1 Introduction
As already discussed in several previous publications, the taxonomic history related to the genus Opisthacanthus Peters, 1861 is rather complex [1,2]. The genus was created by Peters [3], based on the species Ischnurus elatus Gervais, 1844 [= Opisthacanthus elatus (Gervais, 1844)], a species distributed from Colombia to Panama. Subsequently, the discovery in Africa of species with morphology closely related to O. elatus led some authors to accept the existence of this genus on both continents. By the end of the 19th century, the notion of Gondwanian groups was yet inexistent and consequently this model of discontinuous distribution appeared difficult to explain by that time. This complex situation led Pocock [4], in a new classification of scorpion, to propose a new genus Opisthocentrus for the African species, whereas Opisthacanthus remained associated exclusively with the Neotropical species. Nevertheless, in the following years Pocock himself took different decisions and first placed both American and African species under Opisthacanthus [5,6], but a few years later he split the species of the two continents again in two genera [7]. Other authors such as Kraepelin [8] considered Opisthocentrus as a synonym of Opisthacanthus, a position followed by several authors [9,10].
During the 1970s the question around the issue of one or two genera associated with Opisthacanthus was raised again by two authors: Newlands [11,12] and Francke [13]. Newlands [11,12] suggested the existence of a single genus, Opisthacanthus over two continents (America and Africa), but rejected any hypothesis about a Gondwanian distribution; based on the theory of “transoceanic rafting on driftwood”, as defined by Darlington [14], Newlands [11,12] proposed the hypothesis of recent dispersion from Africa to America. To the contrary, Francke [13] rejected the idea of a single genus over two continents and revalidated the genus Opisthocentrus. However, since Opisthocentrus was already occupied by a fish genus, Opisthocentrus Kner, 1868 (Actinopterygii: Pholidae), he introduced a new replacement name Nepabellus Francke, 1974 for the African species.
The arguments suggested both by Newlands [11,12] and Francke [13] were entirely rejected by Lourenço [1,2], and a single genus Opisthacanthus was defined for all the American and African species, based on a Gondwanian model of distribution (cf. Lourenço [1,2] for further details). It is important to note that in their different analysis during the 1970s, authors such as Newlands [11,12] and Francke [13] totally ignored the existence of the Malagasy species Opisthacanthus madagascariensis described by Kraepelin, 1894 and the fact that this last species presents more morphological traits in common with American than African species. At the time of their analysis, O. madagascariensis was the only species described in the genus. Subsequently, in 1995, Lourenço [15] confirmed the model of a single Gondwanian genus, Opisthacanthus, but with two subgenera Opisthacanthus for the American species including also one species from Occidental Africa, Opisthacanthus lecomtei (Lucas, 1858) and Nepabellus Francke, 1974 for the other African species. The ever growing knowledge on the Malagasy scorpion fauna [16] led to the creation of a third subgenus for the Malagasy species of Opisthacanthus: Monodopisthacanthus Lourenço, 2001 [17].
One important question can however be addressed concerning the real phylogenetic affinities of the three different lineages defined at present. One group, Opisthacanthus, is clearly defined for the American species, with however one species in Occidental Africa. The rest of the African species, placed in the subgenus Nepabellus, constitute a rather homogeneous group. Finally, Monodopisthacanthus has some particularities isolating the group in Madagascar. Back to the original diagnosis proposed by Pocock [4] and Francke [13] for Opisthocentrus and Nepabellus, it can be observed that for a number of morphological characters, size of the excision on the anterior border of carapace, size and shape of genital operculum, in particular those of females, morphology of pectines, and size and morphology of the anterior tubercle of pedipalp patella, two species could not be classified among the other African species: Opisthacanthus lecomtei and Opisthacanthus madagascariensis. The discoveries of numerous new species both in Madagascar but also in the Neotropical region brought confirmation to the observed morphological traits of both American and Malagasy species [18,19]. Based on the characters listed above, it can be suggested that the species of the subgenus Opisthacanthus show a greater affinity with those of the subgenus Monodopisthacanthus. The morphological traits observed for these two groups appear as less primitive in relation to those of the subgenus Nepabellus, in particular if compared to the single known fossil record which can be associated with elements of Opisthacanthus: Protoischnurusaxelrodorum Carvalho & Lourenço, 2001 (family Protoischnuridae) from Cretaceous Santana Formation from Crato area in Brazil [20] (Fig. 1).
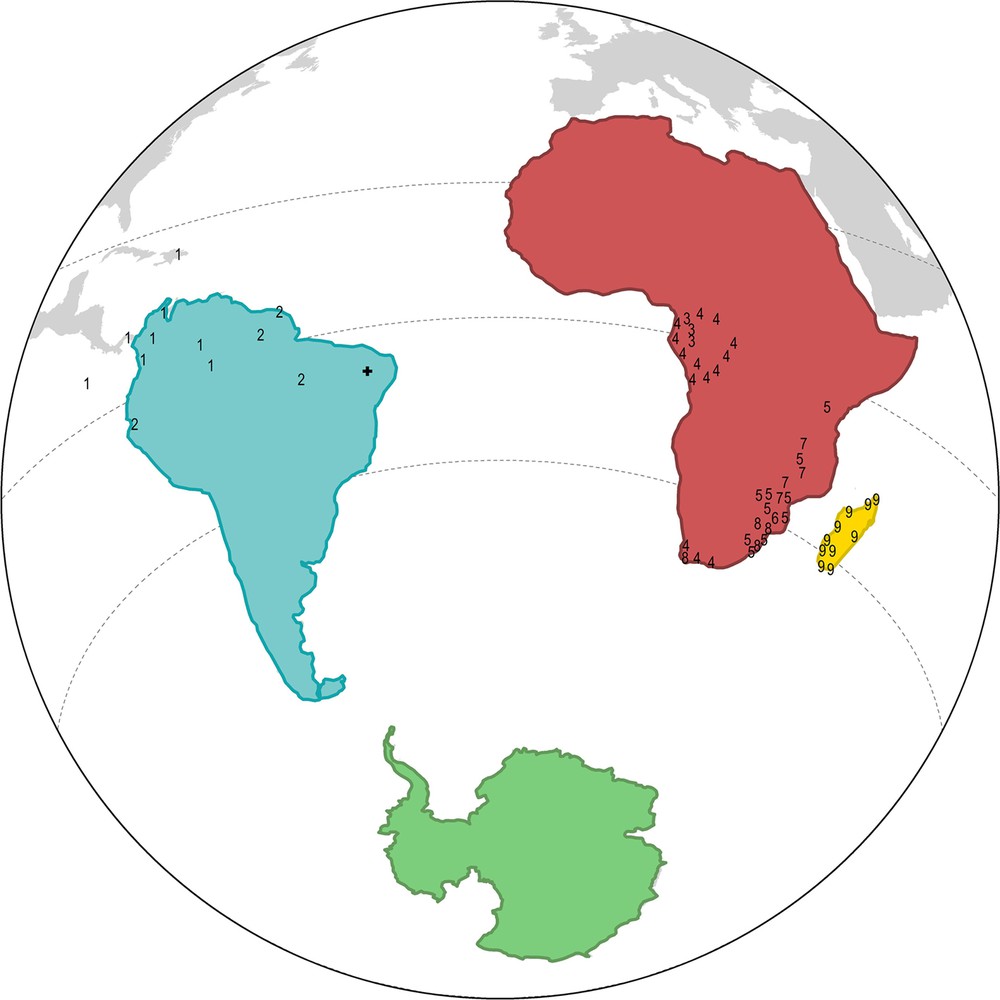
Current known distribution of the groups of Opisthacanthus in Central and South-America, sub-Saharan Africa and Madagascar. (the groups are numbered from 1 to 9 as following: 1: O. cayaporum + O. heurtaultae + O. surinamensis + O. weyrauchi; 2: O. autanensis + O. borboremai + O. brevicauda + O. elatus + O. lepturus + O. valerioi; 3: O. lecomtei; 4: O. africanus + O. capensis + O. diremptus; 5: O. asper + O. basutus + O. rugiceps; 6: O. laevipes; 7: O. lamorali + O. rugulosus; 8: O. validus + O. piscatorius; 9: O. ambanja + O. andohahela + O. antsiranana + O. darainensis + O. lavasoa + O. lucienneae + O. maculatus + O. madagascariensis + O. milloti + O. pauliani + O. piceus + O. titanus sp.; the cross marks the type locality of the Cretaceous Protoischnurus axelrodorum).
