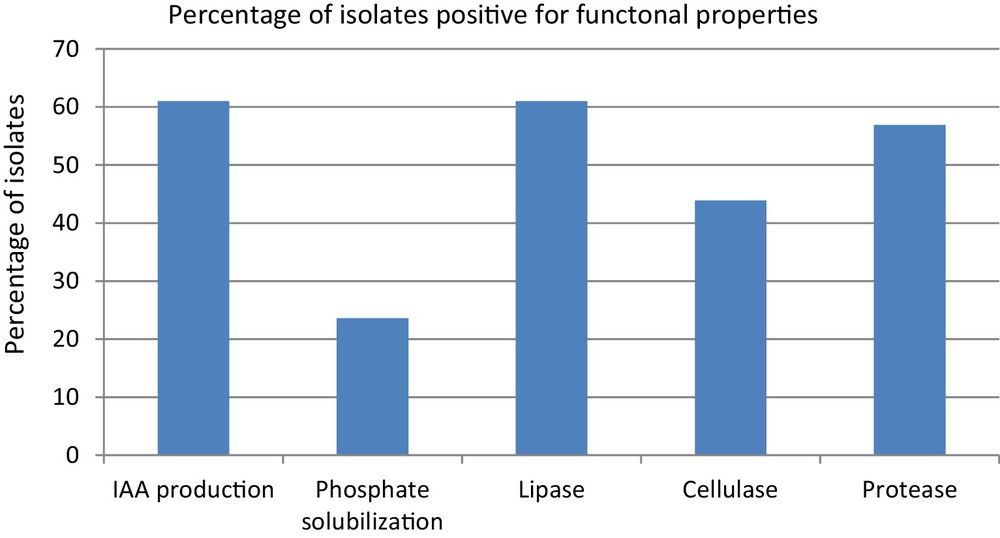
Percentage of isolates positive for PGP and extracellular enzyme properties.
3.4 Extracellular enzymes production
The extracellular enzyme production by hot spring isolates showed that 61.0% (75) isolates produced lipase, 56.9% (70) isolates produced protease, and 43.9% (54) isolates produced cellulase (Fig. 1). A total of 21 isolates (17.1%) showed production of all the three enzymes tested. The results show that the hot spring isolates had multienzyme production capabilities. Further, these extreme environments could be explored as multipotent enzymes producers for industrial purposes. Many researchers reported that production of extra cellular enzymes by rhizobacteria is another mechanism by which it can inhibit phytopathogens [13–15].
3.5 Extreme properties
The extreme properties such as salt tolerance and temperature tolerance showed that half of the isolates were tolerant to 5% NaCl (w/v), whereas 48.8% (60) isolates showed temperature tolerance at 70 °C. The isolates TNA3, TNB14, JNA2, TNB13, TNA4, JNA4, JRA13, URA12, JRA12, JRB7 URB7, URA9, and URA7 tolerated heat up to 100 °C. There are several reports that indicate the impacts of halophilic microorganisms on plant growth. Many researchers [16,17] reported that halophilic bacteria show abiotic stresses and plant growth promotion activities such as indole-3-acetic acid production, phosphate solubilization, and potential nitrogen fixation. These halophilic bacteria also produced intracellular antifungal enzymes and halotolerant and thermotolerant chitinases [18]. Different studies proved that temperature is one of the major conditions for affecting the growth rate of the antagonists [19]. Most of the isolates showed tolerance to both salinity and temperature. These isolates could be used as potential plant growth promoters in saline and arid soils.
3.6 Plant yield parameters
On the basis of their multiple PGP aptitudes, 12 most potential isolates were selected for evaluation of their plant growth potential under pot growth conditions (Table 1). The PGP potential of all 12 isolates was determined by bacterization in tomato seeds. It was observed that the isolate URB8 (130.41%) had the highest root length followed by URB3, JTB13, URB13, URA10, JRB10, and TNA8 compared to control (Table 2). For shoot length, isolates JRB9 and URA13 showed a statistically significant increase compared to control (Table 2). A corresponding increase in the root and shoot biomass was also observed with JRA81-, JRB13-, URA10-, and TRA11-bacterized seedlings. The results of the present study are consistent with the previous findings [2,11] that hot springs isolates could be used as bioinoculants.