The present study of new samples of hormurid scorpions of the genus Opisthacanthus, subgenus Monodopisthacanthus from Madagascar, has resulted in the discovery of one new species. These were collected from the East of the Island, in the Torotorofotsy Forest (dense humid forest), and are in part related to Opisthacanthus madagascariensis Kraepelin, 1894 which is known from the western portion of the island and Opisthacanthus lavasoa Lourenço, Wilmé & Waeber, 2016 only known from the extreme southeast of the island. Nevertheless, O. madagascariensis is exclusively found in spiny forest thickets and grassland formations. Comments are proposed on the geographic distribution and ecology of the Malagasy species of Opisthacanthus. Given the global distribution of this ancient group of scorpions, this paper explores potential scenarios of origin and distribution.
2 Methods
Illustrations and measurements were made with the aid of a Wild M5 stereo-microscope equipped with a drawing tube (camera lucida) and an ocular micrometer. Measurements follow Stahnke [21] and are given in mm. Trichobothrial notations follow Vachon [22] and morphological terminology mostly follows Hjelle [23].
3 The present composition of the genus Opisthacanthus
The classification given below takes into account the most recent taxonomic modifications proposed for the genus [18,19,24,25]. Since most historical aspects concerning Malagasy species of Opisthacanthus have already been presented in previous publications [19,24–27], these will not be further discussed here.
Subgenus Opisthacanthus Peters, 1861.
1. cayaporum group
Opisthacanthus cayaporum Vellard, 1932.
Opisthacanthus heurtaultae Lourenço, 1980.
Opisthacanthus surinamensis Lourenço, 2017.
Opisthacanthus weyrauchi Mello-Leitão, 1948.
2. lepturus group
Opisthacanthus autanensis González-Sponga, 2006.
Opisthacanthus borboremai Lourenço & Fé, 2003.
Opisthacanthus brevicauda Rojas-Runjaic, Borges & Armas, 2008.
Opisthacanthus elatus (Gervais, 1844).
Opisthacanthus lepturus (Beauvois, 1805).
Opisthacanthus valerioi Lourenço, 1980.
3. lecomtei group
Opisthacanthus lecomtei (Lucas, 1858).
Subgenus Nepabellus Francke, 1974.
4. africanus group
Opisthacanthus africanus africanus Simon, 1876.
Opisthacanthus africanus pallidus Lourenço, 2003.
Opisthacanthus capensis Thorell, 1876.
Opisthacanthus diremptus (Karsch, 1879).
5. asper group
Opisthacanthus asper (Peters, 1861).
Opisthacanthus basutus Lawrence, 1955.
Opisthacanthus rugiceps Pocock, 1897.
6. laevipes group
Opisthacanthus laevipes (Pocock, 1893).
7. rugulosus group
Opisthacanthus lamorali Lourenço, 1981.
Opisthacanthus rugulosus Pocock, 1896.
8. validus group
Opisthacanthus piscatorius Lawrence, 1955.
Opisthacanthus validus Thorell, 1876.
Subgenus Monodopisthacanthus Lourenço, 2001.
9. madagascariensis group
Opisthacanthus ambanja Lourenço, 2014.
Opisthacanthus andohahela Lourenço, 2014.
Opisthacanthus antsiranana Lourenço, 2014.
Opisthacanthus darainensis Lourenço & Goodman, 2006.
Opisthacanthus lavasoa Lourenço, Wilmé & Waeber, 2016.
Opisthacanthus lucienneae Lourenço & Goodman, 2006.
Opisthacanthus maculatus Lourenço & Goodman, 2006.
Opisthacanthus madagascariensis Kraepelin, 1894.
Opisthacanthus milloti Lourenço & Goodman, 2008.
Opisthacanthus pauliani Lourenço & Goodman, 2008.
Opisthacanthus piceus Lourenço & Goodman, 2006.
Opisthacanthus titanus sp. n.
The genus Opisthacanthus is currently distributed in Madagascar, sub-Saharan Africa, and the Caribbean, Central and South America (Fig. 1). The range of the genus in Africa is almost 7.5 million km2, it is ca. 3.5 million km2 in the New World, and less than 0.45 million km2 in Madagascar. Despite its small size, Madagascar is home to 12 species, as much as Africa, but Africa shows the highest diversity of groups, with as many as 6 groups VS. one group in Madagascar, and only two groups in the New World (Fig. 2). The fossil material for these groups is limited to a single specimen collected in the Cretaceous of Brazil (Fig. 1) [20].
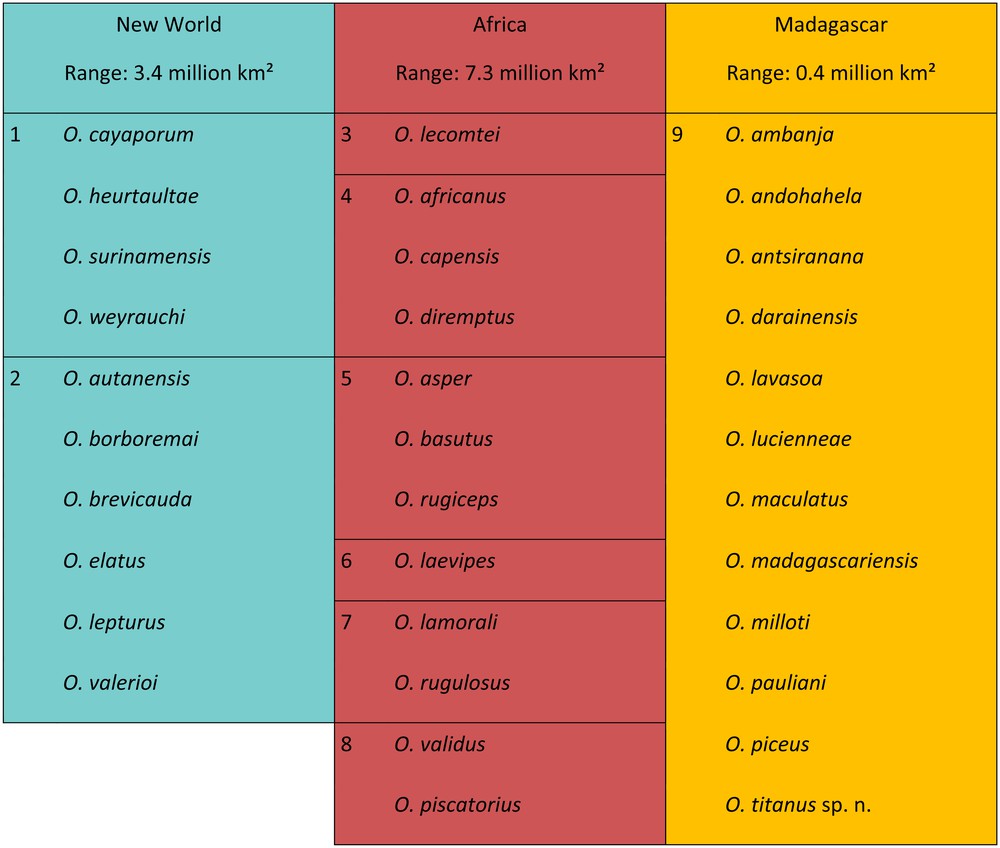
Distribution of the 34 known species of Opisthacanthus in nine groups (color pattern as in Fig. 1).
4 Taxonomic treatment
Family Hormuridae Laurie, 1896.
Genus Opisthacanthus Peters, 1861.
Subgenus Monodopisthacanthus Lourenço, 2001.
Opisthacanthus (Monodopisthacanthus) titanus sp. n. (Figs. 3–7).
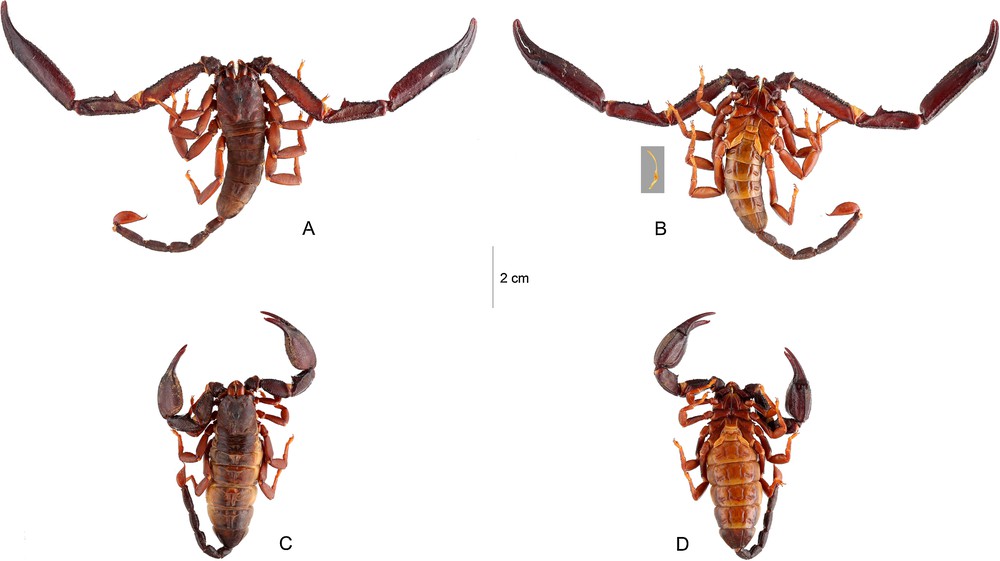
Opisthacanthus (Monodopisthacanthus) titanus sp. n. A–B. Male holotype. C–D. Female paratype, dorsal and ventral aspects. Hemispermatophore is highlighted nearby the male holotype.
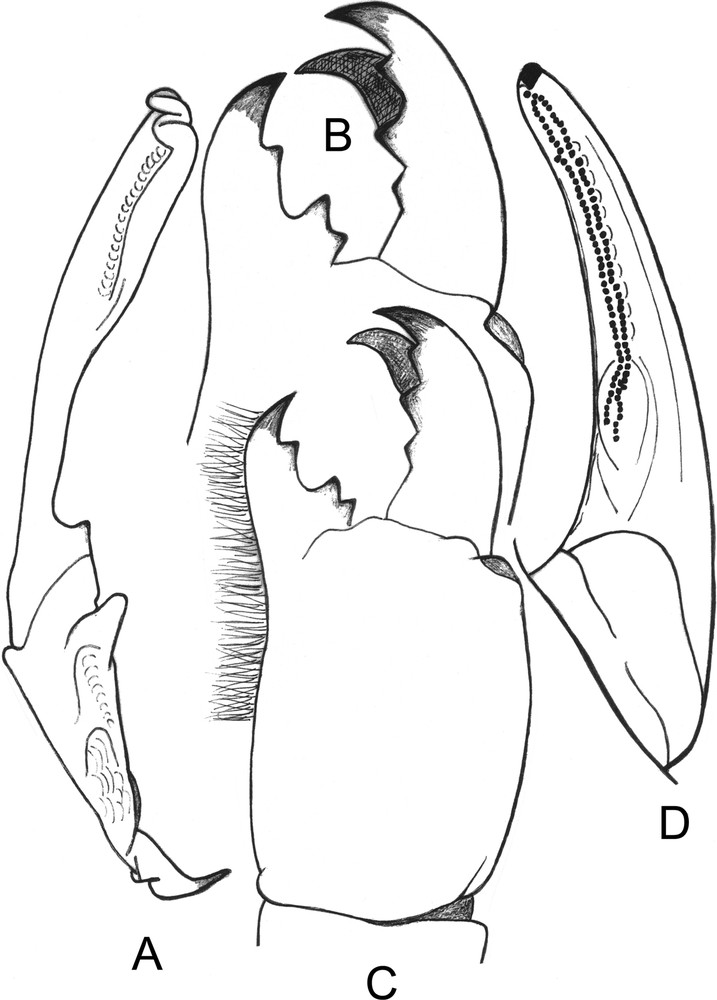
Opisthacanthus (Monodopisthacanthus) titanus sp. n. Male holotype. A. Hemispermatophore, lateral aspect. B-C. Chelicera, dorsal aspect with teeth in detail. D. Cutting edge of movable finger, showing the two series of granulations.