Functional properties of selected isolates from hot spring sites.
| Isolate name | Organisms identified | Accession No. | Extreme properties | Extracellular enzymes production | PGP properties | aBiocontrol properties (% of inhibition) | ||||||||
| Growth on 5% Nacl (w/v) | Growth on temp | Protease | Cellulase | Lipase | PO4 | IAA | Ff | Sr | Ss | Rb | Rs | |||
| URB3 | A. aneurinilyticus | KT221506 | + | 70 | + | + | + | − | + | 37.8 | 44.4 | 20.0 | 24.4 | 46.7 |
| URA10 | A. aneurinilyticus | KT221504 | + | 60 | + | − | + | − | − | 42.2 | 31.1 | 26.6 | 53.3 | 44.4 |
| TRA11 | A. aneurinilyticus | KT221502 | + | 60 | − | + | −– | − | − | 26.7 | 22.2 | 40.0 | 44.4 | 40.0 |
| TNA8 | A. aneurinilyticus | KT221501 | + | 80 | + | − | + | + | − | 44.4 | 44.4 | 26.7 | 17.8 | 46.7 |
| JRB9 | A. aneurinilyticus | KT221498 | + | 70 | + | + | + | + | − | 42.2 | 44.4 | 40.0 | 44.3 | 35.6 |
| JRA81 | B. cereus | KT221497 | + | 80 | + | + | + | − | + | 26.7 | 17.8 | 42.2 | 24.4 | 37.8 |
| JRB10 | B. cereus | KT221499 | + | 60 | − | − | − | − | − | 42.2 | 46.7 | 17.8 | 40.0 | 31.1 |
| JRB13 | B. cereus | KT221500 | + | 80 | + | + | + | + | − | 51.1 | 48.9 | 20.0 | 44.4 | 4.4 |
| URA13 | B. cereus | KT221505 | + | 70 | + | – | + | + | + | 44.4 | 46.7 | 42.2 | 26.7 | 40.0 |
| TRA2 | B. anthracis | KT221503 | + | 80 | + | + | + | – | + | 37.8 | 22.2 | 42.2 | 42.2 | 44.4 |
| URB2 | B. subtilis | KU745403 | + | 60 | + | + | + | + | + | 26.7 | 35.5 | 31.1 | 40.0 | 37.8 |
| URB8 | B. cereus | KU745404 | + | 60 | − | − | − | − | + | 48.9 | 31.1 | 33.3 | 31.1 | 17.8 |
a Mean of three replicates; − negative; + positive; Ff, F. fuski; Sr, R. solani; Ss, S. rolfsii; Ss, S. sclerotiorum, and Rb, R. bataticola.
Plant-growth-promoting properties of selected isolates on tomato plants.
| Isolates name | Root length (cm) | Root wet weight (mg) | Root dry weight (g) | Shoot length (cm) | Shoot wet weight (mg) | Shoot dry weight (mg) |
| URB 3 | 8.03 ± 0.06b | 330 ± 0.11c | 30 ± 0.11cdef | 10.53 ± 0. 15b | 3300 ± 0.20df | 650 ± 0.08bc |
| URA 10 | 7.50 ± 0.10c | 150 ± 0.43g | 30 ± 0.11cdef | 0.9.43 ± 0.12b | 5400 ± 0.05a | 700 ± 0.01a |
| TRA 11 | 5.10 ± 0.10f | 160 ± 0.36g | 50 ± 0.20bc | 09.83 ± 0.06c | 3900 ± 0.10b | 690 ± 0.01b |
| TNA 8 | 6.23 ± 0.15d | 200 ± 0.01efg | 20 ± 0.15def | 10.36 ± 0.15b | 3100 ± 0.10e | 520 ± 0.02f |