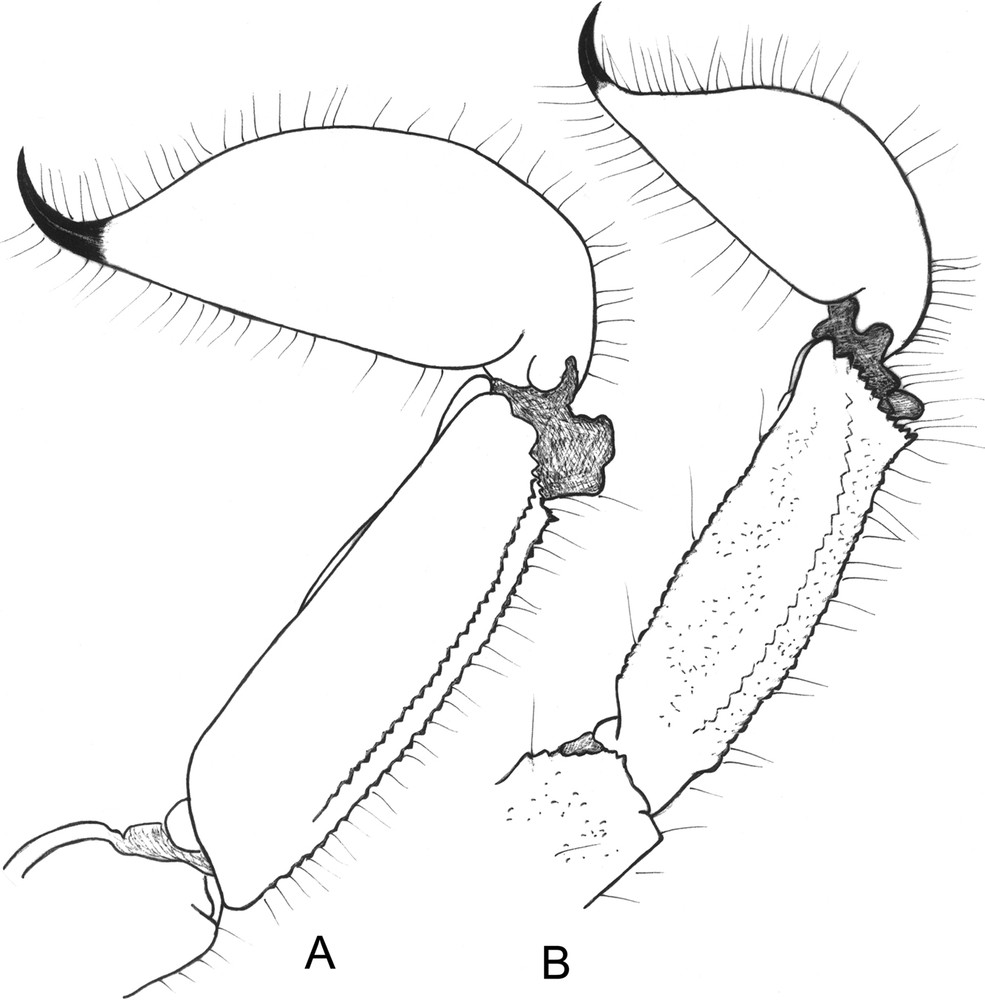
Opisthacanthus (Monodopisthacanthus) titanus sp. n. Metasomal segment V and telson, lateral aspect. A. Male holotype. B. Female paratype.
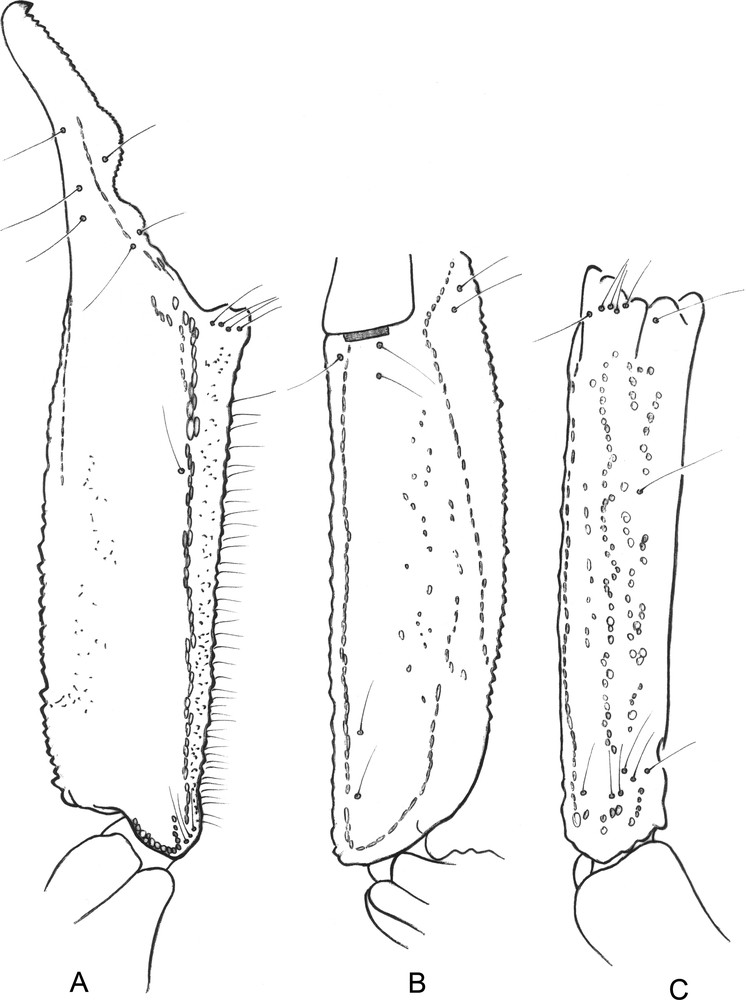
Opisthacanthus (Monodopisthacanthus) titanus sp. n. Male holotype. Trichobothrial pattern. A–C. Chela, dorso-external, ventral and external aspects.

Opisthacanthus (Monodopisthacanthus) titanus sp. n. Male holotype and female paratype. Trichobothrial pattern. A–B. Femur, dorsal aspect (male and female). C–E. Patella, dorsal, external and ventral aspects (male).
Madagascar, ex-Province of Toamasina, Region of Alaotra-Mangoro, District of Moramanga, Torotorofotsy Forest (dense humid forest), 1020 m, XI/1974 (J.-M. Betsch–RCP-225): 1 male holotype, 1 female paratype (MNHN) (Fig. 8).
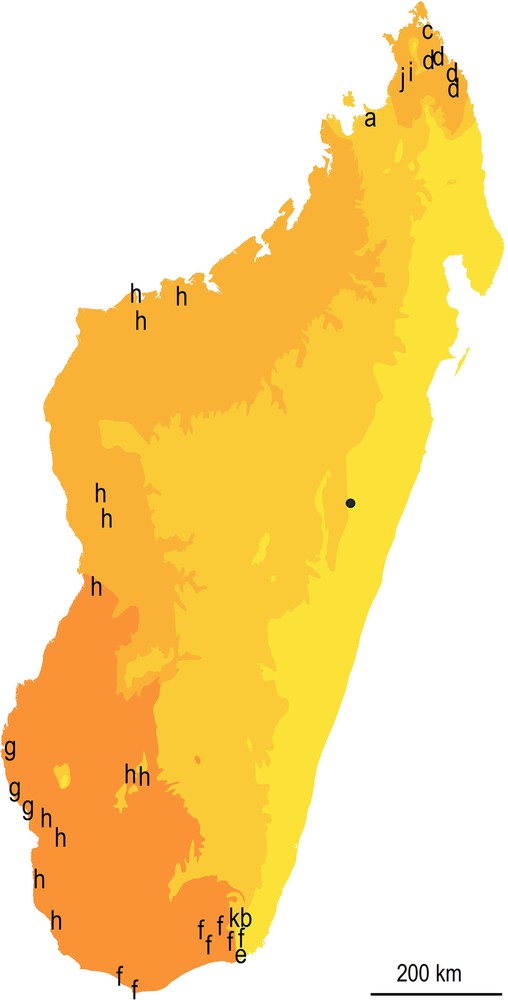
Distribution of the 12 species of Opisthacanthus in Madagascar according to the simplified bioclimates proposed by Cornet in 1974 [30]. (yellow to dark orange for humid, subhumid, dry to subarid; the dark spot for Torotorofotsy, the type locality of Opisthacanthus titanus sp. n. Other species of Opisthacanthus as follow: a: O. ambanja; b = O. andohahela; c: O. antsiranana; d: O. darainensis; e: O. lavasoa; f: O. lucienneae; g: O. maculatus; h: O. madagascariensis; i: O. milloti; j: O. pauliani; k: O. piceus).
Etymology: The specific name derives from the mythological Titans and conveys the idea of giant, powerful.
Diagnosis: Scorpions of large size: male and female with respectively 96.6 and 88.0 mm in total length. Coloration reddish to reddish-brown, with blackish zones over carinae and some dark variegated zones on chelicerae. Pectines with 8–8 teeth in male and 8–7 in female. Hemispermatophore moderately slender with an elongated distal lamina, moderately enlarged. Genital operculum in male formed by two semi-oval to round plates, with a minute incision in the base; in female with a more or less hexagonal shape. Trichobothrial pattern of type C, orthobothriotaxy.
Description based on holotype and paratype.
Coloration. Basically reddish to reddish-brown with blackish zones over carinae and some dark variegated zones on chelicerae. Carapace reddish-brown; median and lateral eyes surrounded with black pigment. Tergites reddish-brown. Metasomal segments reddish-brown to dark brown; vesicle reddish with a reddish-brown lateral strip; aculeus dark reddish to blackish. Chelicerae reddish-brown; base of fingers darker; the whole surface with diffuse variegated spots; fingers yellowish-brown with dark red teeth. Pedipalps reddish to reddish-brown; most carinae blackish; chela fingers reddish-brown to blackish. Venter and sternites reddish-yellow; pectines and genital operculum yellow, much paler than sternites, sternum and coxapophysis reddish; legs reddish to reddish-yellow.
Morphology. Carapace with some granulations, better marked on female; male with punctuation and smooth zones; furrows moderately shallow. Anterior margin with a strong concavity reaching as far as the level of the 2nd lateral eye. Median ocular tubercle flattened and almost in the center of the carapace; median eyes moderate to small, separated by one ocular diameter; three pairs of large lateral eyes. Sternum pentagonal, wider than long. Genital operculum formed by two semi-oval to round plates in male, with a minute incision in the base; with a more or less hexagonal shape in female. Tergites with a moderate to weak median carina, smooth and with punctuations. Pectinal tooth count 8–8 in male holotype (8–7 in female paratype). Sternites smooth and shiny; VII acarinate with very few punctuations. Metasomal segments I to V longer than wide, with some thin but intense granulations in female and mainly punctuations in male. All carinae moderately marked in segments I-IV; segment V rounded with some weak spinoid granules on the ventral surface. All segments with moderate chetotaxy. Telson with a pear-like shape; smooth and covered with strong chetotaxy. Pedipalps: femur with dorsal internal, dorsal external, ventral internal and ventral external carinae strong, tuberculate; dorsal face with thin granulation; ventral face with minute granulation; internal face moderately granulose better marked on female. Patella with internal and external faces moderately granulated; dorsal and ventral faces weak to moderately granulated; dorsal internal, ventral internal, ventral external and external carinae strong; other carinae less well marked. Chela moderately to strongly granular excepted on ventral face; dorsal marginal, external secondary, ventrointernal and ventral median carina strong; other carinae less well marked. Chelicerae typical of Scorpionoidea [28]; teeth strongly sharp. Trichobothriotaxy type C; orthobothriotaxic [22]. Legs: tarsi with two lateral rows of spines, surrounded by a few long setae. Spurs moderate. Hemispermatophore moderately slender with an elongated and bi-lobated distal lamina, weakly enlarged.
Relationships: The new species seems to be associated with Opisthacanthus madagascariensis and with O. lavasoa but can be distinguished from these two species by a number of characters:
- • bigger global size;
- • quite distinct morphometric values, and;
- • hemispermatophore with a distinct morphology–slender compared to that of O. madagascariensis [26] and with a bi-lobate distal lamina.
It should be noted that the types of forests in which O. madagascariensis and O. lavasoa are encountered include subhumid forests according to Moat & Smith [29] in the subhumid bioclimate according to Cornet [30], and the type locality of the new species is at the edge of the humid bioclimate on the eastern humid belt of the island (Fig.8).