| JRA 81 | 4.77 ± 0.12g | 460 ± 0.01a | 60 ± 0.15ab | 09.40 ± 0.10d | 3700 ± 0.26bc | 500 ± 0.02g |
| JRB 10 | 7.47 ± 0.15c | 260 ± 0.03d | 40 ± 0.15bcd | 09.80 ± 0.10c | 3560 ± 0.15cd | 560 ± 0.01d |
| JRB 13 | 7.50 ± 0.10c | 390 ± 0.01b | 73 ± 0.00a | 10.40 ± 0.10b | 3330 ± 0.25df | 280 ± 0.01f |
| URA 13 | 7.50 ± 0.10c | 210 ± 0.01ef | 20 ± 0. 01ef | 11.13 ± 0.12a | 1870 ± 0.15i | 190 ± 0.01g |
| JRB 9 | 6.27 ± 0.29d | 170 ± 0.01fh | 20 ± 0.00f | 11.16 ± 0.15a | 2460 ± 0.11g | 320 ± 0.01h |
| TRA 2 | 5.53 ± 0.06e | 160 ± 0.02g | 10 ± 0.00cdef | 07.36 ± 0.25f | 3230 ± 0.25f | 540 ± 0.01e |
| URB 2 | 5.67 ± 0.15e | 220 ± 0.02e | 30 ± 0.01def | 08.56 ± 0.21e | 1600 ± 0.17h | 160 ± 0.01g |
| URB 8 | 9.47 ± 0.06a | 160 ± 0.00h | 23 ± 0.00cde | 06.60 ± 0.10g | 5130 ± 0.01j | 540 ± 0.06de |
| Control | 4.11 ± 0.28 | 110 ± 0.6 | 12 ± 0.002 | 06.40 ± 0.32 | 1200 ± 0.07 | 130 ± 0.02 |
| CD | 0.8 | 0.45 | 0.12 | 0.82 | 0.45 | 0.39 |
3.7 Identification of potential isolates
A subset of 12 isolates that showed maximum functional properties were identified based on 16S rRNA gene sequencing (Fig. 2). The results showed that all the isolates belonged to Aneurinibacillus aneurinilyticus (URB3, URA10, TRA11, TNA8, and JRB9), Bacillus cereus (JRA81, JRB10, JRB13, URA13, and URB8), B. subtilis (URB2), and B. anthracis (TRA2). The sequences were submitted to NCBI, GenBank (accession numbers KT221497–KT221506 and KU745403–KU745404). Many reports depicted that Bacillus and Bacillus-derived genera with functional properties were the predominant bacteria isolated from many extreme environments [2,20].
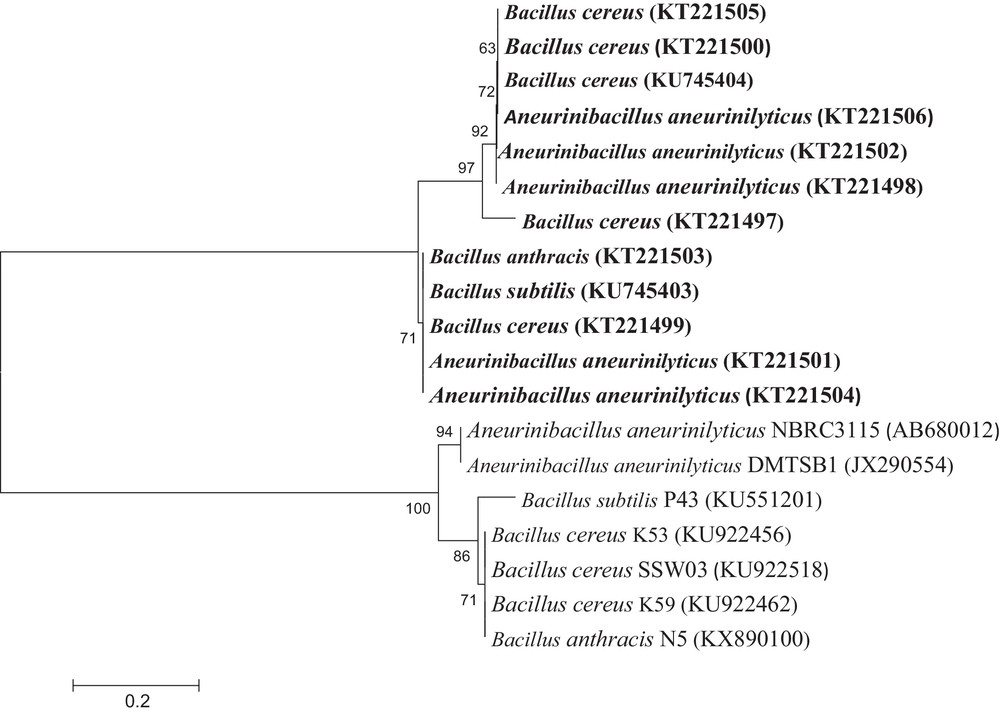
Phylogenetic tree based on the neighbour-joining method of 16S rDNA sequences. The bootstrap values are indicated on their nodes.
4 Conclusion
From this study, it can be concluded that bacteria isolated from hot spring sites possess multipotential functional properties. These organisms can be exploited for their plant growth promotion and biocontrol properties in arid and saline soils. In addition, the investigated bacterial strains have high salt tolerance and significant enzyme activities, which can improve soil nutrient cycling and fertility.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgment
The authors thank UTU Management and Director, CGBIBT for constant support and providing necessary facilities to carry out the work.