Morphometric values (in mm) of male holotype and female paratype. Total length (including telson) 96.6/88.0. Carapace: length 15.5/13.4; anterior width 10.7/8.7; posterior width 15.7/13.2. Mesosoma length 31.8/35.6. Metasomal segment I: length 5.8/4.5, width 3.6/3.8; II: length 6.8/5.3, width 2.9/3.3; III: length 7.2/5.7, width 2.6/2.9; IV: length 8.0/6.3, width 2.3/2.6; V: length 10.2/8.4, width 2.3/2.5, depth 3.2/2.9. Telson length 11.3/8.8. Vesicle: width 3.3/2.5, depth 4.2/3.2. Pedipalp: femur length 24.7/11.4, width 6.4/5.4; patella length 21.6/12.2, width 6.1/5.9; chela length 42.6/24.4, width 7.8/8.8, depth 8.4/10.2; movable finger length 16.4/12.9.
Reports: Male total length 96.6/Pedipalp span 177.8. Female total length 88.0/Pedipalp span 96.0.
5 Biogeographic considerations for the new Madagascar species
The Madagascar Opisthacanthus belong to a single group and are extremely diversified at the species level. This is not unusual in the global Malagasy faunal and floral context, i.e. high species diversity and endemism, and also in the scorpion context.
Lourenço and colleagues [19] detailed the biogeography of the subgenus Monodopisthacanthus at the time of the description of Opisthacanthus (Monodopisthacanthus) lavasoa. The 12th Madagascar species O. titanus sp. n. described has several characteristics outlined earlier for several species of Madagascar scorpions:
- • the species belongs to an old bradytelic group with slow evolution, sensu Lourenço et al. [19]);
- • the species is known from a mountain located in the rainshadow on the leeward side of the humid Betsimisaraka mountains, therefore experiencing a local climate with lower rainfall as compared to most humid forests found along the Eastern belt of the island [31–34];
- • the species is known from a locality sharing bioclimate aspects with localities where O. ambanja, O. andohahela, O. lavasoa, and O. piceus have been collected, i.e. in the subhumid bioclimate as defined by Cornet [30] (Fig.8).
Opisthacanthus madagascariensis as it is currently circumscribed, is distributed in the subarid and dry bioclimates, and in pockets of subhumid climate. In the dry bioclimate, the species has been collected in the tsingy of Namoroka, and the tsingy of Bemaraha where subhumid forests occur in the canyons [29].
Lourenço et al. [19] looked at the climatic context of Madagascar since the Cretaceous–Paleogene (K–Pg) and proposed that following the K–Pg mass extinction at the end of the Cretaceous some 66 Ma, and during the early Paleogene, the ancestral Monodopisthacanthus was assumed present in Madagascar and survived the K–Pg. Madagascar was located at some 7 to 8 degrees to the south of its current position (its southern tip at 32.9°S vs. current 25.6°S; its northern tip at 20.1°S vs. current 12.0°S [35]. During the Paleocene (66.0–56.0 Ma), Madagascar was isolated from Africa and India; the South-Indian drift was affecting the eastern coast of Madagascar which increased the rainfall, and Northern Madagascar entered the monsoon belt. At the end of the Paleocene (56.0 Ma), a major global increase in temperature occurred, the Paleocene–Eocene thermal maximum (PETM), which lasted some 200,000 years [36]. Only by the mid Oligocene, 29 Ma, has Madagascar completed its drift out of the arid belt and entered completely the trade wind regime [35,37,38] and references therein (Fig. 9).
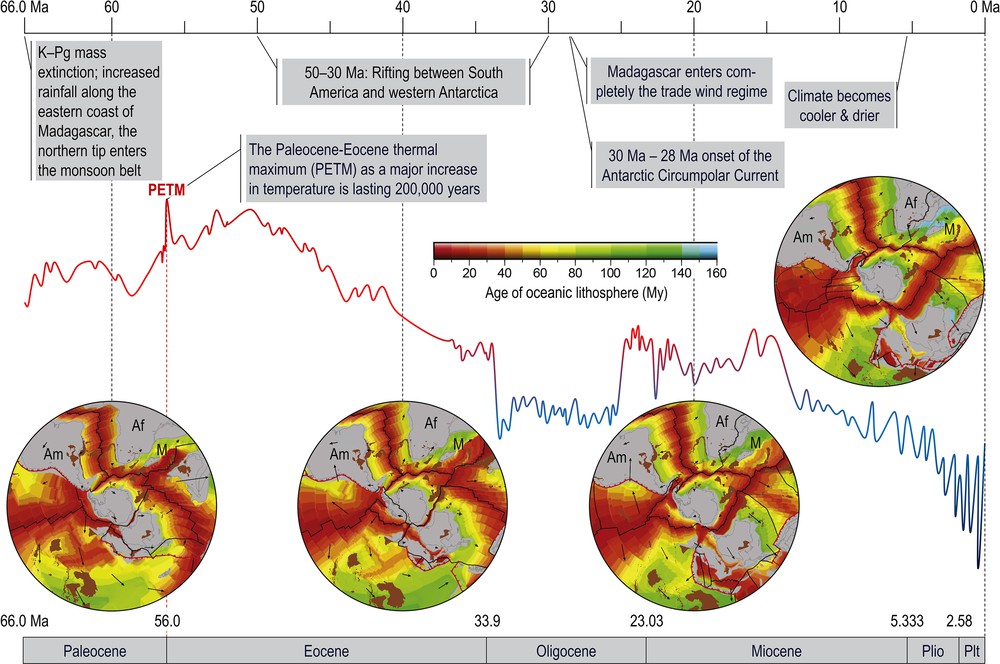
Breakup of Gondwana and changing palaeoclimate during the Cenozoic after the K–Pg mass extinction. (modified from [19,46]); Af: Africa; Am: South America; M: Madagascar.
At the time of the PETM, the ancestral population of O. madagascariensis and O. titanus sp. n. would have been able to cope with dry conditions but would have been extirpated from the driest regions of the island, or could have survived in some refugia of these regions [39]. At the time of the PETM, Southern Madagascar was still in the arid belt and thus the conditions to find humid refugia were reduced.
Adaptation to dry conditions of the Madagascar Monodopisthacanthus lineage can be inferred from a combination of the following: its current distribution in the dry biome of western and southern Madagascar (Fig. 8), the wide range of O. madagascariensis in western and southern Madagascar, and the ancient age of the group in Madagascar when the climate was a lot warmer and drier during the Paleogene. At the time, a common ancestor of O. madagascariensis and O. titanus sp. n. is assumed to have been distributed over the eastern belt of the island which was likely covered with some types of dry forests and thickets [35]. At the same time, some populations may have been able to cope with increased drought, at least in some refugia of western and southern Madagascar.
Lourenço et al. [19] proposed that the ancestral Monodopisthacanthus populations adapted to the dry conditions during the Paleocene in eastern Madagascar (Fig. 9), but could have disappeared from the eastern belt of the island when Madagascar entered the trade wind regime with increased humidity along this portion of the island. Some populations could have survived at the limit of the new rainforest belt where humidity was limited. Several species in the genus Opisthacanthus seem to reflect this scenario, as O. andohahela and O. piceus occurring in the humid subtropical forest of Andohahela, O. madagascariensis in the subhumid forests occurring in the western tsingy formations as encountered in Namoroka, or Bemaraha, O. pauliani in the subhumid forests of Ankarana, and finally O. titanus sp. n. in the forest of Torotorofotsy in the rainshadow of the Betsimisaraka mountains (Fig. 8).
The current distribution of the groups of Opisthacanthus and the affinities of the genus with the Cretaceous Protoischnurus axelrodorum in Brazil date back to distant times in a Gondwana model. The origins are thus considered during the Jurassic (201 Ma–145 Ma) and Lower Cretaceous (145 Ma–100 Ma) when the southern continental landmasses were still connected.
6 Further support for the Gondwanian model
After evolution of the respective populations of ancestral Opisthacanthus on the isolated landmasses, the resulting lineages with the most affinities would be those that would have been in contact in the most recent times. At the end of the Cretaceous, Antarctica, Africa and Madagascar were isolated from each other; South America was still connected with Antarctica, Madagascar was within the 30-degree subtropical arid belt [35] and peninsular India was detached from Madagascar. Peninsular India did only collide with Asia during the Eocene, between ca. 54 and 40 Ma [40,41]. We infer that ancestral Opisthacanthus survived the K–Pg at least in Africa, Madagascar and South America. Vicariance will take place during the following Cenozoic characterized by the breakup and changing palaeo-climate of Gondwana, at least during the Oligocene between 30 Ma and 28 Ma when the southern land masses where isolated (Fig. 9). To better understand the extant distribution of Opisthacanthus, we use a scenario approach to highlight possible branching of sister clades.
6.1 The Gondwana breakup
During the Jurassic Madagascar has drifted away Africa and Africa has drifted away Antarctica, while South America was still connected to both Africa and Antarctica. Indo-Madagascar started rifting away Africa during the lower Jurassic 182 million years ago (Ma), as early as 206 Ma [42] to as late as 160 Ma [43]. At the same time, Africa started rifting away Antarctica contiguously but at a lower starting velocity on the other side of the Gondwana. A land route of dispersal still existed between Antarctica and Indo-Madagascar (via the Kerguelen plateau), Antarctica and South America, Southern Africa and southern South America, and Western and Central Africa and northern South America (Fig. 10). Micro-continental blocks bridged Southern South America and Antarctica until the Lower Cretaceous [145–100 Ma], including Tierra del Fuego, South Georgia, and South Orkney [44]. Through the Jurassic and during the Lower Cretaceous 170–120 Ma, Southern Africa was disconnected from southern South America, while Indo-Madagascar's position differed with relation to Antarctica (Fig. 10). The cessation of the rifting between Madagascar and Africa occurred at 120 Ma [43]. At the time of the Middle Aptian at ca. 120 Ma, the connections between Indo-Madagascar and Antarctica were limited to a small fraction of the Kerguelen plateau that emerged [45,46]. Continuous land between terminal southern South-America and the Antarctic peninsula was maintained from the Upper Cretaceous [100–66 Ma] through Eocene [56–34 Ma] [47], and possibly until 30 Ma [48].
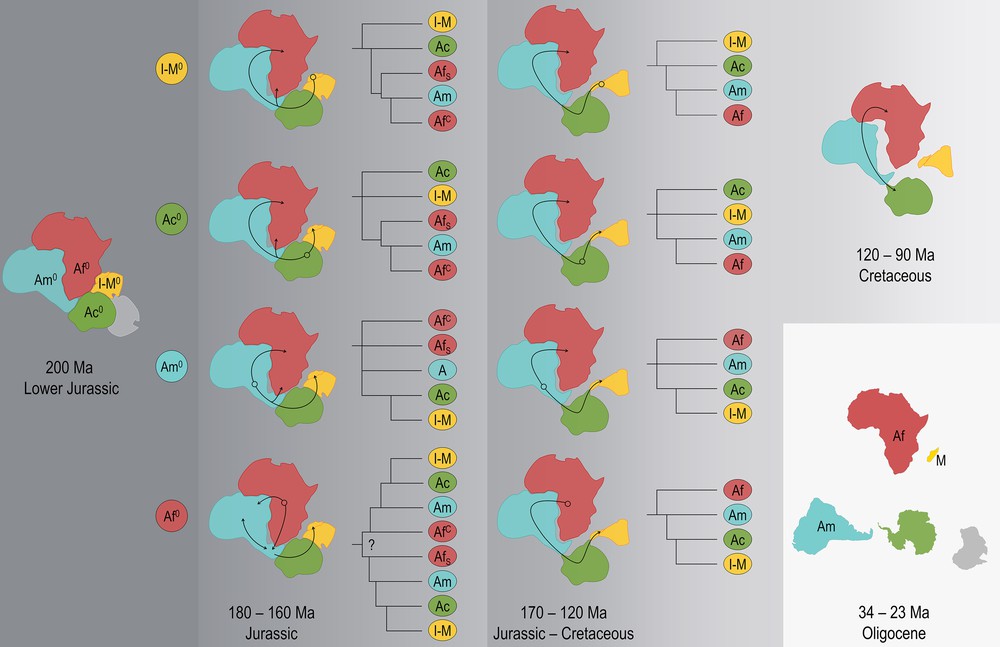
Scenarios of origin and potential dispersal of Opisthacanthus using the connected land masses after the breakup of the Gondwana some 200 Ma. The initial breakup of the Gondwana occurred simultaneously between Indo-Madagascar, Antarctica and Australia on one side, and between South-America and Africa on the other side. Indo-Madagascar started rifting away Africa during the lower Jurassic 182 million years ago (Ma), when Africa started rifting away Antarctica (2nd column from left). Between 140 and 120 Ma, a rapid increase of spreading velocity occurred between Africa and Antarctica, and Western Gondwana started breaking up with South America rifting away from Africa (column 3). Micro-continental blocks bridged Southern South America and Antarctica until the Lower Cretaceous [145–100 Ma]. The final breakup of Gondwanaland occurred in southern South America and the Antarctic peninsula. Origin in Indo-Madagascar or Madagascar = I-M0; in Antarctica = Ac0, in Southern America = Am0, in Africa = Af0. The resulting groups in Africa could have evolved in Western or Central Africa (AfC) or Southern Africa (AfS). As from the Oligocene, Africa (Af), South America (Am), and Madagascar (M) were all isolated from each other.
6.2 Scenarios of origin
We propose vicariant events during the Mesozoic with several scenarios of origins of the ancestral populations in the Gondwana, which are based on the slow evolution of scorpions in general, the ancestrality of the members of the family Hormuridae in particular, and the current distribution of the genus Opisthacanthus. We infer the phylogeny of the resulting populations in the isolated landmasses after the breakup of the Gondwana. Cenozoic over-water dispersal is not considered in the scenarios proposed.
Scenario 1: Madagascar origin
Antarctica is bridging Africa, South America and Madagascar; as long as Southern Africa was still connected to southern South America, an ancestral Indo-Madagascar or Madagascar Opisthacanthus (I-M0) could have dispersed clockwise from Indo-Madagascar towards Antarctica, South America, and from South America towards Southern Africa, or Western or Central Africa (Fig. 10). In this scenario, the American groups are more closely related to the African groups, and the Madagascar group has more affinities with the American groups than with the African groups. However, the split would have occurred during the Cretaceous (Fig. 10). The scenario with a Madagascar origin would imply that the youngest Opisthacanthus would occur in Africa, and the latest split between the lineages would have occurred between the South American and African lineages. The Madagascar lineage would show the least affinities with the two other lineages. The presence of a single group of Opisthacanthus in Madagascar can be explained by the small size of the island as compared to Africa, but the low group diversity in the New World as compared to the high group diversity observed in sub-Saharan Africa seems incompatible with a late arrival of an ancestral Opisthacanthus in Africa.
Scenario 2: Antarctic origin
The scenario with an ancestral Antarctic Opisthacanthus (Ac0) results with the same affinities between the current lineages as described for a Madagascar origin (e.g., closer affinities between Madagascar and New World lineages) (Fig. 10). The absence of Opisthacanthus in Antarctica is explained by the glaciation of the southern continent, and its absence from India could be explained by the global mass extinction at the K–Pg, or the local extinction occurring during the fast drifting of the Indian peninsula towards the North during the Upper Cretaceous. The absence of Opisthacanthus in Australia is more difficult to explain, especially because Antarctica has been proposed as a major dispersal pathway between South America and Australia during the Cenozoic [48]. The group could have been exterminated at the time of the K–Pg, or could have been outcompeted by a modern group of scorpions.
Scenario 3: American origin
An ancestral American Opisthacanthus (Am0) would imply the closest affinities between the American lineages and the African lineages, with possible routes of dispersal to Central and Southern Africa (Fig. 10). By the Lower Cretaceous, South America drifted away from Southern Africa, the latter was isolated from other land masses in Southern Gondwana, but Antarctica was still bridging South America and Madagascar, thus allowing dispersal of ancestral Opisthacanthus (Am0) to Indo-Madagascar via the Antarctic (Fig. 10). Such a scenario implies a Madagascar lineage closer to the South American lineage than to any other, as well as long branches, i.e. early split between the African and the New World lineages, which would therefore share some characteristics, e.g., a similar diversity in species and groups.
Scenario 4: African origin
Antarctica is bridging Africa, South America and Madagascar. An ancestral African Opisthacanthus (Af0) could have dispersed over Southern and Central Africa, and from there towards northern South America, as well as to Antarctica, before dispersing to Indo-Madagascar. From Central or Western Africa, Af0 could have dispersed towards northern South America, later to Antarctica, and Indo-Madagascar. After the drifting of South America during the Lower Cretaceous, the land dispersal routes would be limited to a route starting in Central or Western Africa towards Madagascar in anti-clockwise direction. This scenario is based on the assumption that the ancestral populations colonizing Madagascar should have done so during the Cretaceous, i.e. before Madagascar drifted away Antarctica. This also implies early dispersal from Africa towards South America.
The global affinities observed between the groups, as well as the high diversity of the genus in Africa with six groups widely distributed on the continent, including the africanus group occurring both in Central Africa and Southern Africa, would point towards an African origin of Opisthacanthus. The South American fossil (Protoischnurus axelrodorum (family Protoischnuridae) from Cretaceous Santana Formation from Crato area in Brazil [20] would therefore support an early dispersal to the New World during the Upper Jurassic, Lower Cretaceous, with further vicariance after the separation of the continents. Finally, the affinities shown between the groups of the New World and Madagascar would also be best supported by a later dispersal from the New World to Madagascar when Antarctica was still bridging these land masses during the Jurassic–Cretaceous 170–120 Ma (Fig. 10).
7 The Gondwanian model in other groups
There are many more cases of Gondwanian distribution in the Madagascar context, and they mostly relate to archaic or extinct groups. For instance, the primitive family Winteraceae R. Br. ex Lindl. of trees and shrubs is absent from Africa, represented in Malesia, Oceania, the Neotropics and in Madagascar with the monotypic narrow endemic monotypic genus Takhtajania M. Baranova & J.-F. Leroy [49].
The extinct Cretaceous frog Beelzebufo ampinga Evans, Jones, & Krause, 2008 from Madagascar has its closest living relatives, the members of the Subfamily Ceratophryinae Tschudi, 1838 occuring in South America [50–52]. Based on an explicit phylogenetic analysis, Ruane and colleagues [51] proposed a South American origin of the group.
The most relevant example to illustrate an ancient Gondwana lineage comes from an endemic beetle of Madagascar representing an ancient lineage with origins from Upper Triassic to Lower Jurassic (226 Ma–187 Ma). After the K–Pg, Madagascar served as a refugia for this beetle, Heterogyrus milloti Legros, 1953 which is currently the new case recognized for vicariance given that it diverged from its closest continental relative before the breakup of the Gondwana [53].
8 Conclusion
The implications of the biogeographic scenarios outlined above can be surprising given that they show vicariant events between the most distant land masses. The Gondwanian model to explain the origin of the ancestral Opisthacanthus is justified by the Cretaceous sister clade, or stem group, from Brazil. To fully understand the tree of the Opisthacanthus groups in the New World, Africa and Madagascar, one would need a larger sample of the entire clade, DNA sequence data, and more fossil material from various places to calibrate the phylogeny of the Opisthacanthus. With the information currently at hand, to explain the distribution and pattern of the Opisthacanthus groups, rather than the Cenozoic overwater dispersal, we privilege the Mesozoic vicariance with an origin in Africa, and Madagascar serving as a dead-end within Southern Gondwana.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgements
We are most grateful to Élise-Anne Leguin (Muséum, Paris) for her contribution to the preparation of the photos and plates.


